Abstract
Study Design
Retrospective subgroup analysis of prospectively collected data according to treatment received.
Objective
The purpose of this study is to determine if the duration of symptoms affects outcomes following the treatment of spinal stenosis (SS) or degenerative spondylolisthesis (DS).
Summary of Background Data
The Spine Outcomes Research Trial (SPORT) study was designed to provide scientific evidence on the effectiveness of spinal surgery versus a variety of non-operative treatments.
Methods
An as-treated analysis was performed on patients enrolled in the Spine Patient Outcomes Research Trial (SPORT) for the treatment of SS or DS. A comparison was made between patients with SS with ≤12 months (n=405) and those with >12 months (n=227) duration of symptoms. A comparison was also made between patients with DS with ≤12 months (n=397) and those with >12 months (n=204) duration of symptoms. Baseline patient characteristics were documented. Primary and secondary outcomes were measured at baseline and at regular follow-up time intervals up to 4 years. The difference in improvement among patients whose surgical or nonsurgical treatment began less than or greater than 12 months after the onset of symptoms was measured. In addition, the difference in improvement with surgical versus nonsurgical treatment (treatment effect) was determined at each follow-period for each group.
Results
At final followup, there was significantly less improvement in primary outcome measures in SS patients with >12 months symptom duration. Primary and secondary outcome measures within the DS group did not differ according to symptom duration. There were no statistically significant differences in treatment effect of surgery in SS or DS patients.
Conclusions
Patients with spinal stenosis with fewer than twelve months of symptoms experienced significantly better outcomes with surgical and nonsurgical treatment relative to those with symptom duration greater than twelve months. There was no difference in outcome of patients with degenerative spondylolisthesis according to symptom duration.
Keywords: Surgery, Lumbar, Spinal Stenosis, Degenerative Spondylolisthesis, Outcomes
Introduction
The Spine Outcomes Research Trial (SPORT) study was designed to provide scientific evidence on the effectiveness of spinal surgery versus a variety of non-operative treatments. The prospective collection of data in the SPORT study provides a unique opportunity to correlate symptom duration with outcome. The purpose of this study was to assess the correlation between the duration of symptoms (DOS) prior to treatment and the outcome following the treatment of spinal stenosis (SS) and degenerative spondylolisthesis (DS).
The hypothesis of this study was that there exists a specific duration of symptoms of SS and DS beyond which clinical outcomes would be less favorable than with earlier intervention.
Materials and Methods
Study Design
SPORT was conducted at thirteen multidisciplinary spine practices in eleven states across the United States. The details of methods have been reported previously.19
Patient Population
All patients had neurogenic claudication or radicular leg pain with associated neurological signs, SS seen on cross-sectional imaging, symptoms that had persisted for at least twelve weeks, and physician confirmation that they were a surgical candidate. Patients with DS seen on standing lateral radiographs were included in a separate analysis.
Patients with adjacent levels of stenosis were eligible, but those with spondylolysis and isthmic spondylolisthesis were not. Pre-enrollment nonoperative care was not specified but included physical therapy (68%), epidural injections (55%), chiropractic care (25%), anti-inflammatory medications (63%), and opioid analgesics (30%).
Enrollment began in March 2000 and ended in February 2005.
Study Interventions
Patients were offered participation in either a randomized or observational cohort. Participants in the randomized cohort received computer-generated random treatment assignments blocked by center; those in the observational cohort chose their treatment with their physician. The protocol surgery consisted of a standard posterior decompressive laminectomy with or without bilateral single-level fusion (autogenous iliac crest bone-grafting with or without posterior pedicle screw instrumentation).
The non- operative protocol was “usual recommended care,” which includes, at least, active physical therapy, education and counseling with instructions regarding home exercise, and nonsteroidal anti-inflammatory drugs if the patient could tolerate them.
Because of extensive crossover in the randomized cohort (that is, some patients randomized to nonoperative care received operative care and vice versa) and similar baseline characteristics and outcomes between randomized and observational patients when analyzed by treatment, the two groups were combined in this “as-treated” analysis.
Study Measures
Data used in this study were obtained from patient questionnaires completed at baseline, six weeks, three months, six months, one year, two years, and four years after enrollment or surgery.
Primary outcome measures included the bodily pain, physical function domains, and mental status domains of the SF-36 [22] and the American Academy of Orthopaedic Surgeons MODEMS (Musculoskeletal Outcomes Data Evaluation and Management System) version of the Oswestry Disability Index [23]. Secondary measures included patient self-reported improvement, work status, and satisfaction with current symptoms [1]. Symptom severity was measured by the low back pain bothersomeness scale, the sciatica bothersomeness index (SBI), and leg pain bothersomeness index [10] [24]. The SF-36 scales and the ODI range from 0 to 100, the SBI from 0 to 24, and the low back pain bothersomeness scale from 0 to 6. Higher scores indicated more severe symptoms on the ODI, SBI, and low back pain bothersomeness scale, whereas higher scores indicated less severe symptoms on the SF-36.
Comparison
Differences in baseline characteristics were compared between patients with less than12 months or greater than or equal to 12 months duration of symptoms prior to enrollment. The primary analyses compared changes in the clinical outcome measures from baseline as a function of the timing of surgery within each treatment arm (i.e., surgery or nonoperative). The treatment effect of surgery was defined to compare the improvement after surgical intervention. It was defined as the change in outcome measure after surgical treatment minus the change in outcome measure after nonoperative treatment [25]
Statistical Analysis
Statistical modeling was performed with use of SAS software (version 9.1; SAS Institute, Cary, North Carolina), with the procedures PROC MIXED, and S-PLUS software (version 6.2; Insightful, Seattle, Washington) was used for all other calculations. Significance was defined as a p value of 0.05 on the basis of a two-sided hypothesis test.
Results
Of the SS patients, there were four hundred and five patients with symptom duration < 12 months (SS <12 Months Patients). There were two hundred and twenty nine patients in the group with ≥12 months (SS >12 Months Patients). There was a significant baseline difference (Table 1) in unlisted comorbidities (SS <12 Months Patients 31% vs SS >12 Months Patients 41%, p=0.009), patient self-assessed health trend getting worse (57% vs 64%, respectively, p=0.003), and treatment preference for surgery at baseline (42% vs 49%, p=0.038) favoring duration of symptoms < 12 months. There were significant differences in incidence of a positive straight leg raise (24% vs 16%, p=0.023) and lateral recess stenosis (77% vs 84%, p=0.027). At baseline, there was no instability or spondylolisthesis in the SS patient groups, as described in the methods (Table 1).
Table 1.
