Abstract
A one-pot synthesis of polyrotaxanes has been developed. The method employs a supramolecular monomer comprising a polymerizable ammonium salt and crown ether, in combination with dynamic ADMet polymerization. Ultimately, highly efficient complexation, polymerization, and end-capping were accomplished in a single operation to yield polyrotaxanes with Mws up to 19.3 kDa and > 80% of the repeat units being complexed.
Advanced supramolecular and mechanically interlocked polymers such as polyrotaxanes, polycatenanes, and polypseudorotaxanes offer enticing synthetic targets and the promise of unique characteristics.1 The ability to construct these complicated architectures in an efficient, scalable, and modular fashion is essential to realizing their full potential. A particular challenge in the synthesis of main-chain polyrotaxanes, for example, is achieving a combination of polymerization, threading or “clipping”, and end-capping to secure the overall interlocked nature of the ensemble – feats often executed in a step-wise fashion. Inspired by elegant examples that have successfully accomplished these objectives,2 we sought a system of high efficiency in which polyrotaxanes could be generated in one-pot from readily available, modular building blocks via a dynamic polymerization/end-capping strategy. We envisioned that incorporating end-caps during polymerization, while also allowing threading to occur, would benefit from a rapidly equilibrating polymerization such as acyclic diene metathesis (ADMet) polymerization.3 Herein we describe a method to accomplish a one-pot synthesis of polyrotaxanes via multi-component ADMet polymerization.
Starting from commercially available tert-butyl carbamate 1, acyclic dienyl ammonium salts 2 were prepared in three steps as depicted in Scheme 1. Specifically, alkylation of 1 using NaH and a bromoolefin in DMF furnished the corresponding dialkyl carbamate intermediates (not shown). Subsequent deprotection using TFA in CH2Cl2, followed by anion metathesis with NH4PF6 provided the desired dialkenyl ammonium salts bearing 6- or 11-carbon chains (2a and 2b, respectively).
Scheme 1.
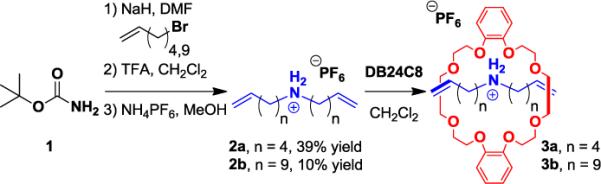
Synthesis of dialkenyl ammonium salts 2 and complexation with DB24C8 to provide supramolecular monomers 3.
Efficient threading of 2 using dibenzo[24]crown-8 ether (DB24C8) was confirmed via 1H NMR spectroscopy of a 1:1 molar ratio of the two species in CD2Cl2 (0.01 M in each, initially).4 The CH2 protons alpha to the ammonium moiety in 2 displayed resonances at δ = 3.0 ppm, and moved downfield to δ = 3.2 ppm in the presence of DB24C8, indicating threading to form supramolecular monomers 3 (Scheme 1). Integration of the two signals correlated to ca 75% threading, and increasing the ratio of DB24C8/2 to 5:1 (0.01 M in 2, initially) resulted in quantitative threading. The supramolecular complexes (3a and 3b) obtained from 1:1 mixtures of 2 and DB24C8 were concentrated under vacuum, and used directly in subsequent ADMet polymerizations. It is expected that the concentrated mixtures comprising 3 contain a higher composition of the supramolecular complexes than the more dilute solutions used for NMR analysis.
Supramolecular monomers 3 (likely as dynamic mixtures with the constituent ammonium salt 2 and DB24C8) were found to readily participate in ADMet polymerization (Scheme 2); key results are summarized in Table 1. Initially, a solution containing 3a was treated with Ru-alkylidene complex 4 and an end-capping chain transfer agent (CTA) 5. Using [3a/4]0 of 40:1 and [3a/5]0 of 2.5:1 (entry 1), the reaction mixture was prepared in dry CH2Cl2 (initially 0.5 M in 3a), sealed under Ar, and vigorously stirred in an oil bath at 50 °C. The viscosity of the solution quickly increased, and after ca 30 min the mixture was removed from heat and placed under vacuum to remove all volatiles including ethylene byproduct formed during the polymerization. Fresh CH2Cl2 was then added under Ar, and the sealed reaction mixture was again heated at 50 °C. The process of adding CH2Cl2 and subsequently removing the solvent under vacuum was repeated after 1, 2, and 12 h intervals. After a total reaction time of ca 24 h, the mixture was concentrated under vacuum to give a thick tan foam. Analysis of the polyrotaxane via 1H NMR spectroscopy indicated ca 72% of the repeat units remained threaded, and GPC analysis using multi-angle laser light scattering (MALLS) revealed a Mw of 13.2 kDa.
