Abstract
Background
Obesity and diabetes are epidemic in the predominantly minority Harlem community. To address them, a coalition of community and academic leaders tested the effectiveness of a peer-led weight loss course.
Methods
The coalition developed Project HEAL: Healthy Eating, Active Lifestyles through extensive collaboration with community members and experts in nutrition, exercise, and peer education. We piloted the course in a local church and assessed its impact through pre and post course weights, self-reported behaviors and quality of life.
Results
Twenty-six overweight and obese African American adults lost a mean of 4.4 pounds at 10 weeks, 8.4 pounds at 22 weeks, and 9.8 pounds at 1year. Participants reported decreased fat consumption and sedentary hours, and improved health related quality of life.
Conclusions
A peer-led, community-based course can lead to weight loss and behavior change. The minority communities most affected by obesity and diabetes may benefit from this low-cost, culturally appropriate intervention.
Keywords: Diabetes, obesity, weight loss, peer education, nutrition, physical activity, community-based participatory research
Obesity and diabetes in the U.S. are burgeoning public health problems that lead to significant economic cost, medical complications, and increased mortality.1–5 Communities of color bear a disproportionate share of the national burden of obesity and diabetes.6–9 Weight loss decreases diabetes incidence and mortality,10–20 even eliminating racial and ethnic disparities in diabetes incidence.10 However, health providers frequently lack the time or training to work with obese patients.21–23 Physicians are spending less time counseling obese patients to lose weight, even as obesity rates rise.24 Nutritionists are often monolingual, and not readily available in the non-White, low-income communities that need them most.25–27
In contrast, peer educators are widely available, cost-effective, and culturally attuned, and could be of great benefit in communities with limited resources and a history of medical mistrust.28–34 Peer programs may be sustained long past the period of the grant funding that demonstrates their effectiveness, distinguishing them from short-term projects that collapse when funding sources are withdrawn.
East Harlem (EH) presents a unique opportunity to study the potential role of peer education in facilitating weight loss. A low-income community, EH is 36% Black and 52% Latino, and has the highest prevalence of obesity and of diabetes in New York City. Compared with their predominantly White, high-income neighbors on the Upper East Side, EH residents suffer 4-fold greater rates of obesity, 7-fold greater rates of diabetes, and over 5-fold greater diabetes mortality.35–38
The East Harlem Diabetes Center of Excellence is a community-academic coalition that came together to address this epidemic.39–41 The coalition operates on the principles of community-based participatory research, an egalitarian collaboration among community, clinical, and public health leaders that recognizes the unique strengths of all partners and equitably involves all partners in research.42–44 To evaluate the effectiveness of a peer-led nutrition and physical activity course in leading to weight loss, the coalition sought to marry elements of successful weight loss interventions with the practical and culturally and economically appropriate elements of peer education.
Methods
Intervention development
The coalition formed a sub-committee of local nutritionists, health professionals, and outreach workers to develop a program patterned after the proven-effective Stanford University Chronic Disease Self-Management Program.30–31 Stanford uses peer leaders with backgrounds similar to those of the participants, and includes weekly action plans, group feedback and support to inspire change and to model self-management and problem-solving. Participants need not be literate. We worked with Stanford’s developers to focus their program to address weight loss.