Baseline Characteristics, Comorbidities and Health Status Measures for Patients with Lumbar Spinal Stenosis (SpS)
| Characteristics SpS |
One year or less (n=405) |
More than one year (n=229) |
p-value | 6 months or less (n=266) |
7 to 12 months (n=139) |
More than one year (n=229) |
p-value |
|---|---|---|---|---|---|---|---|
| Mean Age (SD) | 64.6 (11.5) | 64.7 (12) | 0.90 | 64.7 (11.6) | 64.2 (11.2) | 64.7 (12) | 0.90 |
| Female - no.(%) | 164 (40%) | 85 (37%) | 0.45 | 110 (41%) | 54 (39%) | 85 (37%) | 0.63 |
| Ethnicity: Not Hispanic | 387 (96%) | 218 (95%) | 0.99 | 252 (95%) | 135 (97%) | 218 (95%) | 0.54 |
| Race - White† | 346 (85%) | 187 (82%) | 0.26 | 227 (85%) | 119 (86%) | 187 (82%) | 0.46 |
| Education - At least some college | 264 (65%) | 137 (60%) | 0.21 | 173 (65%) | 91 (65%) | 137 (60%) | 0.40 |
| Marital Status - Married | 284 (70%) | 162 (71%) | 0.94 | 183 (69%) | 101 (73%) | 162 (71%) | 0.71 |
| Work Status | 0.12 | 0.11 | |||||
| Full or part time | 143 (35%) | 73 (32%) | 87 (33%) | 56 (40%) | 73 (32%) | ||
| Disabled | 31 (8%) | 29 (13%) | 21 (8%) | 10 (7%) | 29 (13%) | ||
| Retired | 187 (46%) | 109 (48%) | 132 (50%) | 55 (40%) | 109 (48%) | ||
| Other | 44 (11%) | 18 (8%) | 26 (10%) | 18 (13%) | 18 (8%) | ||
| Compensation - Any‡ | 28 (7%) | 20 (9%) | 0.50 | 17 (6%) | 11 (8%) | 20 (9%) | 0.61 |
| Mean Body Mass Index (BMI), (SD)§ | 29.4 (5.7) | 29.7 (5.6) | 0.51 | 29.4 (5.8) | 29.4 (5.5) | 29.7 (5.6) | 0.80 |
| Smoker | 35 (9%) | 27 (12%) | 0.25 | 24 (9%) | 11 (8%) | 27 (12%) | 0.41 |
| Comorbidities - no.(%) | |||||||
| Hypertension | 172 (42%) | 116 (51%) | 0.057 | 112 (42%) | 60 (43%) | 116 (51%) | 0.14 |
| Diabetes | 57 (14%) | 39 (17%) | 0.38 | 42 (16%) | 15 (11%) | 39 (17%) | 0.25 |
| Osteoporosis | 38 (9%) | 22 (10%) | 0.96 | 27 (10%) | 11 (8%) | 22 (10%) | 0.76 |
| Heart Problem | 103 (25%) | 62 (27%) | 0.72 | 70 (26%) | 33 (24%) | 62 (27%) | 0.77 |
| Stomach Problem | 83 (20%) | 56 (24%) | 0.29 | 54 (20%) | 29 (21%) | 56 (24%) | 0.51 |
| Bowel or Intestinal Problem | 49 (12%) | 37 (16%) | 0.19 | 35 (13%) | 14 (10%) | 37 (16%) | 0.25 |
| Depression | 43 (11%) | 27 (12%) | 0.75 | 35 (13%) | 8 (6%) | 27 (12%) | 0.071 |
| Joint Problem | 210 (52%) | 136 (59%) | 0.081 | 139 (52%) | 71 (51%) | 136 (59%) | 0.18 |
| Other¶ | 125 (31%) | 95 (41%) | 0.009 | 83 (31%) | 42 (30%) | 95 (41%) | 0.026 |
| SF-36 scores, mean (SD)†† | |||||||
| Bodily Pain (BP) | 33.4 (19.9) | 33.6 (19.5) | 0.87 | 33.1 (20.5) | 33.8 (18.7) | 33.6 (19.5) | 0.94 |
| Physical Functioning (PF) | 35.3 (23.3) | 34 (23.2) | 0.53 | 35.8 (24.1) | 34.2 (21.7) | 34 (23.2) | 0.65 |
| Mental Component Summary (MCS) | 49 (12) | 49.9 (11.8) | 0.40 | 48.8 (12.4) | 49.4 (11.1) | 49.9 (11.8) | 0.63 |
| Oswestry (ODI) (SD)‡‡ | 42 (18) | 43 (19.3) | 0.53 | 42.4 (18.9) | 41.4 (16.3) | 43 (19.3) | 0.72 |
| Stenosis Frequency Index (0–24) (SD)§§ | 13.8 (5.9) | 14 (5.7) | 0.74 | 13.8 (6.1) | 13.8 (5.4) | 14 (5.7) | 0.95 |
| Stenosis Bothersome Index (0–24) (SD)§§ | 14.3 (5.7) | 14.4 (5.8) | 0.84 | 14.3 (5.9) | 14.2 (5.5) | 14.4 (5.8) | 0.96 |
| Low Back Pain Bothersomeness (0–6) (SD)¶¶ | 4 (1.8) | 4.1 (1.8) | 0.46 | 4.1 (1.8) | 4 (1.9) | 4.1 (1.8) | 0.70 |
| Leg Pain Bothersomeness (0–6) (SD)¶¶ | 4.4 (1.6) | 4.2 (1.8) | 0.32 | 4.4 (1.5) | 4.3 (1.8) | 4.2 (1.8) | 0.55 |
| Satisfaction with symptoms - very dissatisfied | 281 (69%) | 152 (66%) | 0.49 | 180 (68%) | 101 (73%) | 152 (66%) | 0.44 |
| Patient self-assessed health trend - no.(%) | 0.003 | <0.001 | |||||
| Getting better | 40 (10%) | 6 (3%) | 32 (12%) | 8 (6%) | 6 (3%) | ||
| Staying about the same | 129 (32%) | 74 (32%) | 93 (35%) | 36 (26%) | 74 (32%) | ||
| Getting worse | 232 (57%) | 146 (64%) | 138 (52%) | 94 (68%) | 146 (64%) | ||
| Treatment preference at baseline - no.(%) | 0.038 | 0.086 | |||||
| Preference for non-surg | 161 (40%) | 68 (30%) | 111 (42%) | 50 (36%) | 68 (30%) | ||
| Not sure | 73 (18%) | 48 (21%) | 48 (18%) | 25 (18%) | 48 (21%) | ||
| Preference for surgery | 170 (42%) | 113 (49%) | 106 (40%) | 64 (46%) | 113 (49%) | ||
| Pseudoclaudication - Any | 321 (79%) | 187 (82%) | 0.53 | 210 (79%) | 111 (80%) | 187 (82%) | 0.75 |
| SLR or Femoral Tension | 96 (24%) | 36 (16%) | 0.023 | 64 (24%) | 32 (23%) | 36 (16%) | 0.057 |
| Pain radiation - any | 326 (80%) | 173 (76%) | 0.17 | 215 (81%) | 111 (80%) | 173 (76%) | 0.33 |
| Any Neurological Deficit | 227 (56%) | 122 (53%) | 0.55 | 151 (57%) | 76 (55%) | 122 (53%) | 0.73 |
| Reflexes - Asymmetric Depressed | 110 (27%) | 58 (25%) | 0.68 | 69 (26%) | 41 (29%) | 58 (25%) | 0.66 |
| Sensory - Asymmetric Decrease | 117 (29%) | 65 (28%) | 0.97 | 78 (29%) | 39 (28%) | 65 (28%) | 0.96 |
| Motor - Asymmetric Weakness | 121 (30%) | 56 (24%) | 0.17 | 87 (33%) | 34 (24%) | 56 (24%) | 0.073 |
| Listhesis Level | |||||||
| L3–L4 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| L4–L5 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Stenosis Levels | |||||||
| L2–L3 | 116 (29%) | 63 (28%) | 0.83 | 76 (29%) | 40 (29%) | 63 (28%) | 0.95 |
| L3–L4 | 262 (65%) | 158 (69%) | 0.31 | 172 (65%) | 90 (65%) | 158 (69%) | 0.55 |
| L4–L5 | 364 (90%) | 215 (94%) | 0.11 | 242 (91%) | 122 (88%) | 215 (94%) | 0.13 |
| L5–S1 | 107 (26%) | 66 (29%) | 0.58 | 61 (23%) | 46 (33%) | 66 (29%) | 0.075 |
| Stenotic Levels (Mod/Severe) | 0.32 | 0.63 | |||||
| None | 8 (2%) | 7 (3%) | 5 (2%) | 3 (2%) | 7 (3%) | ||
| One | 155 (38%) | 79 (34%) | 106 (40%) | 49 (35%) | 79 (34%) | ||
| Two | 158 (39%) | 83 (36%) | 102 (38%) | 56 (40%) | 83 (36%) | ||
| Three+ | 84 (21%) | 60 (26%) | 53 (20%) | 31 (22%) | 60 (26%) | ||
| Stenosis Locations | |||||||
| Central | 347 (86%) | 196 (86%) | 0.93 | 235 (88%) | 112 (81%) | 196 (86%) | 0.11 |
| Lateral Recess | 310 (77%) | 193 (84%) | 0.027 | 206 (77%) | 104 (75%) | 193 (84%) | 0.057 |
| Neuroforamen | 128 (32%) | 79 (34%) | 0.51 | 77 (29%) | 51 (37%) | 79 (34%) | 0.22 |
| Stenosis Severity | 0.68 | 0.49 | |||||
| Mild | 8 (2%) | 7 (3%) | 5 (2%) | 3 (2%) | 7 (3%) | ||
| Moderate | 180 (44%) | 102 (45%) | 126 (47%) | 54 (39%) | 102 (45%) | ||
| Severe | 217 (54%) | 120 (52%) | 135 (51%) | 82 (59%) | 120 (52%) | ||
| Instability | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Received surgery* | 255 (63%) | 158 (69%) | 0.15 | 168 (63%) | 87 (63%) | 158 (69%) | 0.31 |
Race or ethnic group was self-assessed. Whites and blacks could be either Hispanic or non-Hispanic.
This category includes patients who were receiving or had applications pending for workers compensation, Social Security compensation, or other compensation.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Other indicates problems related to stroke, cancer, lung, fibromyalgia, chronic fatigue syndrome, post traumatic stress disorder, alcohol, drug dependency, liver, kidney, blood vessel, nervous system, migraine, anxiety.
The SF-36 scores range from 0 to 100, with higher score indicating less severe symptoms.
The Oswestry Disability Index ranges from 0 to 100, with lower scores indicating less severe symptoms.
The Stenosis Bothersomeness index and the Stenosis Frequency index range from 0 to 24, with lower scores indicating less severe symptoms.
The Low Back Pain Bothersomeness and the Leg Pain Bothersomeness Scale range from 0 to 6, with lower scores indicating less severe symptoms.
