Abstract
Platelets interact with fibrin polymers to form blood clots at sites of vascular injury1–3. Bulk studies have shown clots to be active materials, with platelet contraction driving the retraction and stiffening of clots4. However, neither the dynamics of single-platelet contraction nor the strength and elasticity of individual platelets, both of which are important for understanding clot material properties, have been directly measured. Here we use atomic force microscopy to measure the mechanics and dynamics of single platelets. We find that platelets contract nearly instantaneously when activated by contact with fibrinogen and complete contraction within 15 min. Individual platelets can generate an average maximum contractile force of 29 nN and form adhesions stronger than 70 nN. Our measurements show that when exposed to stiffer microenvironments, platelets generated higher stall forces, which indicates that platelets may be able to contract heterogeneous clots more uniformly. The high elasticity of individual platelets, measured to be 10 kPa after contraction, combined with their high contractile forces, indicates that clots may be stiffened through direct reinforcement by platelets as well as by strain stiffening of fibrin under tension due to platelet contraction. These results show how the mechanosensitivity and mechanics of single cells can be used to dynamically alter the material properties of physiologic systems.
At sites of vascular injury, platelets and fibrin polymers interact to form blood clots that prevent haemorrhage. During clot formation, platelets bind to the fibrin network and aggregate. Actomyosin-based contraction of individual platelets then leads to substantial decreases in clot size and exerts significant strains on fibrin scaffolds2, altering clot organization and stiffness. Indeed, the addition of platelets increases the elastic moduli of fibrin gels by approximately tenfold, according to bulk studies of isometrically contracting clots4,5. As clots are exposed to a wide range of external forces in a haemodynamic environment, clot mechanical properties affect numerous aspects of haemostasis and thrombosis6, and recent clinical studies have correlated altered clot mechanics with disease states. For example, clots in young heart attack patients are much stiffer and resistant to degradation than in healthy subjects7. Conversely, clots in patients with bleeding disorders such as haemophilia are much softer and prone to degradation than in healthy subjects8. Interest in the factors that control clot mechanics has therefore focused attention on the role of platelets and fibrin.
Assays developed over the past few decades have provided measurements of the total contraction force exerted during clot retraction and of the mechanical properties of fibrin gels with and without platelets, but only at the bulk clot level4,9. As platelets drive clot contraction, single-cell measurements are required to obtain a mechanistic understanding of the retraction process and to identify specific therapeutic targets for disease states in which platelet/clot retraction is pathologically altered. However, the complexity of clots makes isolation and investigation of individual platelet and fibrin polymer behaviour difficult. Recent in vitro studies have provided new insight into the mechanical properties of fibrin polymers10,11, particularly the high extensibility of fibrin. Less is known about single-platelet mechanics, owing in part to platelets’ small size and their propensity to rapidly activate, adhere, and spread onto flat surfaces12. The elastic modulus of a contracted platelet remains unknown, and basic biophysical characteristics of individual contracting platelets, such as timescale, maximum contraction forces, and adhesion strength, have not been measured.