Scheme 2.
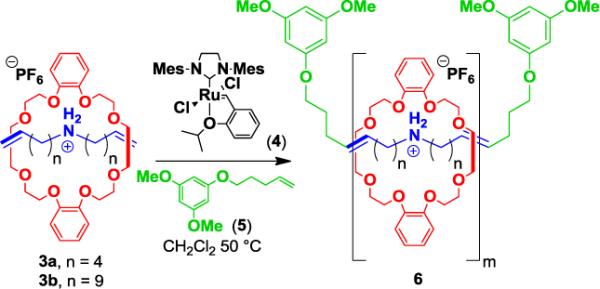
ADMet polymerization of supramolecular monomers 3 to form polyrotaxanes 6 (shown as an idealized, fully threaded polyrotaxane).
Table 1.
Data for ADMet polymerization of 2 to form polyrotaxanes. 6.a
| Entry | Monomer | Yield (%)b | Threading (%)c | M w (kDa)d | PDId |
|---|---|---|---|---|---|
| 1 | 3a | 85 | 72 | 13.2 | 1.58 |
| 2 | 3b | 94 | 82 | 11.0 | 1.42 |
| 3e | 2b | 80 | 82 | 19.3 | 1.18 |
| 4f | 2b | 50 | 80 | 14.9 | 1.37 |
Reactions were conducted under Ar atmosphere at 50 °C using [2/4]0 or [3/4]0 = 40:1 and [2/5]0 or [3/5]0 = 2.5:1.
Isolated yield after precipitation, based on 2 or 3 accordingly.
Determined by 1H NMR spectroscopy of the isolated product.
Determined by gel-permeation chromatography using multi-angle laser light scattering.
Using [2b/DB24C8]0 = 1:1.
Using [2b/DB24C8]0 = 5:1.
To improve the efficiency of the ADMet polymerization, we next focused on monomer 3b bearing longer undecenyl groups in comparison with 3a. The reduced viscosity of the resulting reaction mixture indeed appeared to facilitate the polymerization, reaching full monomer conversion in ca 2 h (cf. 90% conversion at 6 h when 3a was employed). After ca 12 h and three cycles of solvent removal/addition, the polyrotaxane was concentrated to yield a thick viscous oil (entry 2). Analysis as before revealed ca 82% threading, and Mw = 11.0 kDa.
Encouraged by the ability to efficiently polymerize the congested supramolecular monomers via ADMet, and the rapid threading observed from ammonium salts 2 and DB24C8, we next attempted the polyrotaxane synthesis without discrete pre-assembly to form 3 (Scheme 3). Accordingly, 2b, DB24C8, and CTA 5 were combined as a heterogeneous mixture and a solution of catalyst 4 in CH2Cl2 was added. The polymerization and solvent cycling were conducted as described above. Analysis of the resulting polyrotaxane revealed similar threading (82%) and an increased Mw of 19.3 kDa (entry 3) in comparison with using the pre-assembled 3b monomer (entry 2). Notably, the amount of threading did not benefit from the addition of 5 equiv of DB24C8 relative to monomer 2b (entry 4), suggesting that the maximum amount of threading for this particular monomer structure had been reached.
Scheme 3.
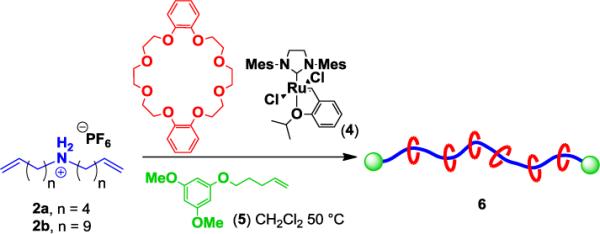
One-pot ADMet polymerization to form polyrotaxanes.