Patients received surgery were classified according to whether they received surgical treatment during the first 4 years of enrollment.
Operative details of the SS patients are described in Table 2. The majority of patients underwent decompression alone (90% SS <12 Months Patients and 85% SS >12 Months Patients). There were no statistically significant differences in types of fusion or use of instrumentation. There were no statistically significant differences in number of fusions. A higher percentage of patients in SS >12 Months Patients had decompression at L4-L5 (90% vs 97%, p=0.02). There were no statistically significant differences in operative details or complications such as blood loss, dural tear, or wound complication.
Table 2.
Operative treatments, complications and events.
| SpS | One year or less (n=252) |
More than one year (n=159) |
p-value | 6 months or less (n=168) |
7 to 12 months (n=84) |
More than one year (n=159) |
p-value |
|---|---|---|---|---|---|---|---|
| Specific procedures† | 0.11 | 0.13 | |||||
| Decompression only | 224 (90%) | 131 (85%) | 152 (92%) | 72 (87%) | 131 (85%) | ||
| Non-instrumented fusion | 9 (4%) | 13 (8%) | 6 (4%) | 3 (4%) | 13 (8%) | ||
| Instrumented fusion | 15 (6%) | 10 (6%) | 7 (4%) | 8 (10%) | 10 (6%) | ||
| Multi-level fusion | 9 (4%) | 7 (4%) | 0.87 | 6 (4%) | 3 (4%) | 7 (4%) | 0.91 |
| Decompression level | |||||||
| L2–L3 | 86 (35%) | 61 (39%) | 0.44 | 60 (37%) | 26 (31%) | 61 (39%) | 0.49 |
| L3–L4 | 167 (68%) | 115 (74%) | 0.23 | 112 (68%) | 55 (66%) | 115 (74%) | 0.41 |
| L4–L5 | 222 (90%) | 151 (97%) | 0.02 | 147 (90%) | 75 (90%) | 151 (97%) | 0.04 |
| L5–S1 | 91 (37%) | 62 (40%) | 0.63 | 57 (35%) | 34 (41%) | 62 (40%) | 0.54 |
| Levels decompressed | 0.31 | 0.64 | |||||
| None | 5 (2%) | 3 (2%) | 4 (2%) | 1 (1%) | 3 (2%) | ||
| 1 | 64 (25%) | 29 (18%) | 41 (24%) | 23 (27%) | 29 (18%) | ||
| 2 | 79 (31%) | 49 (31%) | 54 (32%) | 25 (30%) | 49 (31%) | ||
| 3+ | 104 (41%) | 78 (49%) | 69 (41%) | 35 (42%) | 78 (49%) | ||
| Operation time, minutes (SD) | 129.8 (65.7) | 127.2 (66.1) | 0.70 | 124.3 (61.9) | 140.5 (71.8) | 127.2 (66.1) | 0.17 |
| Blood loss, cc (SD) | 304.7 (342.7) | 321.9 (487.7) | 0.68 | 303.9 (342.6) | 306.3 (345) | 321.9 (487.7) | 0.92 |
| Blood Replacement | |||||||
| Intraoperative replacement | 27 (11%) | 12 (8%) | 0.40 | 18 (11%) | 9 (11%) | 12 (8%) | 0.60 |
| Post-operative transfusion | 13 (5%) | 7 (4%) | 0.93 | 6 (4%) | 7 (8%) | 7 (4%) | 0.25 |
| Length of hospital stay, days (SD) | 3.3 (2.6) | 3.1 (2.1) | 0.50 | 3.2 (2.5) | 3.5 (2.8) | 3.1 (2.1) | 0.50 |
| Intraoperative complications§ | |||||||
| Dural tear/ spinal fluid leak | 25 (10%) | 13 (8%) | 0.68 | 13 (8%) | 12 (14%) | 13 (8%) | 0.21 |
| Other | 2 (1%) | 1 (1%) | 0.68 | 1 (1%) | 1 (1%) | 1 (1%) | 0.86 |
| None | 224 (89%) | 144 (91%) | 0.65 | 153 (92%) | 71 (85%) | 144 (91%) | 0.17 |
| Postoperative complications/events¶ | |||||||
| Wound hematoma | 3 (1%) | 1 (1%) | 0.97 | 2 (1%) | 1 (1%) | 1 (1%) | 0.86 |
| Wound infection | 6 (2%) | 3 (2%) | 0.98 | 3 (2%) | 3 (4%) | 3 (2%) | 0.64 |
| Other | 14 (6%) | 10 (6%) | 0.90 | 9 (5%) | 5 (6%) | 10 (6%) | 0.93 |
| None | 217 (87%) | 137 (88%) | 0.88 | 147 (89%) | 70 (83%) | 137 (88%) | 0.48 |
| Post-operative mortality (death within 6 weeks of surgery) |
0 (0%) | 1 (0.6%) | 0.82 | 0 (0%) | 0 (0%) | 1 (0.6%) | 0.45 |
| Post-operative mortality (death within 3 months of surgery) |
0 (0%) | 1 (0.6%) | 0.82 | 0 (0%) | 0 (0%) | 1 (0.6%) | 0.45 |
| Additional surgeries (1-year rate)|| | 11 (4%) | 11 (7%) | 0.26 | 7 (4%) | 4 (5%) | 11 (7%) | 0.52 |
| Additional surgeries (2-year rate)|| | 15 (6%) | 17 (10%) | 0.08 | 10 (6%) | 5 (6%) | 17 (10%) | 0.22 |
| Additional surgeries (3-year rate)|| | 21 (8%) | 25 (15%) | 0.02 | 14 (8%) | 7 (8%) | 25 (15%) | 0.07 |
| Additional surgeries (4-year rate)|| | 24 (9%) | 30 (19%) | 0.01 | 17 (10%) | 7 (8%) | 30 (19%) | 0.02 |
| Recurrent stenosis / progressive listhesis | 9 (4%) | 15 (9%) | 7 (4%) | 2 (2%) | 15 (9%) | ||
| Pseudarthrosis / fusion exploration | 0 | 0 | 0 | 0 | 0 | ||
| Complication or Other | 8 (3.2%) | 10 (6.3%) | 5 (3.1%) | 3 (3.5%) | 10 (6.3%) | ||
| New condition | 3 (1.2%) | 5 (3.2%) | 2 (1.2%) | 1 | 5 (3.2%) |
Surgical information was available for 168 patients with duration of symptoms 6 months or less, 84 patients with duration of symptoms 7 to 12 months and 159 patients with duration of symptoms one year or more.
Specific procedure data were available for 165 patients with duration of symptoms 6 months or less, 83 patients with duration of symptoms 7 to 12 months and 154 patients with duration of symptoms more than one year.
No cases were reported of aspiration into the respiratory tract, vascular injury or operation at wrong level.
Complications or events occurring up to 8 weeks after surgery are listed. There were no reported cases of bone-graft complication, cerebrospinal fluid leak, nerve root injury, paralysis, cauda equina injury, pseudarthrosis, wound dehisence.
Rates of repeated surgery at 1, 2,3,and 4 years are Kaplan-Meier estimates. P values were calculated with the use of the log-rank test. Numbers and percentages are based on the first additional surgery if more than one additional surgery.
There was an increased percentage of additional surgeries in SS >12 Months Patients at two (6% vs 10%, p=0.08), three (8% vs 15%, p=0.02), and four years (9% vs 19%, p=0.01). Of the additional surgeries performed, the majority were to address recurrent stenosis or spondylolisthesis (4% vs 9%). A smaller number were addressed as a new condition (1.2% vs 3.2%).
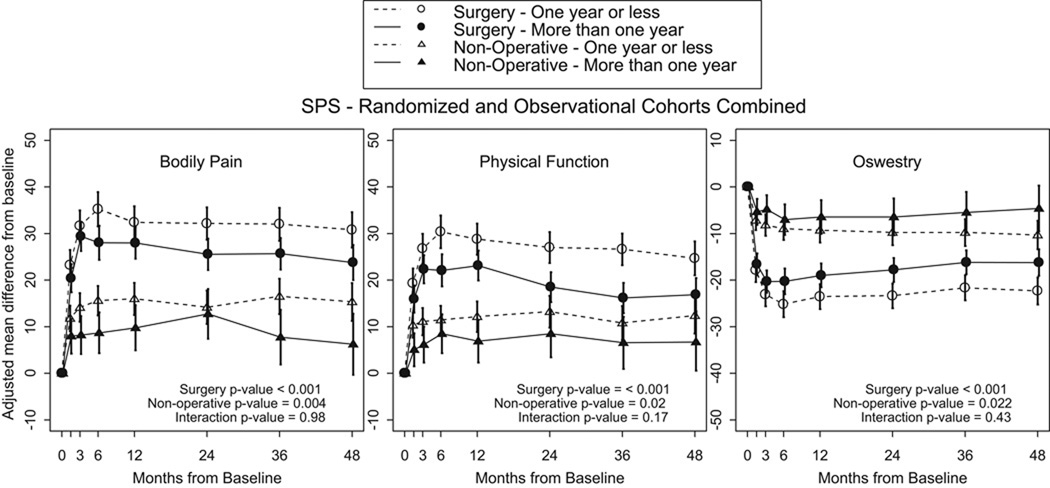
Change in outcome measures in surgically treated patients is displayed in Table 3. Averaged over four years, there was statistically significant less improvement in SS >12 Months Patients versus SS <12 Months Patients in SF36 BP (p<0.001), SF36 PF (p<0.001), and ODI (p<0.001). Specifically at the four year endpoint, the surgically treated patients with duration of symptoms < 12 months (SS <12 Months Patients) demonstrate more improvement than SS <12 Months Patients vs SS >12 Months Patients in SF36 BP (30.8 vs 23.8, p=0.007),, SF36 PF (24.7 vs 16.9, p=0.002), ODI (−22.3 vs −16.2, p=0.002), and patient satisfaction (70.1% vs 55%, p=0.015). (Table 3 and Figure 1).