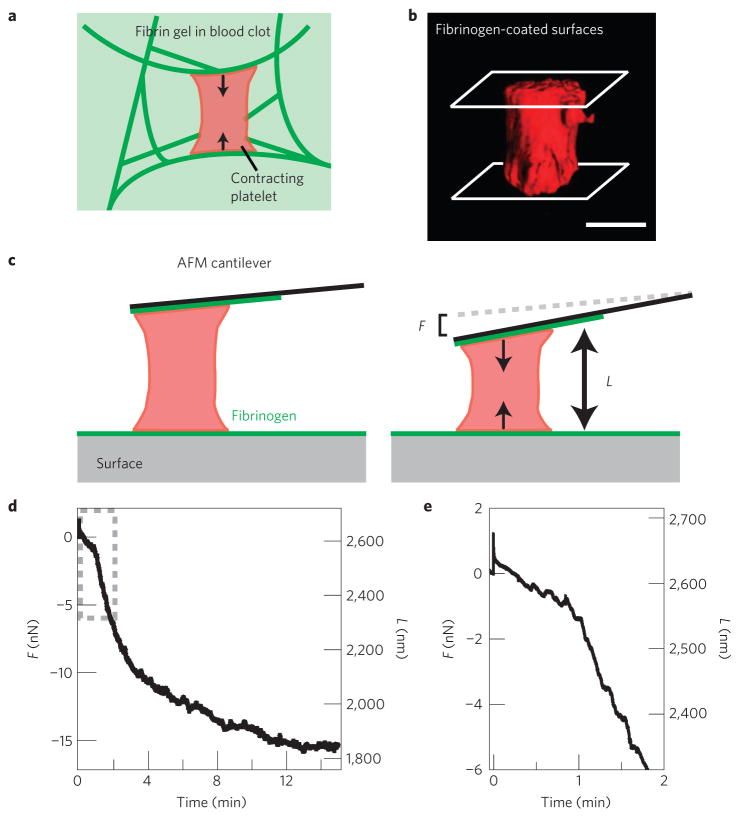
To measure the contraction, mechanics and dynamics of single platelets, we used a custom-built atomic force microscope (AFM) with an integrated ‘side-view’ fluorescence microscope13. Fluorescently labelled single platelets suspended in a buffer solution were positioned between a fibrinogen-coated cantilever and a fibrinogen-coated surface, emulating the geometry of a platelet within a fibrin gel containing pore sizes from 1 to 5 μm (Fig. 1a; ref. 12). Confocal microscopy showed that as a platelet spreads between two opposing fibrinogen-coated surfaces, actin structures are formed between those surfaces (Fig. 1b, Supplementary Fig. S1) and enable the platelet to contract (see the Methods section).
Figure 1. Measuring the contraction of single platelets with AFM.
a, Cartoon of platelet contraction in a blood clot. The blood clot consists of fibrin gel, single contracting platelets, and platelet aggregates (not shown). Activated platelets adhere to fibrin polymers and contract, driving contraction of the blood clot as a whole. b, Three-dimensional isosurface rendering of multiple confocal microscopy planes shows a single thrombin-activated, membrane fluorescently labelled platelet, attached and spread between a fibrinogen-coated cantilever and a fibrinogen-coated glass surface, simulating the experimental set-up of the side-view AFM system. Scale bar = 1 μm. c, Cartoon of the experimental set-up used to measure single-platelet contraction. A fibrinogen-coated AFM cantilever is pressed slightly against an activated platelet just as it lands on a fibrinogen-coated surface. Contraction of the platelet then pulls the cantilever down towards the surface to a length, L, until the force of the cantilever, F, stalls further contraction. d, Force and platelet length measurement during a typical experiment. Tensional force, or force against platelet contraction due to the cantilever deflection towards the surface, is negative here. e, Zoom in of the first two minutes of the experiment from d showing a small compressional force applied to the platelet to initiate contact, and instantaneous contraction of the platelet.
To prevent further bleeding, clots must form efficiently and promptly; therefore, all platelet processes, especially contraction, must occur rapidly. Using AFM to monitor platelet contractile force and dynamics, we found that activated single platelets began contracting nearly instantaneously on contact with the fibrinogen-coated cantilever and surface. In a typical experiment, the platelet reached a maximum rate of contraction 2–3 min after contact and then stalled after 10–15 min as it exerted a maximum contraction force of 15 nN (Fig. 1c–e, Supplementary Video S1). After the platelet reached maximum contraction, it was able to sustain that tension for many minutes until the end of the experiment (Fig. 1c). This rapid timescale of platelet contraction was seen in all experiments (Supplementary Fig. S2), even when an external load was applied (Supplementary Information). Interestingly, the timescale we observed is consistent with the minimum reported time for platelet-induced bulk clot retraction of 15 min, although retraction times up to 120 min have been reported14–16. This broad range of clot retraction timescales may be due to differences in the concentration and/or organization of the fibrin, platelet concentrations, or platelet activation at different time points within the clot in those systems.