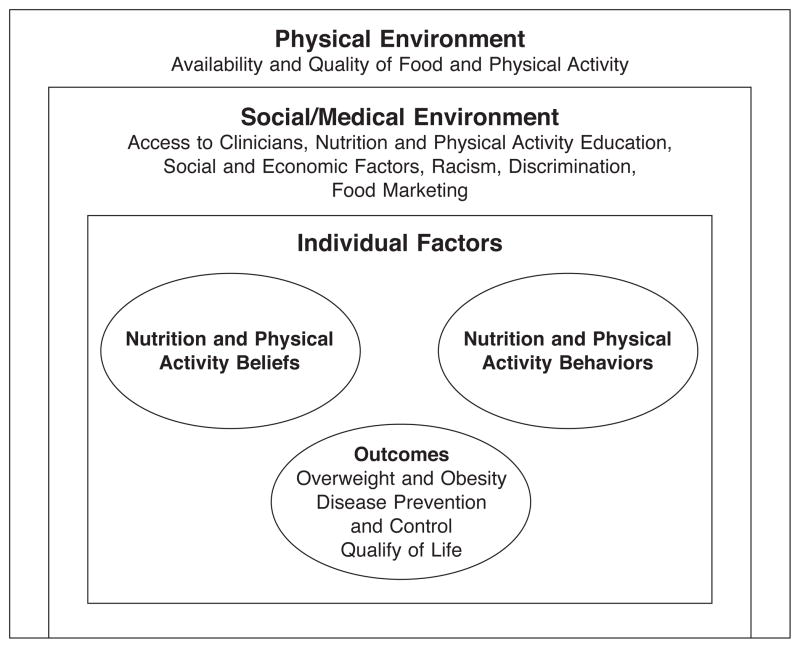
Using local survey and focus group data and their own experiences, sub-committee members built a conceptual model that details barriers to weight loss in EH (Figure 1).39,40,45 The subcommittee identified relevant diet and exercise messages by reviewing weight loss curricula and consulting with experts in nutrition, physical activity, community outreach, and education, to include content that conformed to accepted guidelines and to take into account the environmental, economic, and cultural realities of EH. The group identified key messages, including 1) portion control; 2) filling half the plate with fruits and vegetables of multiple colors at each meal; 3) drinking calorie-free beverages; 4) cutting fat; 5) making daily life more active; and 6) eating healthy food on a budget and at fast food venues. We shortened the original Stanford course from 150 to 90 minutes, and added two refresher classes,30 resulting in 8 sessions spread over 10 weeks.
Figure 1.
Conceptual model.
All course elements, from the name to content to program evaluations, were the fruits of community-academic collaboration. The coalition named the course Project HEAL: Healthy Eating, Active Lifestyles. The Mount Sinai Institutional Review Board approved the pilot course.
Assessment
The primary outcome was change in weight. To assess body mass index (BMI), we measured weights (on a digital scale) and heights after asking participants what they thought their weights and heights were. To assess secondary outcomes, including changes in knowledge, attitudes, and behaviors, we chose validated scales for domains including knowledge about healthy eating, exercise, and weight;46 food choice;47 total fat, fruit and vegetable intake;48–49 physical activity;50–51 sedentary time;52 barriers to healthy eating and exercise;53–54 body image; weight loss locus of control;55 depressive symptoms;56 perceived health-related quality of life;57–59 food security; demographic questions; and self-reported height and weight.60–62 We added items to assess food preparation, food shopping, and perception of neighborhood food quality, that we developed and employed in an earlier survey.39 A post-intervention survey also included items so participants could provide feedback about the course. The survey was at a sixth grade reading level.
Intervention conduct
We pilot-tested the course from April to June 2006 at a local Harlem church. We wanted to test the course in English before developing a Spanish-language version, so we responded to a request by a Black church in Harlem whose congregants had expressed interest in information about losing weight. We posted fliers at the church advertising a free course on how to eat healthier, be more active, and lose weight, and the pastor encouraged congregants to attend. Church leaders were involved in the logistics of recruitment, and they decided the course should be offered to the first 30 individuals who signed up.
After providing written informed consent, course participants completed baseline surveys, and the research team measured their heights and weights without shoes, coats, or sweaters. Two trained peer leaders led the 8 sessions at the church. Ten weeks after enrollment, at the eighth and final session of the course, and at 22 and 32 weeks and 1 year after enrollment, trained research assistants, who were blinded to patients’ baseline weights, surveyed and weighed participants. Participants received a five-dollar gift for each completed survey.
Statistical analysis
We performed analyses using SPSS versions 13 and 14 (SPSS Inc., Chicago, IL). To characterize participants’ baseline characteristics, we used descriptive statistics. To evaluate course impact, we compared pre-course and post-course data using paired samples t-tests at a significance level of p<.05 for continuous data, and a non-parametric Wilcoxon matched-pairs signed rank test at a significance level of p<.05 for categorical data.