Table 3.
Subgroup results from adjusted* as-treated outcome analysis by two groups duration of symptoms (One year or less vs. More than one year) for the randomized and observational cohorts combined patients with lumbar spinal stenosis.
| Outcome |
Duration of Symptoms |
1-Year |
2-Year |
3-Year |
4-Year |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SpS | Surgical | Non- operative |
Treatment Effect† (95% CI) |
Surgical | Non- operative |
Treatment Effect† (95% CI) |
Surgical | Non- operative |
Treatment Effect† (95% CI) |
Surgical | Non- operative |
Treatment Effect† (95% CI) |
|
|
Primary Outcomes |
|||||||||||||
| SF-36 Bodily Pain (BP) (0–100) (SE) |
One year or less |
32.4 (1.8) | 16 (1.8) | 16.4 (11.5, 21.3) |
32.1 (1.8) | 14.2 (1.8) | 18 (13, 22.9) |
32 (1.8) | 16.5 (1.9) | 15.5 (10.3, 20.7) |
30.8 (1.9) | 15.3 (2.1) | 15.5 (10, 21) |
| More than 1 year |
28 (1.7) |
9.8 (2.5) | 18.2 (12.6, 23.8) |
25.5 (1.7) | 12.8 (2.7) | 12.8 (6.7, 18.8) |
25.7 (1.7) | 7.7 (3) | 17.9 (11.4, 24.5) |
23.8 (1.9) | 6.2 (3.3) | 17.5 (10.2, 24.9) |
|
| pvalue | 0.073 | 0.036 | 0.63 | 0.005 | 0.66 | 0.18 | 0.009 | 0.013 | 0.56 | 0.007 | 0.019 | 0.65 | |
| SF-36 Physical Function (PF) (0–100) (SE) |
One year or Less |
28.8 (1.7) | 12.1 (1.7) | 16.6 (12, 21.3) |
27 (1.7) | 13.2 (1.7) | 13.8 (9.1, 18.4) |
26.6 (1.7) | 10.8 (1.8) | 15.8 (10.9, 20.7) |
24.7 (1.9) | 12.4 (2) | 12.3 (7.1, 17.5) |
| More than 1 Year |
23.1 (1.6) | 6.9 (2.3) | 16.2 (11, 21.5) |
18.5 (1.6) | 8.5 (2.6) | 10 (4.4, 15.7) |
16.1 (1.7) | 6.5 (2.8) | 9.6 (3.4, 15.8) |
16.9 (1.8) | 6.7 (3.1) | 10.2 (3.3, 17) |
|
| pvalue | 0.015 | 0.061 | 0.91 | <0.001 | 0.12 | 0.31 | <0.001 | 0.20 | 0.12 | 0.002 | 0.12 | 0.62 | |
| Mental Component Summary (MCS) (0–100) (SE) |
One year or less |
4.9 (0.7) | 2.9 (0.7) | 2 (0, 4.1) |
4.2 (0.7) | 1.4 (0.8) | 2.7 (0.7, 4.8) |
3.7 (0.7) | 1.2 (0.8) | 2.5 (0.3, 4.7) |
2.7 (0.8) | 0.8 (0.9) | 2 (−0.4, 4.3) |
| More than 1 year |
2.3 (0.7) | 1.3 (1) | 0.9 (−1.4, 3.3) |
3 (0.7) | 0.6 (1.1) | 2.4 (−0.1, 5) |
1.6 (0.7) | −0.3 (1.3) | 1.9 (−0.9, 4.7) |
2.1 (0.8) | −1.7 (1.4) | 3.8 (0.7, 6.9) |
|
| pvalue | 0.01 | 0.21 | 0.49 | 0.24 | 0.55 | 0.84 | 0.032 | 0.30 | 0.73 | 0.58 | 0.14 | 0.35 | |
| Oswestry Disability Index (ODI) (0–100) (SE) |
One year or less |
−23.5 (1.4) | −9.3 (1.3) | −14.2 (−17.9, 10.5) |
−23.4 (1.3) | −9.8 (1.4) | −13.6 (−17.3, −9.9) |
−21.7 (1.4) | −9.8 (1.5) | −11.9 (−15.8, −8) |
−22.3 (1.5) | −10.3 (1.6) | −12 (−16.2, -7.9) |
| More than 1 year |
−19 (1.3) | −6.5 (1.8) | −12.5 (−16.7, −8.4) |
−17.8 (1.3) | −6.5 (2) | −11.4 (−15.8, −6.9) |
−16.2 (1.3) | −5.5 (2.2) | −10.7 (−15.6, −5.8) |
−16.2 (1.4) | −4.6 (2.5) | −11.6 (−17.1, −6.2) |
|
| pvalue | 0.014 | 0.19 | 0.55 | 0.002 | 0.16 | 0.44 | 0.002 | 0.10 | 0.71 | 0.002 | 0.049 | 0.91 | |
|
Secondary Outcomes |
|||||||||||||
| Stenosis Bothersomenes s Index (0–24) (SE) |
One year or Less |
−8.4 (0.5) | −3.7 (0.5) | −4.8 (−6.1, −3.4) |
−8.8 (0.5) | −4.4 (0.5) | −4.3 (−5.7, −2.9) |
−8.2 (0.5) | −4.6 (0.5) | −3.6 (−5.1, −2.1) |
−7.9 (0.5) | −4.2 (0.6) | −3.7 (−5.2, −2.1) |
| More than 1 year |
−8.2 (0.5) | −3 (0.7) | −5.1 (−6.6, −3.6) |
−7.2 (0.5) | −2.6 (0.7) | −4.5 (−6.2, −2.9) |
−7 (0.5) | −2.8 (0.8) | −4.2 (−6, −2.4) |
−7.4 (0.5) | −2.5 (0.9) | −4.9 (−6.9, −2.9) |
|
| pvalue | 0.69 | 0.41 | 0.70 | 0.013 | 0.04 | 0.84 | 0.072 | 0.057 | 0.59 | 0.45 | 0.092 | 0.33 | |
| Low Back Pain Bothersomenes s (0–6) (SE) |
One year or less |
−2.2 (0.1) | −0.9 (0.1) | −1.3 (−1.7, −0.9) |
−2.3 (0.1) | −1.1 (0.1) | −1.2 (−1.6, −0.8) |
−2.1 (0.1) | −1.1 (0.2) | −1 (−1.4, −0.6) |
−1.9 (0.2) | −1 (0.2) | −0.9 (−1.3, −0.5) |
| More than 1 year |
−2 (0.1) | −0.7 (0.2) | −1.3 (−1.7, −0.9) |
−1.9 (0.1) | −0.5 (0.2) | −1.3 (−1.8, −0.8) |
−1.8 (0.1) | −0.3 (0.2) | −1.5 (−2, −1) |
−1.7 (0.2) | −0.5 (0.3) | −1.2 (−1.8, −0.6) |
|
| pvalue | 0.30 | 0.34 | 0.99 | 0.027 | 0.05 | 0.71 | 0.082 | 0.002 | 0.15 | 0.43 | 0.047 | 0.27 | |
| Leg pain (0–6) (SE) |
One year or less |
−2.8 (0.2) | −1.3 (0.2) | −1.4 (−1.8, −1) |
−2.8 (0.1) | −1.4 (0.2) | −1.4 (−1.8, −1) |
−2.7 (0.2) | −1.8 (0.2) | −0.9 (−1.4, −0.4) |
−2.5 (0.2) | −1.5 (0.2) | −1 (−1.5, −0.5) |
| More than one year |
−2.6 (0.1) | −1.1 (0.2) | −1.5 (−2, −1) |
−2.4 (0.1) | −0.8 (0.2) | −1.6 (−2.1, −1.1) |
−2.4 (0.1) | −0.9 (0.3) | −1.5 (−2.1, −0.9) |
−2.7 (0.2) | −1.1 (0.3) | −1.6 (−2.2, −1) |
|
| pvalue | 0.34 | 0.40 | 0.98 | 0.072 | 0.027 | 0.53 | 0.30 | 0.005 | 0.081 | 0.27 | 0.32 | 0.14 | |
| Very/somewhat satisfied with symptoms (%) |
One year or less |
70.3 | 29.1 | 41.2 (30.9, 51.6) |
72 | 30.7 | 41.3 (30.6, 51.9) |
71.5 | 41.3 | 30.1 (18.4, 41.9) |
70.1 | 34.5 | 35.7 (23.4, 48) |
| More than 1 year |
66.8 | 24.9 | 41.9 (29.9, 54) |
67.4 | 20.7 | 46.7(34.3, 59.1) | 60.2 | 21.6 | 38.6(24.7, 52.5) | 55 | 25.2 | 29.8 (13.5, 46.1) |
|
| pvalue | 0.51 | 0.53 | 0.90 | 0.38 | 0.17 | 0.50 | 0.04 | 0.029 | 0.38 | 0.015 | 0.33 | 0.69 | |
| Self-rated Progress Major improvement (%) |
One year or less |
70.8 | 25.7 | 45.2 (35.2, 55.1) |
70.2 | 31 | 39.2 (28.6, 49.8) |
64.8 | 33.3 | 31.5 (20.1, 43) |
53.3 | 25.6 | 27.7 (15.7, 39.7) |
| More than 1 year | 66.6 | 22.1 | 44.5 (32.9, 56) |
58.4 | 18.6 | 39.8 (27.6, 52) |
58.7 | 15.1 | 43.6 (31.2, 56) |
54.3 | 12.7 | 41.5 (28.2, 54.9) |
|
| pvalue | 0.43 | 0.57 | 0.99 | 0.031 | 0.093 | 0.73 | 0.27 | 0.04 | 0.16 | 0.89 | 0.17 | 0.18 | |
Adjusted for age, gender, BMI, race, smoking status, compensation, joint, stomach, bowel, osteoporosis, number of moderate/severe stenotic levels, self-assessed health trend at baseline, treatment preference, baseline stenosis bothersomeness, other ** comorbiditiy, baseline score and center.