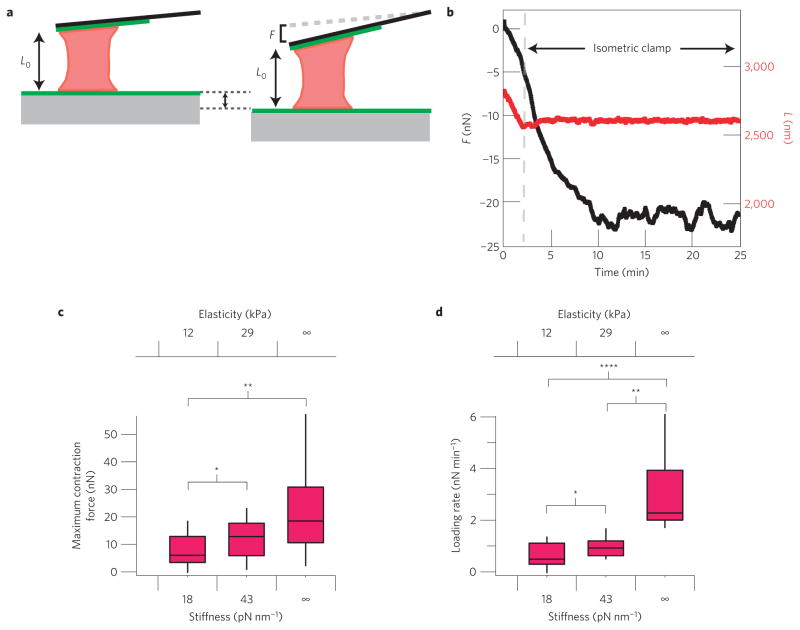
Clot structure can be anisotropic and spatially non-uniform12, leading to a heterogeneity of mechanical microenvironments platelets might encounter. As fibrinogen density correlates with clot stiffness17, we investigated whether platelet contraction force and the rate of increase in contraction force (nN s−1) are influenced by alterations in the stiffness of their surroundings. To conduct these experiments, we used cantilevers with different stiffnesses (either ~18 or ~43 pN nm−1), which correlate with physiologically relevant mechanical properties of fibrin clots (~12 or ~29 kPa, respectively; see Supplementary Information)17. To measure the maximum contraction force of an individual platelet, we also used a feedback algorithm that places the platelet under isometric contraction, also known as a distance or isometric clamp18, which is equivalent to an infinitely stiff microenvironment (Fig. 2a,b, Supplementary Video S2). This feedback algorithm modulates the position of the surface during platelet contraction such that the distance between the cantilever and surface is held constant.
Figure 2. Stiffness dependence and timescale of platelet contraction.
a, Cartoon of isometric clamp experiments that were used to simulate an infinitely stiff environment. As the platelet contracts, the surface is retracted such that the length of the platelet remains constant. b, Typical isometric experiment measurement with the cantilever deflection shown in black and platelet height shown in red. The isometric clamp is turned on after ~2 min. c,d, Distribution of stall forces and contraction rates for platelets pulling against cantilevers with stiffnesses of 18 and 43 pN nm−1, and in an isometric clamp. Medians, quartiles and 90/10 levels are shown, and the * represents a significant difference with a P value of <0.05, ** represents a significant difference with a P value of <0.01, and **** represents a significant difference with a P value of <0.001.
Under these three different microenvironmental stiffness conditions, we found that platelet contraction was significantly altered. As cantilever stiffness increased, platelets exhibited higher contraction forces and rates, and generated more work (Fig. 2c,d and Supplementary Fig. S3). Interestingly, a ~2.5-fold increase in cantilever stiffness led to a nearly 2-fold increase in the median platelet maximum contraction force, so that actual contraction distances were similar. However, this relationship may be different at lower stiffness values. Despite the relative simplicity of platelets, such stiffness-dependent behaviour is similar to that reported for other contractile cells, which pull to a constant fraction of their original height over a certain regime of stiffness.19–21. Physiologically, the ability of platelets to pull with higher forces in stiffer microenvironments, such as areas of high fibrin density, would be expected to lead to a more uniform contraction of a heterogeneous fibrin gel in a blood clot. Stiffness in a fibrin gel has been shown to scale with the 1.67 power of fibrin concentration17. Thus, we estimate that a given platelet will contract with 2.5 times as much force for an increase in fibrin concentration by a factor of 2, and generate 2 times as much work (Supplementary Information). Further studies are needed to correlate contraction force and local fibrin elasticity in clots.
