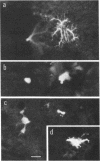
Abstract
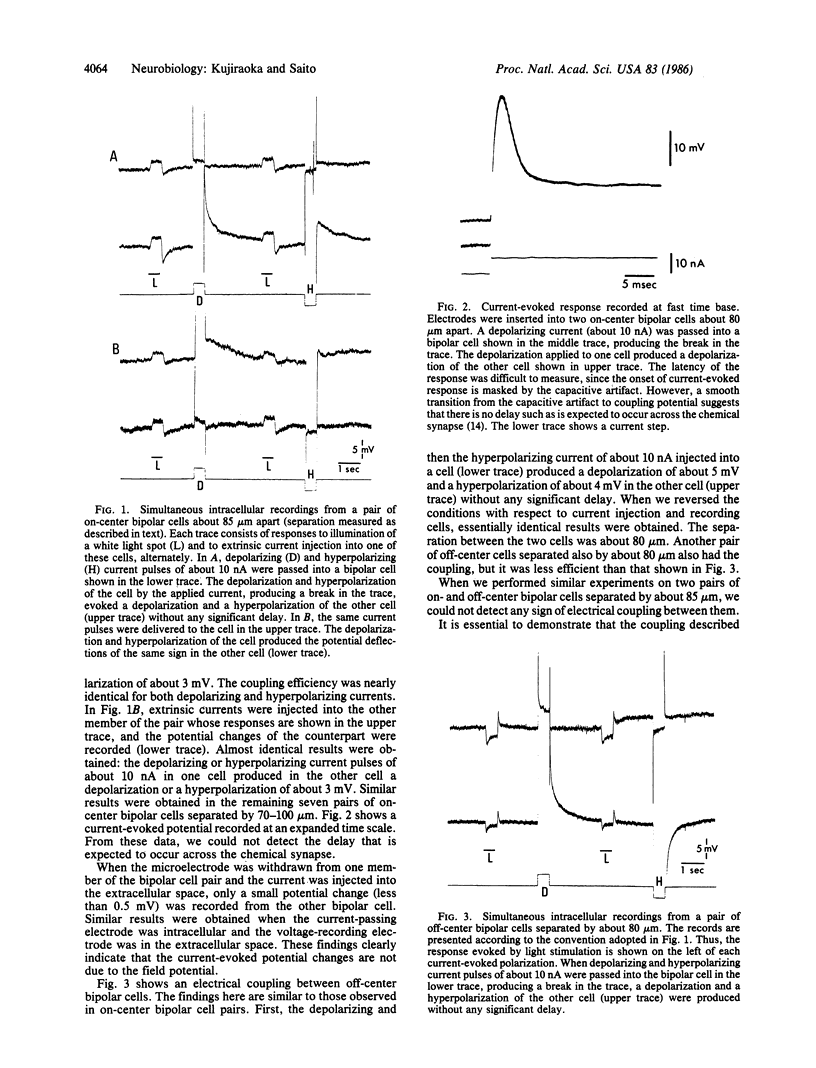
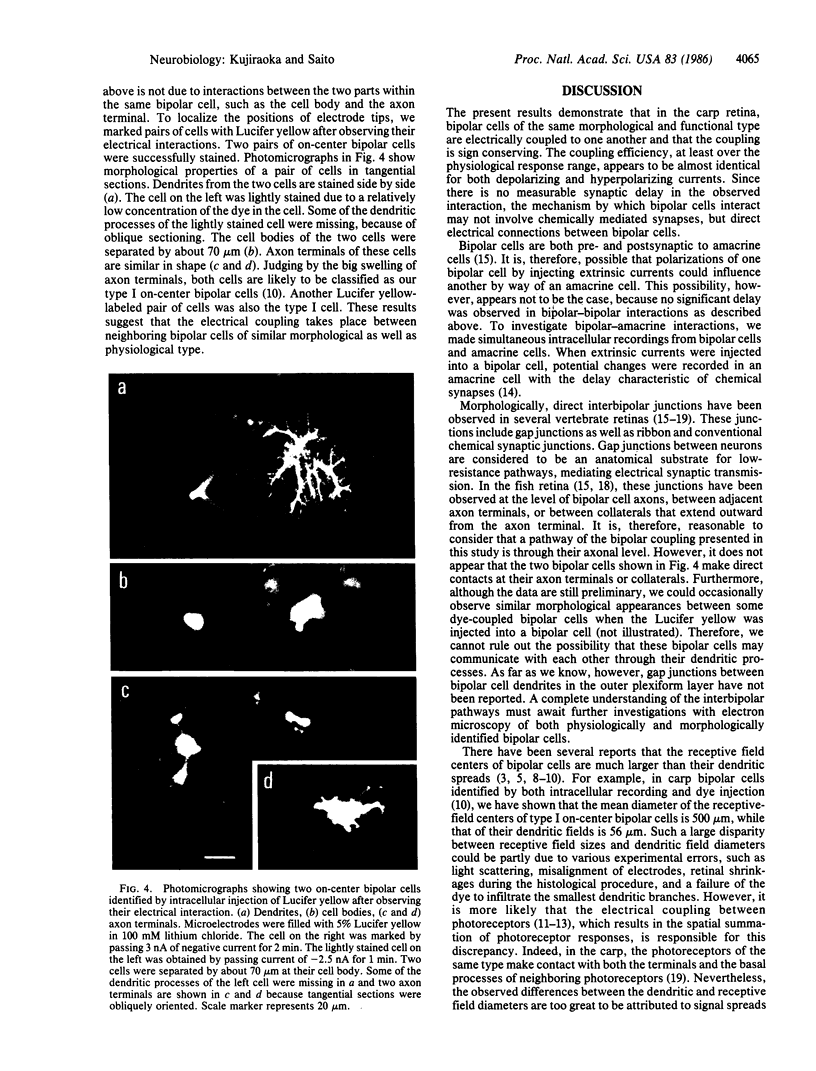
Intracellular recordings were made simultaneously from pairs of neighboring bipolar cells by advancing two independent microelectrodes into retinas of carp (Cyprinus carpio). Bipolar cells were identified by their response properties and in several samples were verified by intracellular injection of Lucifer yellow. Current of either polarity injected into one member of the bipolar cell pair elicited a signconserving, sustained potential change in the other bipolar cell without any significant delay. This electrical coupling was reciprocal, and it was observed between cell types similar in function and in morphology. Our results strongly suggest that there is a spatial summation of signals at the level of bipolar cells, which makes central receptive field areas much larger than their dendritic fields.
Keywords: intracellular recording, Lucifer yellow, receptive field, dendritic field
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Falk G. The single-photon signal in rod bipolar cells of the dogfish retina. J Physiol. 1980 Mar;300:151–166. doi: 10.1113/jphysiol.1980.sp013156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt D. A. Responses and receptive-field organization of cones in perch retinas. J Neurophysiol. 1977 Jan;40(1):53–62. doi: 10.1152/jn.1977.40.1.53. [DOI] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Coupling between rod photoreceptors in a vertebrate retina. Nature. 1976 Mar 4;260(5546):57–59. doi: 10.1038/260057a0. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Werblin F. S. Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. J Neurophysiol. 1969 May;32(3):315–338. doi: 10.1152/jn.1969.32.3.315. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Receptive field organization of bipolar and amacrine cells in the goldfish retina. J Physiol. 1973 Nov;235(1):133–153. doi: 10.1113/jphysiol.1973.sp010381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979 Jun;8(3):295–329. doi: 10.1007/BF01236124. [DOI] [PubMed] [Google Scholar]