Other comorbidities include: stroke, cancer, fibromyalgia, cfs, PTSD, alcohol, drug dependency, lung, liver, kidney, blood vessel, nervous system, migraine, anxiety
Treatment effect is the difference between the surgical and non-operative mean change from baseline. Analysis is done using a mixed model with a random subject intercept term. Treatment is a time-varying covariate where a patients' experience prior to surgery is attributed to the non-operative arm and time is measured from enrollment and his/her post-surgery outcomes are attributed to the surgical arm and time is measured from time of surgery.
Figure 1.
- Surgery p-value compares duration of symptoms one year or less to more than 1 year among Surgery.
- Non-operative p-value compares duration of symptoms one year or less to more than 1 year among Non-operative.
- Interaction p-value compares treatment effect (surgery vs. non-operative) between duration of symptoms one year or less and more than 1 year.
* P-values are time weighted average 4 years (Area Under Curve p-values).
Averaged over four years, there was more improvement in the nonsurgically treated SS <12 Months Patients patient versus SS >12 Months Patients in SF36 BP (p=0.004), SF36 PF (p=0.02), and ODI (p=0.022) (Table 3, Figure 1). At four years, there was more improvement in SS <12 Months Patients versus SS >12 Months Patients in SF36 BP (15.3 vs 6.2, p=0.019), ODI (−10.3 vs −4.6, p=0.049), Low Back Pain Bothersomeness Index (−1 vs −0.5, p=0.047)..
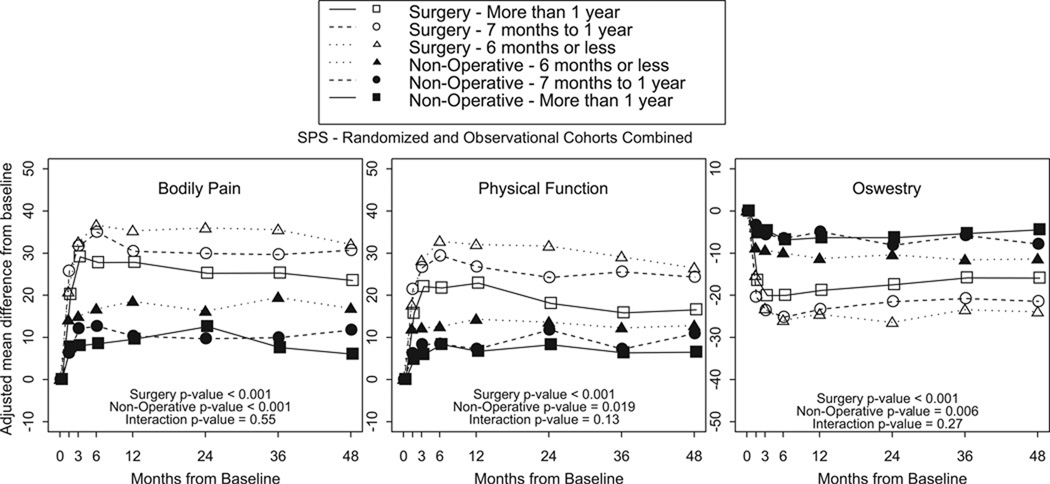
Comparing the change in outcome of the surgically treated patients minus the change in outcome of the nonsurgically treated patients (Table 3, Figure 1), there were no statistically significant differences in treatment effect of surgery between SS <12 Months Patients and SS >12 Months Patients in primary outcome measures or secondary outcome measures averaged over four years or at individual time endpoints. Additonal analysis was performed using a cutoff of 6 months duration of symptoms. There were no significant differences in outcomes between those patients with a duration of symptoms greater or less than 6 months (Table 4, Figure 2).
Table 4.
Subgroup results from adjusted* as-treated outcome analysis by three groups duration of symptoms for the randomized and observational cohorts combined patients with lumbar spinal stenosis.
| Outcome |
Duration of Symptoms |
1-Year |
2-Year |
3-Year |
4-Year |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SpS | Surgical | Non- operative |
Treatment Effect† (95% CI) |
Surgical | Non- operative |
Treatment Effect† (95% CI) |
Surgical | Non- operative |
Treatment Effect† (95% CI) |
Surgical | Non- operative |
Treatment Effect† (95% CI) |
|
|
Primary Outcomes |
|||||||||||||
| SF-36 Bodily Pain (BP) (0- 100) (SE) |
6 months or less |
35.3 (2.6) | 18.5 (2.1) | 16.8 (10.1, 23.5) |
35.9 (2.7) | 16.2 (2.2) | 19.8 (12.8, 26.7) |
35.5 (2.7) | 19.5 (2.4) | 16.1 (8.9, 23.2) |
32.1 (2.8) | 16.8 (2.5) | 15.2 (7.8, 22.6) |
| 7 to 12 months |
30.4 (2.4) | 10.3 (3) | 20.1 (12.6, 27.7) |
29.9 (2.3) | 9.6 (3.1) | 20.2 (12.7, 27.8) |
29.6 (2.4) | 9.9 (3.3) | 19.7 (11.7, 27.7) |
30.7 (2.6) | 11.7 (3.6) | 18.9 (10.3, 27.6) |
|
| More than 1 year |
27.8 (1.7) | 9.6 (2.5) | 18.2 (12.6, 23.8) |
25.2 (1.7) | 12.6 (2.7) | 12.6 (6.6, 18.7) |
25.3 (1.7) | 7.6 (3) | 17.7 (11.2, 24.3) |
23.5 (1.9) | 6 (3.3) | 17.5 (10.1, 24.8) |
|
| pvalue | 0.057 | 0.008 | 0.80 | 0.002 | 0.20 | 0.18 | 0.005 | 0.003 | 0.80 | 0.011 | 0.031 | 0.80 | |
| SF-36 Physical Function (PF) (0–100) (SE) |
6 months or less |
32 (2.6) | 14.2 (2) | 17.8 (11.3, 24.3) |
31.6 (2.6) | 13.6 (2.1) | 18.1 (11.3, 24.8) |
29.1 (2.6) | 12.2 (2.3) | 16.9 (10, 23.7) |
26.5 (2.7) | 12.8 (2.4) | 13.7 (6.6, 20.8) |
| 7 to 12 months |
26.7 (2.3) | 7.2 (2.8) | 19.5(12.3, 26.7) |
24.2 (2.2) | 11.7 (2.9) | 12.4 (5.3, 19.6) |
25.5 (2.3) | 7.2 (3.1) | 18.3 (10.7, 25.9) |
24.3 (2.5) | 10.9 (3.4) | 13.4 (5.1, 21.6) |
|
| More than 1 year |
22.9 (1.6) | 6.7 (2.3) | 16.2 (11, 21.4) |
18.1 (1.6) | 8.3 (2.6) | 9.8 (4.2, 15.5) |
15.8 (1.7) | 6.3 (2.8) | 9.5 (3.3, 15.6) |
16.5 (1.8) | 6.5 (3.1) | 10 (3.2, 16.9) |
|
| pvalue | 0.01 | 0.022 | 0.76 | <0.001 | 0.27 | 0.17 | <0.001 | 0.19 | 0.13 | 0.002 | 0.27 | 0.72 | |
| Mental Component Summary (MCS)(0–100) (SE) |
6 months or less |
5.4 (1.1) | 2.9 (0.9) | 2.5 (−0.3, 5.3) |
4.3 (1.1) | 1.7 (0.9) | 2.6 (−0.3, 5.5) |
3.6 (1.1) | 2.3 (1) | 1.3 (−1.7, 4.2) |
2.8 (1.1) | 1.6 (1.1) | 1.2 (−1.9, 4.3) |
| 7 to 12 months |
4.3 (1) | 2.8 (1.2) | 1.5 (−1.7, 4.6) |
4 (0.9) | 0.8 (1.3) | 3.2 (0, 6.3) |
3.9 (1) | −0.8 (1.4) | 4.6 (1.3, 8) |
2.6 (1.1) | −0.9 (1.5) | 3.5 (−0.2, 7.2) |
|
| More than 1 year |
2.3 (0.7) | 1.3 (1) | 0.9 (−1.4, 3.3) |
3 (0.7) | 0.6 (1.1) | 2.4 (−0.2, 4.9) |
1.6 (0.7) | −0.3 (1.3) | 1.9 (−0.9, 4.7) |
2.1 (0.8) | −1.7 (1.4) | 3.8 (0.7, 6.9) |
|
| pvalue | 0.032 | 0.44 | 0.69 | 0.49 | 0.70 | 0.93 | 0.099 | 0.12 | 0.29 | 0.85 | 0.13 | 0.45 | |
| Oswestry Disability Index (ODI) (0–100) (SE) |
6 months or less |