Our experiments reveal that platelets exert remarkably high contraction forces, with the magnitude of maximum contraction forces ranging from 1.5 to 79 nN (mean: 19 ± 3.1 nN, n = 30, s.e.m.; Fig. 2c). This roughly matches with the prediction of a previous study of bulk clot retraction in which the contraction force of a single platelet was extrapolated to be 20 nN (ref. 22), although the prediction does not capture the large variation in contraction forces or stiffness dependence of platelet contraction that we measure. The forces exerted by individual platelets are remarkable given their small size. Although single myoblast cells have been shown to exert up to 300 nN (ref. 19), they are approximately three orders of magnitude larger in volume than platelets23. Thus, platelets exert a force per volume two orders of magnitude greater than that exerted by myoblasts, or a force per area that is one order of magnitude greater. As single myosin II molecules can generate a maximum of ~6 pN of force24,25 and there are approximately 12,000 myosin II molecules in each platelet2, we estimate the maximum theoretical contractional force of a single platelet to be 72 nN. In our isometric clamp conditions, we measured a median contraction force of 18 nN, which is ~25% of this maximum value. Interestingly, skeletal muscle cells, with highly ordered sarcomere contractile units, also exert maximum contractile forces with 30% of the myosins contracting, although skeletal muscle cells contain muscle myosin II as opposed to the non-muscle myosin II in platelets25. Thus, the contraction forces generated by platelets, without any prior ordering of contractile actomyosin fibres, are surprisingly high.
The large forces exerted by platelets also have implications for the interaction between platelets and fibrin. Protofibrils of fibrin are estimated to unfold at a force of ~75 pN (refs 11,26). Although these protofibrils are linked together into fibres, the large forces exerted by platelets may still be high enough to cause unfolding of some fibrin polymers, potentially leading to permanent alteration of the fibre structure11.
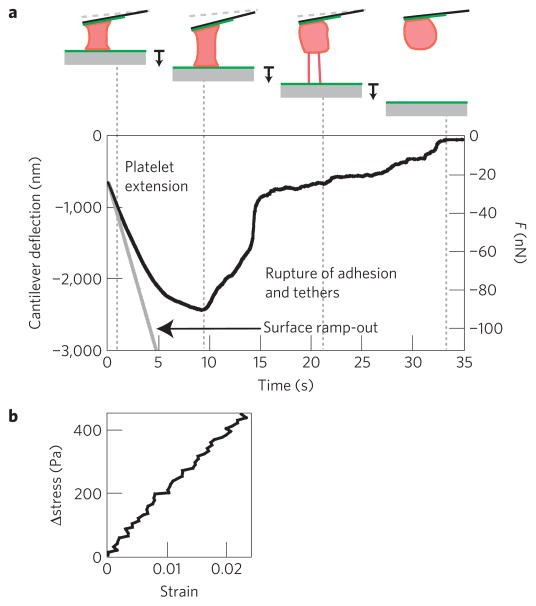
After clot formation and retraction, preservation of clot mechanical integrity is vital to maintaining haemostasis, and contracted platelets have been shown in bulk studies to enhance clot stiffness4,27. To understand how, we investigated the mechanical properties of single contracted platelets. After platelet contraction was completed, we measured the elasticity and extensibility of single platelets, as well as the adhesion strength between the platelet and the fibrinogen-coated surface or cantilever (Fig. 3a–b, Methods, Supplementary Video S3). We found the elasticity of a contracted platelet in this regime to have a mean of 9.85 ± 1.71 kPa (n = 12, s.e.m.). The adhesion force of the platelet, measured when the platelet detached from the surface or cantilever (Fig. 3a), had a mean of 69.0 ± 12.7 nN (n = 11, s.e.m.), corresponding to rupture stresses of approximately 5 kPa. Although this measurement represents a minimum, as rupture could have occurred between the platelet and the fibrinogen or between the fibrinogen and the surface, platelet adhesion force per area is at least 600 times higher than that measured between single leukocytes and endothelial cells13,28. We also found the extensibility of the platelet to have a mean of 1.57 ± 0.22 (n = 11, s.e.m.) before rupture. Interestingly, both platelet elasticity and adhesion strength correlated with maximum contraction force (r = 0.76 and 0.77, respectively, P < 0.05), indicating that the more force a platelet exerts, the stiffer and more adhesive it becomes.