Results
Forty Black church members came to hear about the course, and of these, 32 enrolled in the pilot study. Of the 8 who did not enroll, 2 declined signing informed consent, and 6 did not find the time that was agreed upon by the majority of the group convenient. One person of the 32 enrolled was not overweight (BMI = 21.2 kg/m2), was in fact interested in gaining weight, and was excluded from data analysis. Five completed only the first one or two sessions and were excluded from analysis, but were not significantly different in weight, BMI, race, age, gender, income, or education from those who completed the pilot.
The remaining 26 participants attended an average of 6 (75%) of the 8 sessions. Their mean age was 68 years (Table 1). Most were female, moderately well-educated, retired, and lived in low-income households. Over one quarter received emergency food in the past year. Participants reported having a mean of 2 medical conditions, the most common being hypertension and arthritis. At baseline, mean weight was 194.3 pounds, and mean body mass index (BMI) was obese at 32.7 kg/m2 (range 24.3 to 43.7 kg/m2). Although 25 of the 26 (96.1%) were overweight (BMI 25–29.9 kg/m2) or obese (BMI ≥30kg/m2) one third self-reported as normal weight (BMI 18.5–24.9 kg/m2). Interestingly, when comparing what participants thought they weighed with their measured weights, group members weighed, on average, 10 pounds more than they thought (range −0.5 to 42 pounds). They did correctly estimate their heights.
Table 1.
BASELINE CHARACTERISTICS
| Characteristics | Pilot study participants |
|---|---|
| Total Participants, n | 26 |
| Age, years (SD) | 68.3 (10.0) |
| Female, n (%) | 21 (81%) |
| African American, n (%) | 26 (100%) |
| At least some college education, n (%) | 18 (69%) |
| Employment, n (%) | |
| Working full-time or part-time | 5 (19%) |
| Retired | 19 (73%) |
| Yearly household income, n (%) | |
| Less than $15,000 | 6 (23%) |
| $15,000 to $30,000 | 9 (35%) |
| Greater than $30,000 | 5 (19%) |
| Unknown | 6 (23%) |
| Received emergency food in the past 12 months, n (%) | 7 (27%) |
| Co-morbidities, mean (SD) | 1.9 (1.1) |
| Depressive symptoms, n (%) | 7 (27%) |
| Weight in pounds, SD | 194.3 (38.0) |
| Difference between actual and self-reported weight, pounds (SD) | 10.4 (10.0) |
| Difference between actual and self-reported height, centimeters (SD) | .2 (1.4) |
| Body mass index (BMI), kg/m2 (SD) | 32.7 (5.1) |
| Normal weight (BMI 18.5 to 24.9), n (%) | 1 (4) |
| Overweight (BMI 25.0 to 29.9), n(%) | 7 (27) |
| Obese (BMI ≥30), n (%) | 18 (69) |
Table 2 shows that the 26 participants lost a mean of 4.4 pounds (p<.001) or 2.2% of their body weight at 10 weeks. Additional follow up data for 21 of the 26 participants (81%) at 22 weeks, 32 weeks and one year, reveal that group members continued to lose weight. At one year, the mean weight was 185 pounds and the mean BMI was 30.9 kg/m2 (p=0.001). Participants had lost a mean of nearly 10 pounds, or 5% of their initial body weight (range 1.5% to −17.7%).
Table 2.
MEASURED CHANGES IN WEIGHT
| Weight change from baseline
|
||
|---|---|---|
| Pounds, n (SD) | Weight change, % (SD) | |
| Baseline (n=26) | ||
| 10 weeks (n=21)* | −4.4 (5.0) | −2.2 (2.5) |
| 22 weeks (n=21)* | −8.4 (6.7) | −4.2 (3.2) |
| 32 weeks (n=21)** | −8.4 (12.3) | −4.3 (6.4) |
| 1 year (n=21)*** | −9.8 (11.1) | −5.0 (5.5) |
p<.001,
p=.003,
p=.001.