−24.5 (2) | −11.3 (1.6) | −13.2 (−18.3, −8.1) |
−26.5 (2.1) | −10.4 (1.7) | −16 (−21.4, −10.7) |
−23.4 (2.1) | −11.6 (1.8) | −11.8 (−17.3, −6.4) |
−24 (2.2) | −11.4 (1.9) | −12.6 (−18.3, −6.9) |
| 7 to 12 months |
−23.3 (1.8) | −5 (2.3) | −18.4 (−24.1, - 12.6) |
−21.5 (1.7) | −8.2 (2.3) | −13.4 (−19, −7.7) |
−20.8 (1.8) | −5.9 (2.5) | −15 (−21, −8.9) |
−21.5 (2) | −7.9 (2.7) | −13.6 (−20.1, −7) |
|
| More than 1 year |
−18.8 (1.3) | −6.3 (1.8) | −12.5 (−16.6, −8.4) |
−17.5 (1.3) | −6.3 (2) | −11.2 (−15.6, −6.8) |
−15.9 (1.3) | −5.3 (2.2) | −10.6 (−15.4, −5.7) |
−15.9 (1.4) | −4.5 (2.5) | −11.5 (−16.9, −6) |
|
| pvalue | 0.025 | 0.028 | 0.24 | <0.001 | 0.28 | 0.38 | 0.003 | 0.044 | 0.52 | 0.003 | 0.078 | 0.88 | |
|
Secondary Outcomes |
|||||||||||||
| Stenosis Othersomeness Index (0–24) (SE) |
6 months or Less |
−9.1 (0.7) | −3.9 (0.6) | −5.3 (−7.1, −3.4) |
−9.8 (0.7) | −4.4 (0.6) | −5.3 (−7.3, −3.4) |
−8.7 (0.7) | −4.7 (0.7) | −4 (−6, −2) |
−8.8 (0.8) | −4.4 (0.7) | −4.4 (−6.4, −2.3) |
| 7 to 12 months |
−8 (0.7) | −3.3 (0.8) | −4.8 (−6.8, −2.7) |
−8.1 (0.6) | −4.3 (0.8) | −3.8 (−5.9, −1.7) |
−8 (0.7) | −4.4 (0.9) | −3.7 (−5.9, −1.5) |
−7.2 (0.7) | −3.6 (1) | −3.6 (−6, −1.2) |
|
| More than 1 year |
−8.1 (0.5) | −3 (0.7) | −5.1 (−6.6, −3.6) |
−7.1 (0.5) | −2.6 (0.7) | −4.5 (−6.2, −2.9) |
−7 (0.5) | −2.8 (0.8) | −4.2 (−6, −2.4) |
−7.3 (0.5) | −2.4 (0.9) | −4.8 (−6.8, −2.9) |
|
| pvalue | 0.43 | 0.59 | 0.93 | 0.007 | 0.12 | 0.54 | 0.11 | 0.16 | 0.93 | 0.18 | 0.19 | 0.72 | |
| Low Back Pain Bothersomene s (0–6) (SE) |
6 months or less |
−2.2 (0.2) | −0.9 (0.2) | −1.3 (−1.8, −0.8) |
−2.4 (0.2) | −1.1 (0.2) | −1.4 (−1.9, −0.9) |
−2.2 (0.2) | −1.3 (0.2) | −1 (−1.6, −0.4) |
−2 (0.2) | −1 (0.2) | −1 (−1.6, −0.4) |
| 7 to 12 months |
−2.2 (0.2) | −0.8 (0.2) | −1.4 (−2, −0.8) |
−2.2 (0.2) | −1 (0.2) | −1.2 (−1.8, −0.6) |
−2.1 (0.2) | −0.9 (0.3) | −1.2 (−1.8, −0.6) |
−1.8 (0.2) | −1 (0.3) | −0.8 (−1.5, −0.1) |
|
| More than 1 year |
−2 (0.1) | −0.7 (0.2) | −1.3 (−1.7, −0.9) |
−1.9 (0.1) | −0.5 (0.2) | −1.3 (−1.8, −0.8) |
−1.8 (0.1) | −0.3 (0.2) | −1.5(−2, −1) | −1.7 (0.2) | −0.5 (0.3) | −1.2 (−1.8, −0.6) |
|
| pvalue | 0.49 | 0.55 | 0.96 | 0.04 | 0.14 | 0.88 | 0.14 | 0.003 | 0.43 | 0.49 | 0.13 | 0.57 | |
| Leg pain (0–6) (SE) |
6 months or less |
−2.8 (0.2) | −1.4 (0.2) | −1.4 (−2, −0.8) |
−3 (0.2) | −1.5 (0.2) | −1.5 (−2.1, −0.9) |
−2.9 (0.2) | −1.9 (0.2) | −1 (−1.6, −0.4) |
−2.8 (0.2) | −1.5 (0.2) | −1.2 (−1.8, −0.6) |
| 7 to 12 months |
−2.8 (0.2) | −1.1 (0.3) | −1.7 (−2.3, −1.1) |
−2.7 (0.2) | −1.4 (0.3) | −1.3 (−1.9, −0.7) |
−2.5 (0.2) | −1.6 (0.3) | −0.9 (−1.6, −0.2) |
−2.2 (0.2) | −1.3 (0.3) | −0.9 (−1.7, −0.1) |
|
| More than 1 year |
−2.5 (0.1) | −1.1 (0.2) | −1.5(−2, −1) | −2.4 (0.1) | −0.8 (0.2) | −1.6 (−2.1, −1.1) |
−2.4 (0.1) | −0.9 (0.3) | −1.5 (−2.1, −0.9) |
−2.7 (0.2) | −1.1 (0.3) | −1.6 (−2.2, −1) |
|
| pvalue | 0.52 | 0.42 | 0.78 | 0.08 | 0.085 | 0.70 | 0.16 | 0.011 | 0.35 | 0.14 | 0.50 | 0.39 | |
| Very/somewhat satisfied with symptoms (%) |
6 months or less |
68.6 | 32.6 | 35.9 (21.9, 50) |
76.3 | 29.8 | 46.5 (33.1, 60) |
75.1 | 44 | 31 (15.9, 46.2) |
74.1 | 37.5 | 36.6 (20.9, 52.3) |
| 7 to 12 months |
72.4 | 22.2 | 50.2 (35.6, 64.8) |
68.4 | 32.4 | 36 (19.5, 52.6) |
68.5 | 36.2 | 32.3 (14.3, 50.2) |
66.1 | 28.7 | 37.4 (18.7, 56.1) |
|
| More than 1 year |
66.8 | 24.9 | 41.9 (29.9, 53.9) |
67 | 20.7 | 46.3 (33.9, 58.8) |
59.8 | 21.5 | 38.2 (24.3, 52.1) |
54.5 | 25.2 | 29.3 (13.1, 45.6) |
|
| pvalue | 0.67 | 0.33 | 0.39 | 0.35 | 0.37 | 0.53 | 0.073 | 0.064 | 0.78 | 0.032 | 0.42 | 0.85 | |
| Self-rated Progressmajor Improvement (%) |
6 months or less |
69.4 | 29.1 | 40.3 (26.6, 54) |
72.3 | 30.1 | 42.1 (28.1, 56.1) |
61.6 | 35.7 | 25.9 (10.1, 41.6) |
50.1 | 26.8 | 23.3 (7.3, 39.2) |
| 7 to 12 months |
72.1 | 19.4 | 52.7 (38.7, 66.8) |
68.6 | 32.9 | 35.6 (19.4, 51.9) |
67.9 | 28.8 | 39.1 (22.3, 55.9) |
56.7 | 23.6 | 33.2 (15.2, 51.2) |
|
| More than 1 year |
66.7 | 22.2 | 44.5 (32.9, 56.1) |
58.2 | 18.7 | 39.6 (27.4, 51.8) |
58.8 | 15.1 | 43.7 (31.2, 56.1) |
54.4 | 12.8 | 41.6 (28.2, 55) |
|
| pvalue | 0.70 | 0.35 | 0.46 | 0.075 | 0.22 | 0.80 | 0.41 | 0.092 | 0.19 | 0.75 | 0.37 | 0.31 | |
Adjusted for age, gender, BMI, race, smoking status, compensation, joint, stomach, bowel, osteoporosis, number of moderate/severe stenotic levels, self-assessed health trend at baseline, treatment preference, baseline stenosis bothersomeness, other *
Other comorbidities include: stroke, cancer, fibromyalgia, cfs, PTSD, alcohol, drug dependency, lung, liver, kidney, blood vessel, nervous system, migraine, anxiety
Treatment effect is the difference between the surgical and non-operative mean change from baseline. Analysis is done using a mixed model with a random subject intercept term. Treatment is a time-varying covariate where a patients' experience prior to surgery is attributed to the non-operative arm and time is measured from enrollment and his/her post-surgery outcomes are attributed to the surgical arm and time is measured from time of surgery.