The polydispersity index (PDI) values resulting from the step-growth polymerizations were lower than expected when determined using MALLS analysis (Table 1). It is evident that the polymerizations do not reach full equilibrium, considering the feed ratio of monomer (2 or 3) to end-cap (5) of 2.5:1 would result in an average degree of polymerization (DP) of 5, and those obtained were greater than 5. The PDI values, however, likely reflect poor resolution of the polyelectrolyte structures during elution on the GPC columns that would manifest in artificially low PDIs. For comparison, we determined the average DP (and corresponding Mn values) via 1H NMR analysis by comparing the integration of the CH2 protons alpha to the ammonium moiety with that of the dimethoxyarene end cap. Using the Mw values from MALLS analysis in combination with the Mn values obtained via 1H NMR analysis gives PDIs for entries 1–4 (Table 1) of 1.91, 1.58, 3.55, and 1.65, respectively that are more consistent with other ADMet polymerizations.
The end-capped, mechanically locked nature of the polyrotaxanes was confirmed via 2-D DOSY NMR experiments.5,6 As can be seen in Figure 1, the diffusion rates for both the polyammonium backbone of 6a and the DB24C8 moieties are correlated to one another, with a diffusion rate value of ca 2.5×10−9 m2/s. Moreover, this diffusion rate is distinct from free DB24C8, which displays a faster diffusion rate of ca. 3.5×10−9 m2/s. This supports the successful incorporation of the end-caps, but may also have been ascribed to strong ammonium-DB24C8 interactions. To further support the end-capped nature of 6a, we compared the DOSY spectrum upon 10-fold dilution, which again revealed consistent diffusion rates of the polymer backbone and the interlocked DB24C8 moieties.
Figure 1.
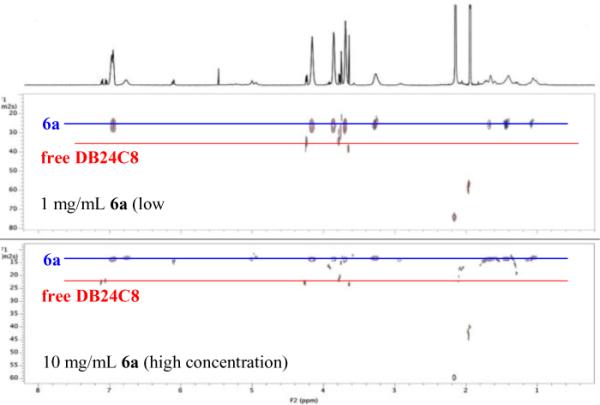
DOSY spectra of polyrotaxane 6a (blue) and free DB24C8 (red) at concentrations of 1 mg/mL (top) and 10 mg/mL (bottom).
In conclusion, we have developed a simple strategy for a onepot, multi-component synthesis of polyrotaxanes using ADMet polymerization that advances the synthetic capabilities toward advanced macromolecular structures. The efficiency and ease with which these mechanically interlocked macromolecules can be assembled should facilitate rapid modulation to achieve versatile polyrotaxane architectures, and can be readily adapted to incorporate a variety of “metathesis-friendly” substrates.
Supplementary Material
Acknowledgment
Financial support of this research by the National Science Foundation (CHE-0809418), Army Research Office (W911NF-09-1-0512), and the Department of Energy (DEFG02-05ER46218). AJB thanks the National Institutes of Health for a postdoctoral fellowship. We are grateful to Dr. David VanderVelde for his assistance with NMR spectroscopy.
