Abstract
Background
The implementation of mass spectrometry to measure serum 25-hydroxyvitamin D [25(OH)D] concentrations has led to concerns regarding the measurement and reporting of the C3-epimer of 25-hydroxyvitamin D3 [3-epi-25(OH)D3], for which there is a near-total lack of data regarding its clinical significance.
Methods
We developed a chromatographic method to resolve (>90%) 3-epi-25(OH)D3 from 25(OH)D3 using a pentafluorophenyl propyl chromatographic column. Using LC-MS/MS, we determined the serum concentrations of 25(OH)D3 and 3-epi-25(OH)D3 in 626 patients aged 3 days to 94 y undergoing routine vitamin D testing.
Results
Comparison between DiaSorin RIA and the new LC-MS/MS method for total 25(OH)D had acceptable agreement. Our data indicate an increase in 25(OH)D3 rather than a reduction in epimer concentration. An average of 3.3 ng/ml of 3-epi-25(OH)D3 was detected in adolescents and adults. Inclusion of 3-epi-25(OH)D3 in the total 25(OH)D3 concentration resulted in 9% (< 1 y) and 3% (1 to 94 y) potential misclassification of patients as vitamin D sufficient.
Conclusions
The new LC-MS/MS method is capable of chromatographically separating 25(OH)D3 and 3-epi-25(OH)D3. It was used to confirm that the contribution of 3-epi-25OHD3 to total 25OHD3 concentrations decreases with age in infants and is detectable in adults.
Keywords: Vitamin D, epimer, Liquid chromatography-tandem mass spectrometry, 25-hydroxy vitamin D
1. Introduction
It has been reported in recent years that commonly used immunoassays and LC-MS/MS based methods for the measurement of 25-hydroxy vitamin D3 [25(OH)D3] do not adequately detect or resolve the C-3 epimeric form of 25(OH)D3, 3-epi-25(OH)D3 [25(OH)D3]. Singh and colleagues demonstrated that elevated concentrations of 25(OH)D3 were reported for many infants by LC-MS/MS assays due to the presence of the C-3 epimer of 25(OH)D3 [1]. The C-3 epimer [3-epi-25(OH)D3] was partially resolved from native 25(OH)D3 with enhanced chromatography, but in that study, no epimer was detected in patients aged 1 to 97 y. It is unclear from where the epimer is derived, but supplements and endogenous metabolism are both possible. Fleet and colleagues have demonstrated reduced activity of 3-epi-1,25(OH)2D3 in a cell culture model of calcium uptake [2]; however, in another model, 3-epi-1,25(OH)2D3 suppressed secretion of PTH from parathyroid cells in vitro [3].
The growing number of potential roles for 25(OH)D status in disease has resulted in an increased awareness of not only the molecule itself, but also of the various methods used in its measurement. Although little is definitively known regarding the in vivo importance of 3-epi-25(OH)D3, clinical laboratories face the decision of whether or not to include 3-epi-25(OH)D3 in the measurement of total 25(OH)D [4,5]. In order to better understand the contribution of 3-epi-25(OH)D3 to total 25(OH)D, we developed a novel method to chromatographically resolve 3-epi-25(OH)D3 from native 25(OH)D3. Here we report key method parameters of this assay and the results of a multi-institutional study on 3-epi-25(OH)D3 contribution to total 25(OH)D3 concentration.
2. Methods
2.1 25(OH)D analysis by LC-MS/MS
Samples were extracted as previously described [6]. Briefly, 200 μl of standard, control, blank or patient specimen was mixed with 200 μl of 1 mol/lNaOH and incubated for 15 min prior to liquid-liquid extraction using 1 ml n-heptane. After freezing in dry ice-acetone bath or a −20°C freezer, the organic layer was transferred to a new vessel, dried down under nitrogen, and resuspended in ethanol. 25(OH)D3 and 3-epi-25(OH)D3 were resolved using a PFP Propyl 100 × 3.2 mm, 5 μm analytical column (Restek) equipped with an XTerra MS C8 2.1 × 10 mm, 3.5 μm guard column using an Acquity UPLC (Waters) and the molecules were detected using a Quattro micro tandem mass spectrometer. Between-day precision was <10% CV and limits of detection were similar to those previously described [6]. Resolution between 25(OH)D3 and 3-epi-25(OH)D3 was >90% (Supplemental Fig. 1). A peak likely corresponding to 3-epi-25(OH)D2 was detected (Supplemental Fig. 1), but no experiments were conducted to confirm the presumed identification of 3-epi-25(OH)D2 due to a lack of commercially available 3-epi-25(OH)D2 material. We validated the presence of 3-epi-25(OH)D3 as illustrated in Supplemental Figure 2.