Participants’ self-reported food intake changed from before to after the course (Table 3). At 10 weeks, participants had significantly decreased daily total fat intake from 87.7 to 80.1 grams (recommended daily fat intake is less than 65 grams based on a 2000 kcal diet), daily saturated fat from 23.4 to 20.6 grams (recommended <20 grams), and daily cholesterol intake from 261.9 to 237.0 mg (recommended <300 mg).38 At 1 year, changes were maintained, but not with statistical significance. Daily servings of fruit and vegetables also increased significantly at all time-points (from 3.7 to 4.4 servings per day from baseline to 1 year).
Table 3.
BASELINE AND POST-INTERVENTION DIETARY HABITS
| Base line n=26 | 10 weeks n=26 | Difference from baseline | 22 Weeks n=21 | Difference from baseline | One year n=21 | Difference from baseline | |
|---|---|---|---|---|---|---|---|
| Daily fat intake-grams (SD) | 87.7 (25.3) | 80.1 (19.8) | p=.046 | 83.7 (20.1) | p=.027 | 78.6 (22.2) | p=.082 |
| Daily saturated fat-grams (SD) | 23.4 (10.5) | 20.6 (8.9) | p=.046 | 22.0 (9.3) | p=.027 | 20.4 (9.3) | p=.082 |
| Total fat, % (SD) | 33.2 (6.4) | 31.2 (5.0) | p=.046 | 32.1 (5.1) | p=.027 | 30.9 (5.6) | p=.082 |
| Dietary cholesterol-mg (SD) | 261.9 (98.3) | 237.0 (85.5) | p=.046 | 248.7 (90.0) | p=.027 | 236.4 (88.3) | p=.082 |
| Servings fruits & veg. Daily, n (SD) | 3.7 (1.7) | 3.9 (1.4) | p=.548 | 4.4 (1.7) | p=.041 | 4.4 (2.1) | p=.039 |
| Fast food, days/week (SD) | 1.5 (1.8) | 1.0 (1.0) | p=.130 | 1.2 (1.0) | p=.505 | 1.3 (1.4) | p=.540 |
Table 4 shows other changes in self-reported knowledge and behaviors. The number of days per week that participants engaged in more than 30 minutes of moderate exercise did not increase significantly.50–51 Amount of sedentary time,52 defined as hours per day that participants spend watching television, videos, or DVDs, decreased by more than 1 hour per day at 10 weeks (p=0.034), and by nearly 3 hours at 1 year (p<.001). Knowledge about diet, exercise, and weight loss,46 using a composite three point scale, increased significantly from 2.6 to 2.8 (p=0.003) at 10 weeks, but these data were not collected subsequently. Weight loss locus of control,55 which revealed a greater internal than external locus of control at baseline, remained unchanged.
Table 4.
BASELINE AND POST-INTERVENTION BEHAVIORS AND KNOWLEDGE
| Base line n=26 | 10 weeks n=26 | Difference from baseline | 22 Weeks n=21 | Difference from baseline | One year n=21 | Difference from baseline | |
|---|---|---|---|---|---|---|---|
| Exercise, days/week (>30 mins/day) (SD) | 3.7 (2.8) | 4.6 (2.2) | p=.115 | 4.2 (1.9) | p=.800 | 3.9 (2.5) | p=.783 |
| Sedentary activity, hours/day (SD) | 5.4 (3.5) | 4.1 (2.3) | p=.034 | 4.5 (2.6) | p=.246 | 2.5 (1.5) | p<.001 |
| Diet and exercise knowledge (Scale 1–3) (SD) | 2.6 (.4) | 2.8 (.2) | p=.003 | not collected | not collected | ||
| Weight loss locus of control (scale 4–16) (SD) | 12.8 (2.3) | 12.8 (2.0) | p=.872 | 12.1 (2.6) | p=.171 | 12.7 (2.3) | p=.553 |
| Health related quality of life | p=.046 | p=.132 | p=.212 | ||||
| Poor or fair | 26.9% | 23.1% | 14.3% | 19.0% | |||
| Very good or excellent | 19.2% | 38.5% | 33.3% | 28.6% |
Perceived health-related quality of life57–59 improved significantly. The number of participants who labeled their health very good or excellent doubled from the start to the end of the course (p=.046). This changed endured, but no longer retained statistical significance at one year. Twenty seven percent of participants screened positive for depressive symptoms56 at baseline, and this did not change significantly.