Figure 3. Elasticity and adhesion measurement for contracted platelets.
a, Typical elasticity and adhesion measurement. The surface (solid grey line) is ramped out at a speed of 500 nm s−1, or 1,000 nm s−1 for a platelet for which contraction has stalled. The cantilever is pulled down until adhesion to the surface or the cantilever is ruptured, giving the adhesion force. Extensibility is defined as the length of the platelet at the rupture point, relative to its length at the beginning of the ramp-out experiment. b, Stress versus strain during the first 0.5 s of the ramp-out experiment in a. These data are fitted with a line to calculate the elasticity of the contracted platelet. Average measured elasticity was 9.85 kPA (n = 12), average extensibility was 1.57 (n = 11) and adhesion force was 69 nN (n = 11).
Previous work has shown that the elasticity of platelet-rich clots (~600 Pa) is 10-fold greater than platelet-free clots (~70 Pa) in an isometric system in which clot length is held constant4,9. Platelets are exerting high contractile forces on the fibrin, so that the strain stiffening of individual fibrin fibres under tension would lead to an increase in overall network elasticity, similar to stiffening of actin networks due to tensional forces exerted by myosin filaments29. Also, platelets are known to reorganize network architecture and to induce additional polymerization of fibrin1,30. However, the contribution of these mechanisms to the enhancement of network elasticity is unclear.
According to our measurements, platelets are two orders of magnitude stiffer than platelet-free clots and one order of magnitude stiffer than platelet-rich clots, indicating that platelets may additionally reinforce the mechanical properties of the clot directly, as in particle-filled elastomers31. Within this context, the high stiffness and adhesion of the platelets to the fibrin matrix may allow the platelet to directly bear some of the load, restrict local deformations of the fibrin matrix, and serve as a crosslinking centre within the fibrin gel (Supplementary Information). We speculate that direct enhancement of clot elasticity by platelets could be important during early phases of clot formation and contraction, when the fibrin gel is relatively sparse and most susceptible to deformation by physiological forces such as blood flow.
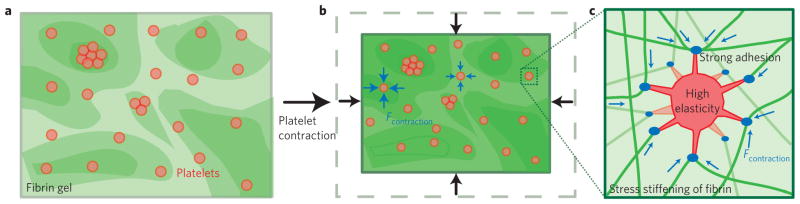
The AFM measurements on single platelets presented here reveal that platelets contract rapidly and generate high contraction and adhesive forces in a stiffness-dependent manner, which, we suggest, may lead to more uniform contraction of the clot as a whole (Fig. 4a). We note that these contraction results have direct implications for platelet aggregation (Supplementary Information) and indicate that mechanics, in addition to biological agonists such as ADP and epinephrine, may affect platelet aggregation. The combined effect of high platelet stiffness, attachment to multiple fibrin polymers, and high detachment forces contributes to the significant enhancement of the elasticity of clots by platelets (Fig. 4b). These two effects illustrate the complex mechanical properties of clots as a composite material, the properties of which are highly dependent on the specific interactions between platelets and the fibrin gel. These experiments improve our overall understanding of clot material properties in haemostasis and thrombosis, and this experimental system could potentially lead to new diagnostic assays and insight for cardiovascular disease and disorders of platelet function.