2.2 Data Collection
Data collected from routine assessment of vitamin D status on 626 healthy outpatients ages 3 days to 94 y seen at Seattle Children’s Hospital or at the University of Arkansas for Medical Sciences were evaluated for the contribution of the 3-epi-25(OH)D3 to the total 25(OH)D3 concentration. To convert from SI units (nmol/l) to conventional units (ng/ml) divide by 2.5.
2.3 Data Analysis
Data were tabulated in a Microsoft Excel spreadsheet, with statistical analyses and graphing performed with the R statistics language [7] and ggplot2 graphics package [8]. Deming regression from the R package MethComp [9] was conducted using 1000 boot-strap iterations.
3. Results
To evaluate the method, we measured 25(OH)D3 and 3-epi-25(OH)D3 in samples collected for routine clinical assessment of vitamin D status in 100 patients at the University of Arkansas for Medical Sciences ages 25–94 y under IRB-approved guidelines. Using the standard C18 column from our original assay [6], the comparison of total 25(OH)D (i.e., 25(OH)D2 and 25(OH)D3) with RIA revealed a proportional bias of 15% while the total 25(OH)D measured with the PFP propyl column had a proportional bias of 17%. After subtracting the 3-epi-25(OH)D3 concentrations calculated from the PFP propyl method, the C18 non-epimeric values had a proportional bias of 1% while the PFP propyl non-epimeric values had a proportional bias of 2% (Supplemental Fig. 3). The results indicate the lack of agreement of LC-MS/MS assays with the RIA method is partially attributable to the presence of 3-epi-25OHD3, a metabolite reported not to cross-react in the Diasorin RIA method [1]. To rule out the possibility that calibration could have resulted in the differences we observed, we also analyzed the SRM 972 reference material from NIST [10], which demonstrated 100–108% (mean 103.3%) recovery of non-epimeric 25(OH)D3.
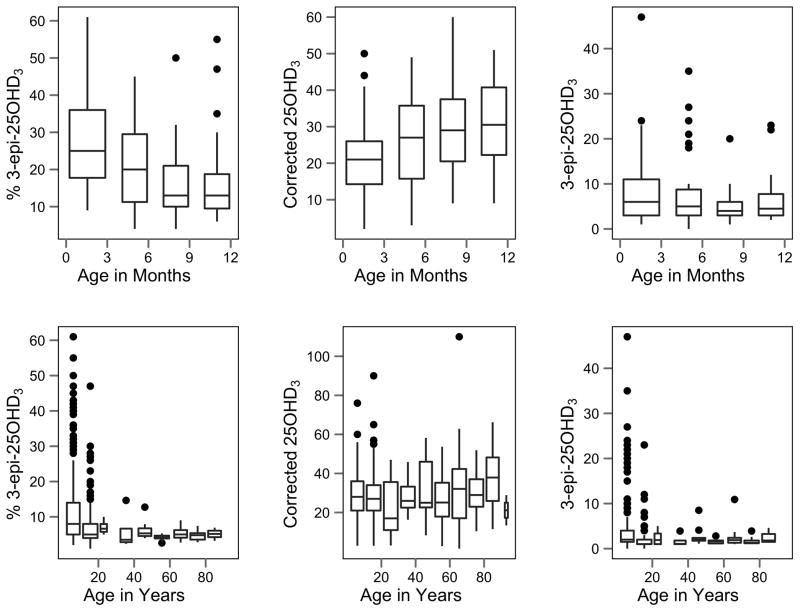
Prior publications in the healthy population have indicated the percent of 3-epi-25(OH)D3 identified by LC-MS/MS decreases substantially with age in infants [1] but that it may still be quantifiable in adults [11]. We analyzed samples collected from 125 patients at Seattle Children’s Hospital aged 3 months to 1 y to determine changes in total 25OHD3 (i.e, 25(OH)D3 and 3-epi-25(OH)D3), non-epimeric 25(OH)D3, 3-epi-25OHD3, and 3-epi-25(OH)D3 as a percentage of the total 25(OH)D3 [% 3-epi-25(OH)D3]. Infants were divided into four groups cut at 3, 6, and 9 months for graphical representation (Fig. 1). To determine the degree of correlation between age and 25(OH)D3 forms, a corresponding Kendall tau nonparametric correlation coefficient and significance level was determined. No statistically significant trend was present for 3-epi-25(OH)D3; however, a significant negative trend for % 3-epi-25(OH)D3 (τ = −0.28, p < 0.001) and a significant positive trend for 25(OH)D3 (τ = −0.27, p = 0.003) were observed, providing evidence that the observed decrease in percent 3-epi-25(OH)D3 in infants is likely due to a constant 3-epi-25(OH)D3 concentration in parallel with an increase in non-epimeric 25(OH)D3 levels.