- Marchiafava P. L. Horizontal cells influence membrane potential of bipolar cells in the retina of the turtle. Nature. 1978 Sep 14;275(5676):141–142. doi: 10.1038/275141a0. [DOI] [PubMed] [Google Scholar]
- Richter A., Simon E. J. Properties of centre-hyperpolarizing, red-sensitive bipolar cells in the turtle retina. J Physiol. 1975 Jun;248(2):317–334. doi: 10.1113/jphysiol.1975.sp010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Kujiraoka T. Physiological and morphological identification of two types of on-center bipolar cells in the carp retina. J Comp Neurol. 1982 Feb 20;205(2):161–170. doi: 10.1002/cne.902050207. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Rod-rod interaction in the retina of the turtle. J Physiol. 1975 Apr;246(3):617–638. doi: 10.1113/jphysiol.1975.sp010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J. I., Tonosaki K. Effect of polarisation of horizontal cells on the on-centre bipolar cell of carp retina. Nature. 1978 Nov 23;276(5686):399–400. doi: 10.1038/276399a0. [DOI] [PubMed] [Google Scholar]
- Van Haesendonck E., Missotten L. Interbipolar contacts in the dorsal inner plexiform layer in the retina of Callionymus lyra L. J Ultrastruct Res. 1983 Jun;83(3):303–311. doi: 10.1016/s0022-5320(83)90137-5. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Shakib M., Ripps H. Interreceptoral junctions in the teleost retina. Invest Ophthalmol. 1974 Dec;13(12):996–1009. [PubMed] [Google Scholar]
- Witkovsky P., Stell W. K. Retinal structure in the smooth dogfish Mustelus canis: electron microscopy of serially sectioned bipolar cell synaptic terminals. J Comp Neurol. 1973 Jul 15;150(2):147–167. doi: 10.1002/cne.901500204. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. T. Synaptic orgnization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol. 1974 Mar;3(1):1–33. doi: 10.1007/BF01111929. [DOI] [PubMed] [Google Scholar]