Figure 2.
- Surgery p-value compares duration of symptoms 6 months or less, 7 to 12 months and more than 1 year among Surgery.
- Non-operative p-value compares duration of symptoms 6 months or less, 7 to 12 months and more than 1 year among Non-operative.
- Interaction p-value compares treatment effect (surgery vs. non-operative) among duration of symptoms 6 months or less, 7 to 12 months and more than 1 year.
* P-values are time weighted average 4 years (Area Under Curve p-values).
In the DS study, there were three hundred and ninety seven patients with duration of symptoms < 12 months (DS <12 Months Patients – DS Table 1). There were two hundred and four patients with duration of symptoms ≥ 12 months (DS >12 Months Patients). There were decreased incidence of unlisted medical comorbidities in DS <12 Months Patients vs DS >12 Months Patients (36% vs 45%, p=0.05). There was worse baseline SF36 MCS (49.1 vs 52.1, p=0.003), Low Back Pain Bothersomeness (4.2 vs 4.5, p=0.039) in DS <12 Months Patients vs DS >12 Months Patients. There was an increased percentage of patients reporting symptoms worsening in DS >12 Months Patients vs DS <12 Months Patients (66% vs 57%, p=0.009). There was an increased percentage of asymmetric depressed reflexes (22% vs 30%, p=0.035) and central stenosis (89% vs 96%, p=0.005) in DS >12 Months Patients.
Operative details of the DS patient group are described in DS Table 2. In contrast to the SS patient population, approximately 94% of the patients in both DS Groups underwent a spinal fusion operation and only 6% underwent isolated decompression. There were no statistically significant differences in the method of fusion (in situ vs. instrumented) between Groups 3 and 4. There were no differences in the number of levels decompressed, fusion levels, or complications. There was a trend toward a higher percentage of patients in DS <12 Months Patients having no complications (73% vs 63%, p=0.051). In contrast to the SS patients, there were no statistically significant differences in the incidence of additional surgeries between treatment groups.
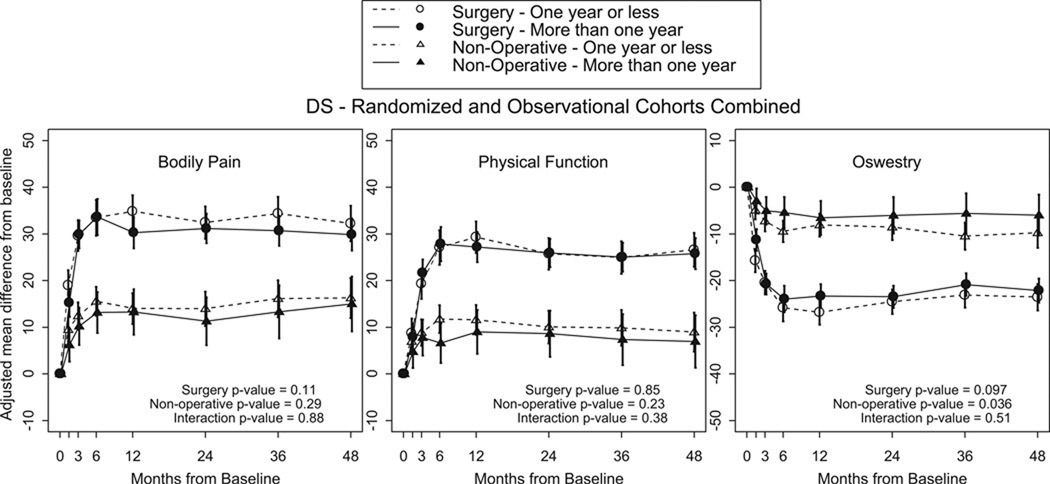
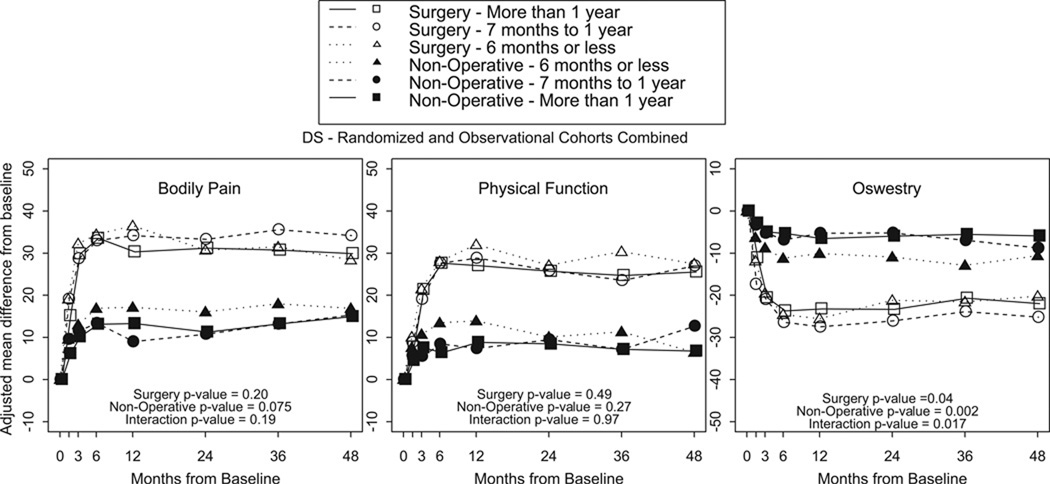
There were no statistically significant differences in primary outcome measures (Figure 1) between surgically treated patients in Groups 3 and 4. There were no statistically significant differences in secondary outcome measures between surgically treated patients in Groups 3 and 4. There were no statistically significant differences in primary or secondary outcome measures in nonsurgically treated patients between Groups 3 and 4. There were no statistically significant differences in treatment effect in primary or secondary outcome measures between DS <12 Months Patients and DS >12 Months Patients. Furthermore, there were no statistically significant differences in all outcome measures between DS patients with > or < 6 months of symptoms (Figure 2)
Discussion
The results of this study show that patients with spinal stenosis symptoms ≥ 12 months have less improvement in outcome, regardless of treatment. The duration of degenerative spondylolisthesis symptoms ≥ 12 months is associated with no difference in outcome of surgical or nonsurgical treatment. The treatment effect of surgery compared to nonoperative treatment is not related to the duration of symptoms.