Figure 1. Distribution of 25(OH)D forms across age groups for 125 infants ranging from birth to 12 months and 624 patients ranging from 1–94 y.
(Top) In the infant population, no statistically significant trend was present for 3-epi-25(OH)D3; however, a significant negative trend for % 3-epi-25(OH)D3 (τ = −0.28, p < 0.001) and a significant positive trend for 25(OH)D3 (τ = −0.27, p = 0.003) were observed. (Bottom) For patients aged 1–94 y, no statistically significant trend was present for corrected 25(OH)D3; however, a significant negative trend was observed for 3-epi-25(OH)D3 (τ = −0.20, p < 0.001) and % 3-epi-25(OH)D3 (τ = −0.27, p < 0.001). Units for 25(OH)D3 and 3-epi-25(OH)D3 are nmol/l.
Previous reports are divided on whether 3-epi-25(OH)D3 is present in normal adult plasma [1,11]. To determine the degree of 3-epi-25(OH)D3 presence in adulthood, we measured total 25(OH)D3, non-epimeric 25(OH)D3, 3-epi-25(OH)D3, and the percent 3-epi-25(OH)D3 in 501 healthy, outpatients aged 1–94 y seen at the Seattle Children’s Hospital or at the University of Arkansas for Medical Sciences. Patients were divided into age groups of 10 y for graphical representation (Fig. 1). Consistent with the current suggestion of detectable epimer in adults, we detected a median value of 8.25 nmol/l across all ages (range 0–117.5 nmol/l). To determine the degree of correlation between age and 25(OH)D3 forms, a corresponding Kendall tau nonparametric correlation coefficient and significance level was determined. No statistically significant trend was present for non-epimeric 25(OH)D3; however, a significant negative trend was observed for 3-epi-25(OH)D3 (τ = −0.20, p < 0.001) and % 3-epi-25(OH)D3 (τ = −0.27, p < 0.001), demonstrating that while 3-epi-25(OH)D3 is relatively constant throughout life, there is a slight decline in later years. Importantly, these are patients commonly afflicted by metabolic bone abnormalities. Supplemental Figure 4 presents a scatter plot of 3-epi-25(OH)D3 vs. non-epimeric 25(OH)D3, which demonstrates a subtle but statistically significant positive correlation between the two forms (r=0.22, p<0.0001).
We observed a wide range of the amount of epimer contributing to total 25(OH)D3 across the population. Epidemiologic and other evidence that have guided the development of dietary guidelines for vitamin D sufficiency have relied on the DiaSorin RIA assay, which has been reported to have no cross-reactivity with the epimeric form of 25(OH)D3 (reviewed in [5]). Given the potential for 3-epi-25(OH)D3 to substantially increase the total 25(OH)D3 concentations measured by LC-MS/MS, we determined the frequency that a deficient patient could be misclassified as sufficient due to the inclusion of the epimer concentration in an LC-MS/MS assay compared with RIA. Classifications were first made using 50 nmol/l of total 25(OH)D3 followed by reclassification using 50 nmol/l of the non-epimeric 25(OH)D3. Comparing the results of the two classification schemes, 84 infants (67%) were classified as sufficient, 30 infants (24%) were classified as deficient, while 11 (9%) were misclassified as sufficient using total 25(OH)D3 when their non-epimeric 25(OH)D3 level indicated deficiency. For children and adults (1–94 y), 388 patients (77%) were classified as sufficient, 96 (15%) were classificed as deficient, while 17 (3%) were misclassified using total 25(OH)D3 as sufficient when their non-epimeric 25(OH)D3 level indicated deficiency.
4. Discussion
The implementation of novel, LC-MS/MS based methods in the clinical laboratory has been challenging due to the lack of agreement frequently seen with current antibody-based methods of detection. The measurement of 25(OH)D is an interesting example where a majority of published LC-MS/MS methods are incapable of separating the C-3 epimer and non-epimer forms. While it is possible that 3-epi-25(OH)D will be an important biomarker in adult medicine, the population studies needed to demonstrate its importance still need to be performed. Importantly, the presented method allows for the quantification of 25(OH)D3 with adequate separation of the epimeric form to begin performing these studies.