Figure 4. Proposed effects of platelets on clot retraction and mechanics.
a, During the initial formation of a clot, activated platelets are interspersed in what is probably a heterogeneous fibrin gel. Areas of higher density of fibrin are indicated with darker shading, and exhibit higher stiffnesses. b, The stiffness dependence of platelet contraction probably results in increased forces of contraction in areas of higher stiffness (that is, higher fibrin density), possibly leading to a more uniform contraction of the clot as a whole and an increase in overall elasticity. c, We suggest that the high elasticity of contracted platelets and large adhesion forces between the platelets and the fibrin gel may allow for platelets to reinforce the mechanical properties of the clot directly, by acting as a multi-point crosslinker, restricting deformation and flow of the fibrin gel around the platelet, and by bearing some of the load. Tension on fibrin fibres due to large forces of platelet contraction also may lead to stress stiffening of the fibres under tension, further contributing to stiffening of the clot as a whole.
Methods
Experimental geometry and platelet capture in side-view AFM
Our experiments measured the contraction force applied by a single cell between opposing sides of the cell body and in a direction normal to the surface of attachment. Similarly, platelets embedded in a fibrin network must exert forces across their cell body to contract and pull fibrin fibres together, although typically with multiple points of contact22. In that regard, the configuration of our AFM measurements provides the simplest geometry possible within a fibrin scaffold, representing a platelet spanning two bundles of fibrin polymers and exerting contractile forces along the axis normal to the two fibres. This quasi-three-dimensional configuration positions a platelet for uniaxial contraction and therefore provides a convenient method to directly measure the maximum or total possible contraction force that a platelet embedded in a fibrin network can exert. In dense fibrin networks, the total force generated by actomyosin contraction would probably be distributed in multiple directions for platelets with multiple attachment points.
To capture platelets before they contacted a surface, we used a custom-built AFM with an integrated ‘side-view’ fluorescence microscope13. This system was important for our experiments, as platelets rapidly activate on contact with fibrinogen-coated surfaces32, and conventional bottom-view epifluorescence microscopy does not easily discriminate between platelets near the surface and platelets that have just come into contact with the surface but have not yet spread. The side-view imaging path was used to rapidly locate platelets that were near the surface but had not yet contacted, and then to initiate contact between the platelet and both the glass surface and AFM cantilever surface simultaneously (see Supplementary Video S4). When a diffusing platelet was positioned so that it contacted the surface and cantilever simultaneously, it contracted and pulled the flexible cantilever towards the surface (Fig. 1c). Side-view imaging provided visual confirmation of platelet contraction (Supplementary Video S1). The cantilever deflection during platelet contraction was detected with an optical lever, providing sub-nanometre-scale resolution of cantilever position and piconewton-scale resolution of cantilever force. AFM cantilevers behave like Hookean springs for small deflections, so that force is proportional to deflection.
Platelet activation in AFM experiments
Two signalling pathways mediate platelet contraction, both of which ultimately converge and result in actomyosin contraction. Binding of fibrin/fibrinogen to integrin αIIbβ3 (glycoprotein IIb/IIIa) receptors on the platelet surface initiates the main pathway, leading to calcium mobilization and activation of myosin light-chain kinase2. In addition, thrombin, a potent platelet activator produced during the clotting process, binds to specific receptors and initiates the rho kinase signalling pathway that inhibits myosin light-chain phosphatase, resulting in increased myosin activation15. In the results reported here, we used thrombin-activated platelets because thrombin is present during clot formation and retraction in vivo, and is used in in vitro bulk clot retraction studies. Control experiments confirmed that platelet contraction in our system was mediated by fibrinogen and integrin αIIbβ3, and was actomyosin and calcium-dependent, which is consistent with the present understanding of clot retraction (see Supplementary Information, Fig. S4). In addition, experiments with a fluorescent calcium indicator confirmed that platelet activation was maximum on contact with the cantilever and glass surfaces (Supplementary Fig. S5), although we cannot rule out some pre-activation on exposure to thrombin.