Discussion
Community and academic partners from a diabetes coalition developed and tested a peer-led nutrition and physical activity course in Harlem. Pre-post comparisons among the 26 African American participants in the pilot showed that participants lost nearly 10 pounds (5% body weight) at one year, increased food and exercise knowledge, changed food and exercise related behaviors, and reported improved health-related quality of life. Weight loss was maintained at one-year after enrollment, making it unlikely that weight loss was due to seasonal variations.
In the past, peer education interventions have been shown to change knowledge and behaviors.28–34 This is one of the first studies to our knowledge (if not the first) to show that a peer-led program can lead to weight loss. This represents a promising approach to combat the obesity and diabetes epidemics across the nation, in a feasible, low-cost way. Previous studies that have demonstrated weight loss required significant resources, and therefore the programs tested were often not sustainable past their period of grant funding. Project HEAL harnessed the experience and knowledge of professionals and provided this information to laypeople in an easily accessible and easily understood form. By targeting existing social networks to enroll participants, by locating programs in places where residents already gather, and by utilizing community members who already have the trust of their neighbors as teachers, Project HEAL sought to weave itself into the fabric of the vibrant Harlem community and avoid the pitfalls of so-called drive-by, or helicopter research.
The study is limited in that it is a small pilot study with a relatively homogeneous pool of participants with follow up only to one year, and with a pre-post design that lacks a control group. Follow-up data were available for 21 of 26 (81%) participants, which limits both generalizability and the sample size with which to gauge statistical significance. Future research should evaluate a larger and more diverse population in a randomized-controlled trial. The study may have selected out participants who were more motivated than average, as some church members had expressed to the pastor that congregants wanted information about losing weight, and all volunteered to come to the class.
Our primary and most objective measure, change in weight, was achieved and was maintained at one year of follow-up. Interestingly, more subjective process measures, including self-reported exercise and food intake, did not all parallel weight loss. The small sample size may have precluded statistical significance in some cases, and we used brief screening tools, which were not consistently sensitive to change in other studies.48–52 The food frequency questionnaires did not capture changes in portion sizes, and despite significant decreases in estimated total fat and saturated fat intake, participants’ consumption continued to exceed daily recommendations even at the end of the study.
Next steps include continuing to follow pilot participants and assessing their weights at regular intervals, conducting a randomized controlled trial to evaluate the effectiveness of Project HEAL, developing a parallel Spanish course, and training more participants from the pilot to lead the course so that it can be self-sustaining.
Front line clinicians, charged with helping individuals prevent and treat diabetes and other chronic conditions, are not sufficiently addressing the obesity epidemic due to limited physician time, and limited availability of trained, culturally consonant nutritionists. Project HEAL presents an affordable, sustainable, weight loss option in communities of color with limited resources and most affected by obesity and diabetes.
Acknowledgments
Dr. Goldfinger was supported by a Clinical Research Fellowship from the Doris Duke Foundation. The coalition and research outlined were supported by the Diabetes Prevention and Control Program of the New York State Dept. of Health and the National Center on Minority Health and Health Disparities (R24-MD001691-03).
We gratefully acknowledge Kate Lorig, Bonnie Bruce, and Virginia Gonzalez from the Stanford University peer education team, who worked with us to tailor their model for weight loss in East Harlem; the women and men who participated in the course; Desiree Maldonado for invaluable assistance with the Project HEAL course and data collection; Samprit Chatterjee for statistical assistance; Tara Ragbir for organizing the site for the pilot course; and the East Harlem Diabetes Center of Excellence peer education sub-committee members, including Joseph Edwards, Kenneth Fernandez, Phyllis Kaskel, Sarah Muller, Cathy Nonas, Romina Pulichino, Louise Square, Thomas Vance, and Andrea Zaldivar, for their partnership, knowledge, and support; and other Center of Excellence members and local nutrition and physical activity experts for their guidance.
Contributor Information
Judith Z. Goldfinger, Internal Medicine Resident at Mt. Sinai School of Medicine (MSSM) in New York City.