Footnotes
Supporting Information Available. Detailed experimental procedures and characterization of all new compounds. This materials is available free of charge via the Internet at http://pubs.acs.org
References
- 1).(a) Durola F, Sauvage J-P, Wenger OS. Coord. Chem. Rev. 2010;254:1748. [Google Scholar]; (b) Hänna KD, Leigh DA. Chem. Soc. Rev. 2010;39:1240. doi: 10.1039/b901974j. [DOI] [PubMed] [Google Scholar]; (c) Fang L, Olson MA, Benítez D, Tkatchouk E, Goddard WA, III, Stoddard JF. Chem. Soc. Rev. 2010;39:17. doi: 10.1039/b917901a. [DOI] [PubMed] [Google Scholar]; (d) Coronado E, Gaviña P, Tatay S. Chem. Soc. Rev. 2009;38:1674. doi: 10.1039/b807441k. [DOI] [PubMed] [Google Scholar]; (e) Stoddart JF. Chem. Soc. Rev. 2009;38:1802. doi: 10.1039/b819333a. [DOI] [PubMed] [Google Scholar]; (f) Niu Z, Gibson HW. Chem. Rev. 2009;109:6024. doi: 10.1021/cr900002h. [DOI] [PubMed] [Google Scholar]; (g) Harada A, Hashidzume A, Yamaguchi H, Takashima Y. Chem. Rev. 2009;109:5974. doi: 10.1021/cr9000622. [DOI] [PubMed] [Google Scholar]; (h) Kang S, Berkshire BM, Xue Z, Gupta M, Layode C, May PA, Mayer MF. J. Am. Chem. Soc. 2008;130:15246. doi: 10.1021/ja806122r. [DOI] [PubMed] [Google Scholar]; (i) Green JE, Choi JW, Boukai A, Bunimovich Y, Johnston-Halperin E, Delonno E, Lou Y, Sheriff BA, Xu K, Shin YS, Tseng H-R, Stoddart JS, Heath JR. Nature. 2007;445:414. doi: 10.1038/nature05462. [DOI] [PubMed] [Google Scholar]; (j) Wenz G, Han B-H, Müller A. Chem. Rev. 2006;106:782. doi: 10.1021/cr970027+. [DOI] [PubMed] [Google Scholar]; (k) Takata T. Polymer J. 2006;38:1. [Google Scholar]; (l) Huang F, Gibson HW. Prog. Polym. Sci. 2005;30:982. [Google Scholar]; (m) Sauvage J-P, Dietrich Buchecker CO, editors. Molecular Catenanes, Rotaxanes and Knots. Wiley-VCH; Weinheim: 1999. [Google Scholar]
- 2).(a) Kohsaka Y, Koyama Y, Takata T. Angew. Chem. Int. Ed. 2011;50:10417. doi: 10.1002/anie.201103869. [DOI] [PubMed] [Google Scholar]; (b) Kohsaka Y, Nakazono K, Koyama Y, Asai S, Takata T. Angew. Chem. Int. Ed. 2011;50:4872. doi: 10.1002/anie.201008020. [DOI] [PubMed] [Google Scholar]; (c) Lee Y-G, Koyama Y, Yonekawa M, Takata T. Macromolecules. 2010;43:4070. [Google Scholar]; (d) Wu J, He H, Gao C. Macromolecules. 2010;43:2252. [Google Scholar]; (e) Yamabuki K, Isobe Y, Onimura K, Oishi T. Chem. Lett. 2007;36:1196. [Google Scholar]; (f) Takata T, Kawasaki H, Kihara N, Furusho Y. Macromolecules. 2001;34:5449. [Google Scholar]
- 3).(a) Matloka PP, Wagener KB. J. Mol. Catal. A: Chem. 2006;257:89. [Google Scholar]; (b) Baughman TW, Wagener KB. Adv. Polym. Sci. 2005;176:1. [Google Scholar]; (c) Schwendeman JE, Church AC, Wagener KB. Adv. Synth. Catal. 2002;344:597. [Google Scholar]
- 4).Ashton PR, Campbell PJ, Chrystal EJT, Glink PT, Menzer S, Philp D, Spencer N, Stoddart JF, Tasker PA, Williams DJ. Angew. Chem. Int. Ed. 1995;34:1865. [Google Scholar]
- 5).(a) Johnson CS., Jr. Prog. Nucl. Magn. Reson. Spectrosc. 1999;34:203. [Google Scholar]; (b) Jerschow A, Müller N. Macromolecules. 1998;31:6573. [Google Scholar]; (c) Morris KF, Stilbs P, Johnson CS., Jr. Anal. Chem. 1994;66:211. [Google Scholar]; (d) Morris KF, Johnson CS., Jr. J. Am. Chem. Soc. 1992;114:3139. [Google Scholar]
- 6).Clark PG, Guidry EN, Chan WY, Steinmetz WE, Grubbs RH. J. Am. Chem. Soc. 2010;132:3405. doi: 10.1021/ja9090337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