Elasticity and adhesion measurements
After platelet contraction was completed, we moved the surface away from the cantilever at a constant rate to determine the elasticity and extensibility of single platelets, as well as the adhesion strength between the platelet and the fibrinogen-coated surface or cantilever. As the surface was moved away, the attachment force between the platelet and the surface increased and the platelet elongated. We calculated the elasticity of the contracted platelet from the relationship between the measured platelet force per unit area (stress) and fractional change in platelet length (strain).
Supplementary Material
Acknowledgments
We thank S. Parekh, G. Venugopalan, G. Stephens, P. Andre, D. Phillips, X. Zhao and the Fletcher Lab for their advice and useful discussions. Financial support for this work was provided by an NSF GRFP for O.C., NIH grant K08-HL093360, a UCSF REAC award, and a Biomedical Research Fellowship from The Hartwell Foundation for W.A.L., and an NSF CAREER Award and NIH R01 grants to D.A.F.
Footnotes
Author contributions
W.A.L., O.C., A.C., K.D.W., J.H. and D.A.F. conceived and designed the experiments; W.A.L., O.C., T-D.L. and A.K. carried out the experiments; W.A.L., O.C. and D.A.F. analysed and interpreted the data; and W.A.L., O.C., D.A.F., A.C., K.D.W. and J.H. wrote the manuscript.
The authors declare no competing financial interests.
Supplementary information accompanies this paper on www.nature.com/naturematerials.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions.
References
- 1.Niewiarowski S, Regoeczi E, Stewart GJ, Senyl AF, Mustard JF. Platelet interaction with polymerizing fibrin. J Clin Invest. 1972;51:685–699. doi: 10.1172/JCI106857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartwig JH. In: Platelets. 2. Michelson AD, editor. Elsevier; 2007. pp. 75–97. [Google Scholar]
- 3.Ruggeri ZM. Platelet adhesion under flow. Microcirculation. 2009;16:58–83. doi: 10.1080/10739680802651477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jen CJ, McIntire LV. The structural properties and contractile force of a clot. Cell Motil. 1982;2:445–455. doi: 10.1002/cm.970020504. [DOI] [PubMed] [Google Scholar]
- 5.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature. 2005;435:191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 6.Weisel JW. Biophysics. Enigmas of blood clot elasticity. Science. 2008;320:456–457. doi: 10.1126/science.1154210. [DOI] [PubMed] [Google Scholar]
- 7.Collet JP, et al. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol. 2006;26:2567–2573. doi: 10.1161/01.ATV.0000241589.52950.4c. [DOI] [PubMed] [Google Scholar]
- 8.Hvas AM, et al. Tranexamic acid combined with recombinant factor VIII increases clot resistance to accelerated fibrinolysis in severe hemophilia A. J Thromb Haemost. 2007;5:2408–2414. doi: 10.1111/j.1538-7836.2007.02755.x. [DOI] [PubMed] [Google Scholar]
- 9.Carr ME. Jr Development of platelet contractile force as a research and clinical measure of platelet function. Cell Biochem Biophys. 2003;38:55–78. doi: 10.1385/CBB:38:1:55. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, et al. Fibrin fibers have extraordinary extensibility and elasticity. Science. 2006;313:634. doi: 10.1126/science.1127317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown AE, Litvinov RI, Discher DE, Purohit PK, Weisel JW. Multiscale mechanics of fibrin polymer: Gel stretching with protein unfolding and loss of water. Science. 2009;325:741–744. doi: 10.1126/science.1172484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys Chem. 2004;112:267–276. doi: 10.1016/j.bpc.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri O, Parekh SH, Lam WA, Fletcher DA. Combined atomic force microscopy and side-view optical imaging for mechanical studies of cells. Nature Methods. 2009;6:383–387. doi: 10.1038/nmeth.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rooney MM, Farrell DH, van Hemel BM, de Groot PG, Lord ST. The contribution of the three hypothesized integrin-binding sites in fibrinogen to platelet-mediated clot retraction. Blood. 1998;92:2374–2381. [PubMed] [Google Scholar]