Guedy Arniella, Director of Community Outreach and Health Education at North Central Hospital in NYC.
Judith Wylie-Rosett, Professor in the Department of Epidemiology and Population Health at Albert Einstein College of Medicine in the Bronx.
Carol R. Horowitz, Assistant Professor in the Departments of Health Policy and Medicine at MSSM.
Notes
- 1.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity related health risk factors, 2001. JAMA. 2003 Jan 1;289(1):76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.Hogan P, Dall T, Nikolov P, et al. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003 Mar;26(3):917–32. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 3.Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998 Mar;6(2):97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 4.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000 Aug 12;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999 Oct 7;341(15):1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 6.Narayan KM, Boyle JP, Thompson TJ, et al. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003 Oct 8;290(14):1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi AN, Zaslavsky AM, Schneider EC, et al. Trends in the quality of care and racial disparities in Medicare managed care. N Engl J Med. 2005 Aug 18;353(7):692–700. doi: 10.1056/NEJMsa051207. [DOI] [PubMed] [Google Scholar]
- 8.Wong MD, Shapiro MF, Boscardin WJ, et al. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002 Nov 14;347(20):1585–92. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 9.Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care. 1998 Apr;21(4):518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 10.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002 Feb 7;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001 May 3;344(18):1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 12.Pan X-R, Li G-W, Hu Y-H, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care. 1997 Apr;20(4):537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 13.Boulé NG, Haddad E, Kenny GP, et al. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001 Sep 12;286(10):1218–27. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 14.Brand-Miller J, Hayne S, Petocz P, et al. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2003 Aug;26(8):2261–7. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999 Oct 20;282(15):1433–9. doi: 10.1001/jama.282.15.1433. [DOI] [PubMed] [Google Scholar]
- 16.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991 Sep 28;338(8770):774–8. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 17.UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990 Sep;39(9):905–12. [PubMed] [Google Scholar]
- 18.Williamson DF, Thompson TJ, Thun M, et al. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care. 2000 Oct;23(10):1499–504. doi: 10.2337/diacare.23.10.1499. [DOI] [PubMed] [Google Scholar]
- 19.Yamaoka K, Tango T. Efficacy of lifestyle education to prevent type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2005 Nov;28(11):2780–6. doi: 10.2337/diacare.28.11.2780. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson KF, Lindgärde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmö feasibility study. Diabetologia. 1991 Dec;34(12):891–8. doi: 10.1007/BF00400196. [DOI] [PubMed] [Google Scholar]
- 21.Ashley JM, St Jeor ST, Schrage JP, et al. Weight control in the physician’s office. Arch Intern Med. 2001 Jul 9;161(13):1599–604. doi: 10.1001/archinte.161.13.1599. [DOI] [PubMed] [Google Scholar]
- 22.Galuska DA, Will JC, Serdula MK, et al. Are health care professionals advising obese patients to lose weight? JAMA. 1999 Oct 27;282(16):1576–8. doi: 10.1001/jama.282.16.1576. [DOI] [PubMed] [Google Scholar]
- 23.Wee CC, McCarthy EP, Davis RB, et al. Physician counseling about exercise. JAMA. 1999 Oct 27;282(16):1583–8. doi: 10.1001/jama.282.16.1583. [DOI] [PubMed] [Google Scholar]