For the laboratory, the ability to separately quantify the epimeric forms of 25(OH)D3 generates a new question: do we include 3-epi-25(OH)D3 in the total 25(OH)D3 concentration, exclude it, or report it separately? It has been advocated for patient populations less than one year of age, in which the epimeric form ranges from 4–61% of the total 25(OH)D3 concentration, that the epimer be excluded for medical decision-making [1]. This recommendation is at least partly based on one study in which the active metabolite of 3-epi-25(OH)D3 has been shown to have reduced efficacy in cellular calcium regulation in vitro when compared to the non-epimeric form [2]. We have now demonstrated a wide range of 3-epi-25(OH)D3 concentrations in adults (0–117 nmol/l or 0–61% of the total) and an apparent misclassification rate of 3% in adults compared with 9% in infants. Importantly, no data were gathered regarding vitamin D supplementation, use of hyperalimentation, or medications. However, given the near-total lack of physiological data for the activity of 3-epi-25(OH)D3 in humans, it seems that either excluding 3-epi-25(OH)D3 from 25(OH)D3 or reporting it separately in LC-MS/MS assays may be prudent for adult populations as well.
5. Conclusion
The clinical utility of measuring 3-epi-25(OH)D3 is at present unclear. It was recently decided that the ongoing NHANES study monitoring 25(OH)D would utilize methods capable of separating the epimeric forms of 25(OH)D3, a clear indication that epimer concentration is of continued interest to the fields of nutrition and laboratory testing. In addition, the lack of agreement between 25(OH)D assays in conjunction with the varying concentration of 3-epi-25(OH)D3 in patient samples prompted the development of a traceable reference material by the National Institute of Standards and Technology with a Certificate of Analysis including values for 25(OH)D2, 25(OH)D3 and 3-epi-25(OH)D3 [10]. For now, though the clinical role of 3-epi-25(OH)D3 remains unclear, it is increasingly apparent that laboratories using LC-MS/MS based methods for 25(OH)D measurement, specifically in patients <1 y, should strive to implement methods that separate the epimeric form of 25(OH)D3 in order to provide more complete 25(OH)D results.
Supplementary Material
Highlights.
A new LC-MS/MS assay resolves C-3-epi-25-hydroxyvitamin D from native analyte
Concentrations of C-3-epi-25-hydroxyvitamin D in infants and adults were determined
The concentration of epimer declines with age, but is still present in adults
The new assay correlates well with RIA, unlike many other LC-MS/MS methods
Acknowledgments
This work was partially supported by the University of Washington Nutrition and Obesity Research Center (DK035816).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh RJ, Taylor RL, Reddy GS, Grebe SKG. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. The Journal of clinical endocrinology and metabolism. 2006;91:3055–3061. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 2.Fleet JC, Bradley J, Reddy GS, Ray R, Wood RJ. 1 alpha,25-(OH)2-vitamin D3 analogs with minimal in vivo calcemic activity can stimulate significant transepithelial calcium transport and mRNA expression in vitro. Archives of biochemistry and biophysics. 1996;329:228–234. doi: 10.1006/abbi.1996.0213. [DOI] [PubMed] [Google Scholar]
- 3.Brown AJ, Ritter C, Slatopolsky E, Muralidharan KR, Okamura WH, Reddy GS. 1Alpha,25-dihydroxy-3-epi-vitamin D3, a natural metabolite of 1alpha,25-dihydroxyvitamin D3, is a potent suppressor of parathyroid hormone secretion. J Cell Biochem. 1999;73:106–113. [PubMed] [Google Scholar]
- 4.Schleicher RL, Encisco SE, Chaudhary-Webb M, Paliakov E, McCoy LF, Pfeiffer CM. Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin Chim Acta. 2011;412:1594–1599. doi: 10.1016/j.cca.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Yetley EA, Pfeiffer CM, Schleicher RL, et al. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr. 2010;140:2030S–2045S. doi: 10.3945/jn.110.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoofnagle AN, Laha TJ, Donaldson TF. A rubber transfer gasket to improve the throughput of liquid-liquid extraction in 96-well plates: application to vitamin D testing. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2010;878:1639–1642. doi: 10.1016/j.jchromb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Team RDC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- 8.Wickham H. ggplot2: elegant graphics for data analysis. Springer; New York: 2009. [Google Scholar]
- 9.Carstensen B, Gurrin L. R package version 11–6. 2010. MethComp: Functions for analysis of method comparison studies. [Google Scholar]
- 10.Phinney KW. Development of a standard reference material for vitamin D in serum. The American journal of clinical nutrition. 2008;88:511S–512S. doi: 10.1093/ajcn/88.2.511S. [DOI] [PubMed] [Google Scholar]
- 11.Stepman H, Vanderroost A, Stockl D, Thienpont L. Full-scan mass spectral evidence for 3-epi-25-hydroxyvitamin D(3) in serum of infants and adults. Clin Chem Lab Med. 2010 doi: 10.1515/CCLM.2011.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.