- 15.Suzuki-Inoue K, et al. Involvement of Src kinases and PLCgamma2 in clot retraction. Thromb Res. 2007;120:251–258. doi: 10.1016/j.thromres.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiyoi T, et al. A naturally occurring Tyr143His alpha IIb mutation abolishes alpha IIb beta 3 function for soluble ligands but retains its ability for mediating cell adhesion and clot retraction: Comparison with other mutations causing ligand-binding defects. Blood. 2003;101:3485–3491. doi: 10.1182/blood-2002-07-2144. [DOI] [PubMed] [Google Scholar]
- 17.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophys J. 1999;77:2813–2826. doi: 10.1016/S0006-3495(99)77113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choy JL, et al. Differential force microscope for long time-scale biophysical measurements. Rev Sci Instrum. 2007;78:043711. doi: 10.1063/1.2727478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitrossilis D, et al. Single-cell response to stiffness exhibits muscle-like behavior. Proc Natl Acad Sci USA. 2009;106:18243–18248. doi: 10.1073/pnas.0903994106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allioux-Guerin M, et al. Spatiotemporal analysis of cell response to a rigidity gradient: A quantitative study using multiple optical tweezers. Biophys J. 2009;96:238–247. doi: 10.1529/biophysj.108.134627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajzar A, Cesa CM, Kirchgessner N, Hoffmann B, Merkel R. Toward physiological conditions for cell analyses: Forces of heart muscle cells suspended between elastic micropillars. Biophys J. 2008;94:1854–1866. doi: 10.1529/biophysj.107.115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen I, Gerrard JM, White JG. Ultrastructure of clots during isometric contraction. J Cell Biol. 1982;93:775–787. doi: 10.1083/jcb.93.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satoh H, Delbridge LM, Blatter LA, Bers DM. Surface: Volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: Species-dependence and developmental effects. Biophys J. 1996;70:1494–1504. doi: 10.1016/S0006-3495(96)79711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finer JT, Simmons RM, Spudich JA. Single myosin molecule mechanics: Piconewton forces and nanometre steps. Nature. 1994;368:113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- 25.Piazzesi G, et al. Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell. 2007;131:784–795. doi: 10.1016/j.cell.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 26.Brown AE, Litvinov RI, Discher DE, Weisel JW. Forced unfolding of coiled-coils in fibrinogen by single-molecule AFM. Biophys J. 2007;92:L39–L41. doi: 10.1529/biophysj.106.101261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang T, et al. The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesth Analg. 2009;108:751–758. doi: 10.1213/ane.0b013e3181966675. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, et al. Atomic force microscopy measurement of leukocyte-endothelial interaction. Am J Physiol Heart Circ Physiol. 2004;286:H359–H367. doi: 10.1152/ajpheart.00491.2003. [DOI] [PubMed] [Google Scholar]
- 29.Koenderink GH, et al. An active biopolymer network controlled by molecular motors. Proc Natl Acad Sci USA. 2009;106:15192–15197. doi: 10.1073/pnas.0903974106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leistikow EA. Platelet internalization in early thrombogenesis. Semin Thromb Hemost. 1996;22:289–294. doi: 10.1055/s-2007-999021. [DOI] [PubMed] [Google Scholar]
- 31.Bergstrom JS, Boyce M. C Mechanical behavior of particle filled elastomers. Rubber Chem Technol. 1999;72:633–656. [Google Scholar]
- 32.Bonnefoy A, Liu Q, Legrand C, Frojmovic MM. Efficiency of platelet adhesion to fibrinogen depends on both cell activation and flow. Biophys J. 2000;78:2834–2843. doi: 10.1016/S0006-3495(00)76826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.