- 24.Jackson JE, Doescher MP, Saver BG, et al. Trends in professional advice to lose weight among obese adults, 1994 to 2000. J Gen Intern Med. 2005 Sep;20(9):814–8. doi: 10.1111/j.1525-1497.2005.0172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coonrod BA, Betschart J, Harris MI. Frequency and determinants of diabetes patient education among adults in the U.S. population. Diabetes Care. 1994 Aug;17(8):852–8. doi: 10.2337/diacare.17.8.852. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald JT, Anderson RM, Funnell MM, et al. Differences in the impact of dietary restrictions on African Americans and Caucasians with NIDDM. Diabetes Educ. 1997 Jan–Feb;23(1):41–7. doi: 10.1177/014572179702300104. [DOI] [PubMed] [Google Scholar]
- 27.Raymond NR, D’Eramo-Melkus G. Non-insulin-dependent diabetes and obesity in the black and Hispanic population: culturally sensitive management. Diabetes Educ. 1993 Jul–Aug;19(4):313–7. doi: 10.1177/014572179301900411. [DOI] [PubMed] [Google Scholar]
- 28.Persky V, Coover L, Hernandez E, et al. Chicago community-based asthma intervention trial: feasibility of delivering peer education in an inner-city population. Chest. 1999 Oct;116(4 Suppl 1):216S–223S. doi: 10.1378/chest.116.suppl_2.216s. [DOI] [PubMed] [Google Scholar]
- 29.Rose MA. Evaluation of a peer-education program on heart disease prevention with older adults. Public Health Nurs. 1992 Dec;9(4):242–7. doi: 10.1111/j.1525-1446.1992.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 30.Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999 Jan;37(1):5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001 Nov;39(11):1217–23. doi: 10.1097/00005650-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Shannon BM, Smiciklas-Wright H, Davis BW, et al. A peer educator approach to nutrition for the elderly. Gerontologist. 1983 Apr;23(2):123–6. doi: 10.1093/geront/23.2.123. [DOI] [PubMed] [Google Scholar]
- 33.Lynde BD. Nutrition promotion for mature adults: a case study in peer education. J Nutr Elder. 1992;11(3):19–31. doi: 10.1300/J052v11n03_02. [DOI] [PubMed] [Google Scholar]
- 34.Taylor T, Serrano E, Anderson J, et al. Knowledge, skills, and behavior improvements on peer educators and low-income Hispanic participants after a stage of change-based bilingual nutrition education program. J Community Health. 2000 Jun;25(3):241–62. doi: 10.1023/a:1005160216289. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Census Bureau. Census 2000 Summary File 1 Census of Population and Housing. Washington, DC: U.S. Census Bureau; 2000. Sep, [Google Scholar]
- 36.United Hospital Fund Staff. New York City Community Health Atlas, 2002. New York: United Hospital Fund; 2002. [Google Scholar]
- 37.Agency for Health Research and Quality (AHRQ) Prevention quality indicators: software documentation, Version 2.1—SPSS. (AHRQ Pub No. 02-R0207.) Rockville, MD: AHRQ; 2001. Revision 3 (January 9, 2004). Available at http://www.qualityindicators.ahrq.gov/archives/pqi/pqi_spss_documentation_rev3.pdf. [Google Scholar]
- 38.Bindman A, Grumbach K, Osmond D, et al. Preventable hospitalizations and access to health care. JAMA. 1995 Jul 26;274(4):305–11. [PubMed] [Google Scholar]
- 39.Horowitz CR, Williams L, Bickell NA. A community-centered approach to diabetes in East Harlem. J Gen Intern Med. 2003 Jul;18(7):542–8. doi: 10.1046/j.1525-1497.2003.21028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horowitz CR, Colson KA, Hebert PL, et al. Barriers to buying healthy foods for people with diabetes: evidence of environmental disparities. Am J Public Health. 2004 Sep;94(9):1549–54. doi: 10.2105/ajph.94.9.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horowitz CR, Arniella A, James S, et al. Using community-based participatory research to reduce health disparities in East and Central Harlem. Mt Sinai J Med. 2004 Nov;71(6):368–74. [PMC free article] [PubMed] [Google Scholar]
- 42.Green LW, Mercer SL. Can public health researchers and agencies reconcile the push from funding bodies and the pull from communities? Am J Public Health. 2001 Dec;91(12):1926–9. doi: 10.2105/ajph.91.12.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nyden P. Academic incentives for faculty participation in community-based participatory research. J Gen Intern Med. 2003 Jul;18(7):576–85. doi: 10.1046/j.1525-1497.2003.20350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Israel BA, Schulz AJ, Parker EA, et al. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202. doi: 10.1146/annurev.publhealth.19.1.173. [DOI] [PubMed] [Google Scholar]
- 45.Horowitz CR, Goldfinger JZ, Muller SE, et al. A model for using community based participatory research to address the diabetes epidemic in East Harlem. Mt Sinai J Med. doi: 10.1002/msj.20017. (In press.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heisler M, Bouknight RR, Hayward RA, et al. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002 Apr;17(4):243–52. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steptoe A, Pollard TM, Wardle J. Development of a measure of the motives underlying the selection of food: the food choice questionnaire. Appetite. 1995 Dec;25(3):267–84. doi: 10.1006/appe.1995.0061. [DOI] [PubMed] [Google Scholar]
- 48.Block G, Gillespie C, Rosenbaum EH, et al. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000 May;18(4):284–8. doi: 10.1016/s0749-3797(00)00119-7. [DOI] [PubMed] [Google Scholar]
- 49.Serdula M, Coates R, Byers T, et al. Evaluation of a brief telephone questionnaire to estimate fruit and vegetable consumption in diverse study populations. Epidemiology. 1993 Sep;4(5):455–63. doi: 10.1097/00001648-199309000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Macera CA, Ham SA, Jones DA, et al. Limitations on the use of a single screening question to measure sedentary behavior. Am J Public Health. 2001 Dec;91(12):2010–2. doi: 10.2105/ajph.91.12.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Estabrooks PA, Nelson CC, Xu S, et al. The frequency and behavioral outcomes of goal choices in the self-management of diabetes. Diabetes Educ. 2005 May–Jun;31(3):391–400. doi: 10.1177/0145721705276578. [DOI] [PubMed] [Google Scholar]
- 52.Hu FB, Li TY, Colditz GA, et al. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003 Apr 9;289(14):1785–91. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 53.Shultz JA, Sprague MA, Branen LJ, et al. A comparison of views of individuals with type 2 diabetes mellitus and diabetes educators about barriers to diet and exercise. J Health Commun. 2001 Apr–Jun;6(2):99–115. doi: 10.1080/108107301750254457. [DOI] [PubMed] [Google Scholar]
- 54.Hill-Briggs F, Gary TL, Hill MN, et al. Health-related quality of life in urban African Americans with type 2 diabetes. J Gen Intern Med. 2002 Jun;17(6):412–9. doi: 10.1046/j.1525-1497.2002.11002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saltzer EB. The weight locus of control (WLOC) scale: a specific measure for obesity research. J Pers Assess. 1982 Dec;46(6):620–8. doi: 10.1207/s15327752jpa4606_11. [DOI] [PubMed] [Google Scholar]
- 56.Whooley MA, Avins AL, Miranda J, et al. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med. 1997 Jul;12(7):439–45. doi: 10.1046/j.1525-1497.1997.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Idler EL, Angel RJ. Self-rated health and mortality in the NHANES-I Epidemiologic Follow-up Study. Am J Public Health. 1990 Apr;80(4):446–52. doi: 10.2105/ajph.80.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hennessy CH, Moriarty DG, Zack MM, et al. Measuring health-related quality of life for public health surveillance. Public Health Rep. 1994 Sep–Oct;109(5):665–72. [PMC free article] [PubMed] [Google Scholar]
- 59.Mossey JM, Shapiro E. Self-rated health: a predictor of mortality among the elderly. Am J Public Health. 1982 Aug;72(8):800–8. doi: 10.2105/ajph.72.8.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stein AD, Lederman RI, Shea S. The Behavioral Risk Factor Surveillance System questionnaire: its reliability in a statewide sample. Am J Public Health. 1993 Dec;83(12):1768–72. doi: 10.2105/ajph.83.12.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stein AD, Courval JM, Lederman RI, et al. Reproducibility of responses to telephone interviews: demographic predictors of discordance in risk factor status. Am J Epidemiol. 1995 Jun 1;141(11):1097–105. doi: 10.1093/oxfordjournals.aje.a117375. [DOI] [PubMed] [Google Scholar]
- 62.Bowlin SJ, Morrill BD, Nafziger AN, et al. Validity of cardiovascular disease risk factors assessed by telephone survey: the Behavioral Risk Factor Survey. J Clin Epidemiol. 1993 Jun;46(6):561–71. doi: 10.1016/0895-4356(93)90129-o. [DOI] [PubMed] [Google Scholar]