The disparity between the SS and DS groups may result from improved outcomes observed in the DS patients with > 12 months of symptoms (DS >12 Months Patients) relative to the SS patients with >12 months of symptoms (SS >12 Months Patients). Thus the surgically treated patients with DOS < 12 months with SS and DS achieve similar improvement. However, the surgically treated patients with DS for >12 months achieve a better outcome than patients with SS. Differences between the SS and DS groups in the nonoperatively treated patients with longer symptom duration were significant in the SF36 BP domain. Consequently, the treatment effect of surgery is significantly greater in the DS patients in SF36 PCS, percent satisfaction with symptoms, and trending toward significance in ODI.
There may be pathophysiological reasons for the differential outcome of DS and SS patients with longer symptom duration. DS may result in more episodic symptoms than pure SS. It may be better tolerated for a longer symptom duration because of the dynamic nature of the instability. Additionally, there is a difference in the distribution of stenosis with the DS patients having a higher incidence of central stenosis (89% vs 96%, DS Table 1). Central stenosis may be less likely to result in severe irreversible changes. Another possible explanation for the difference between the groups may result from the different treatment types delivered to each group. The majority (96%) of the DS patients underwent fusion, while only a small percentage of the SS patients (average 10%) underwent fusion. It is possible that fusion may reduce late dynamic instability and secondary nerve pain. Alternatively, surgeons may have performed more aggressive decompression consisting of wider laminectomy or more facectomy in patients who underwent fusion where there was no concern about iatrogenic instability. However, there was no statistically significant difference in the percentage of patients who underwent fusion in the >12 months or <12 months groups. We would expect to find a significant difference if fusion were a major confounder.
At baseline, the SS symptom duration groups were well balanced. There were no clinically significant differences in baseline outcome measures, physical findings, or type of surgical intervention between SS symptom duration groups. The decreased improvement in SS patient outcome is associated with a significantly higher number of revision surgeries in SS >12 Months Patients.
The association between DOS and patient outcome in SS patients may be explained in part by the pathophysiology of chronic nerve compression. Studies of peripheral nerves have demonstrated that an early consequence of chronic nerve compression is local demyelination and remyelination.3 Chronic nerve compression has also been shown to cause neurons within the dorsal root ganglion to adopt a regenerative phenotype, thereby undergoing a phenotypic change.2 These changes may be partly responsible for the alterations in nerve function during the early period post-compression and may account for the decreased improvement and increased revision rate in SS patients with a DOS >12 months. Furthermore, the changes in nerve physiology may be exacerbated by chronic ischemia in the lateral recess. There is a watershed vascular supply between the medullary arterial system of the spinal cord and the radicular arterial system of the nerve roots.24 The relative hypovascularity of the spinal nerve roots renders them particularly susceptible to local ischemia from extrinsic compression. 24, 25 We hypothesize that since degenerative spondylolisthesis results in a dynamic, not constant, compression there is less long term nerve ischemia and demyelination since the nerves may recover if the spondylolisthesis reduces with posture.
Other studies in the literature do not show a significant effect of preoperative DOS on outcome of treatment of SS or DS1,4,6,9,15,16,23 or did not examine DOS as a possible predictor of outcome of treatment on SS or DS.5,8,12,14,20,21,22 Yasar et al report the results of a prospective study of 125 patients with spinal stenosis with 2 year followup. The authors found no correlation between DOS and outcome of treatment, although the incidence of DS is not specified in the population.23 Jönsson and colleagues report on a series of 105 patients approximately 33% DS and 66% SS who underwent decompressive laminectomy for spinal stenosis. The authors report a nonsignificant trend toward a worse outcome associated with DOS > 4 years.7 Among patients with lateral spinal stenosis, the authors also demonstrated that a long preoperative duration of sciatica was associated with poor outcome.10 Several meta analyses have failed to report an effect of DOS on outcome of treatment of spinal stenosis or degenerative spondylolisthesis.17 Finally, Katz et al report no effect of longer DOS on outcome of treatment of SS or DS. The authors report on a series of 99 patients with approximately 25% incidence of DS. There was no effect of DOS on medium term or long term outcome.11
There are several limitations to this subgroup analysis. The original SPORT study was designed and powered to compare the outcome of surgically and nonsurgically treated patients. There may be confounding variables that are not equally distributed between treatment groups in this “as treated” analysis. For instance, there were significant differences in mental status score and perception of worsening at baseline in the DS patients. Although baseline differences were controlled for in the calculation of change in primary outcome measures according to symptom duration, there is the possibility that baseline differences that have not been identified are confounding factors. Another possible difference is in the effect of medical comorbidities on outcome of treatment. Certainly patients with significant medical comorbidities may be considered more appropriate for nonsurgical treatment to reduce the risks of surgery. However, in both patient groups there was no statistically significant difference in the “major” medical comorbidities between groups (SS Table 1 and DS Table 1). There were differences in unlisted comorbidities although further information is not available. It is possible that patients with particular comorbidities may have an improved outcome with shorter or longer duration of symptoms since their underlying medical comorbidities may preclude full participation in therapy and nonsurgical treatment. Furthermore, the SPORT study was not specifically powered to include analysis of specific patient subgroups and consequently there is a possibility of type II error. However, the SPORT represents the largest study to date on SS and DS patients and is most likely to be powered to answer such detailed questions.
In summary, patients with SS who have symptom duration > 12 months have a significantly less improvement in outcome regardless of treatment. Patients who have DS have no difference in outcome associated with DOS. Evidence reveals that surgical intervention is associated with an improved outcome compared to nonsurgical treatment. Prolonged duration of symptoms does not affect the efficacy of surgical intervention.
A retrospective subgroup analysis of the the Spine Outcomes Research Trial to determine if the duration of symptoms affects outcomes following the treatment of spinal stenosis (SS) or degenerative spondylolisthesis (DS).
Key Points.
The study evaluated whether timing of treatment affected outcome in patients with spinal stenosis and degenerative spondylolisthesis.
Regardless of the timing of treatment, patients in the surgery group had improved outcomes at all time points relative to the nonoperative treatment group.
Patients with spinal stenosis with greater than twelve months of symptoms had less improvement relative to those with less than twelve months. Patients treated with degenerative spondylolisthesis did not have different outcomes according to duration of symptoms.
Figure 3.
- Surgery p-value compares duration of symptoms one year or less to more than 1 year among Surgery.
- Non-operative p-value compares duration of symptoms one year or less to more than 1 year among Non-operative.
- Interaction p-value compares treatment effect (surgery vs. non-operative) between duration of symptoms one year or less and more than 1 year.
* P-values are time weighted average 4 years (Area Under Curve p-values).
Figure 4.
- Surgery p-value compares duration of symptoms 6 months or less, 7 to 12 months and more than 1 year among Surgery.
- Non-operative p-value compares duration of symptoms 6 months or less, 7 to 12 months and more than 1 year among Non-operative.
- Interaction p-value compares treatment effect (surgery vs. non-operative) among duration of symptoms 6 months or less, 7 to 12 months and more than 1 year.
* P-values are time weighted average 4 years (Area Under Curve p-values).
Table 5.
Patient Baseline Characteristics, Comorbidities and Health Status Measures for Patients with Lumbar Degenerative Spondylolisthesis (DS)
| Characteristics DS |
One year or less (n=397) |
More than one year (n=204) |
p-value | 6 months or less (n=240) |
7 to 12 months (n=157) |
More than one year (n=204) |
p-value |
|---|---|---|---|---|---|---|---|
| Mean Age (SD) | 66.1 (10.4) | 65.9 (10.2) | 0.79 | 67.1 (10.2) | 64.6 (10.4) | 65.9 (10.2) | 0.062 |
| Female - no.(%) | 281 (71%) | 131 (64%) | 0.12 | 161 (67%) | 120 (76%) | 131 (64%) | 0.038 |
| Ethnicity: Not Hispanic | 390 (98%) | 197 (97%) | 0.32 | 238 (99%) | 152 (97%) | 197 (97%) | 0.14 |
| Race - White† | 329 (83%) | 177 (87%) | 0.26 | 202 (84%) | 127 (81%) | 177 (87%) | 0.32 |
| Education - At least some college | 266 (67%) | 134 (66%) | 0.82 | 155 (65%) | 111 (71%) | 134 (66%) | 0.43 |
| Marital Status - Married | 259 (65%) | 137 (67%) | 0.70 | 155 (65%) | 104 (66%) | 137 (67%) | 0.85 |
| Work Status | 0.70 | 0.051 | |||||
| Full or part time | 142 (36%) | 76 (37%) | 86 (36%) | 56 (36%) | 76 (37%) | ||
| Disabled | 34 (9%) | 17 (8%) | 15 (6%) | 19 (12%) | 17 (8%) | ||
| Retired | 167 (42%) | 90 (44%) | 113 (47%) | 54 (34%) | 90 (44%) | ||
| Other | 54 (14%) | 21 (10%) | 26 (11%) | 28 (18%) | 21 (10%) | ||
| Compensation - Any‡ | 27 (7%) | 14 (7%) | 0.89 | 17 (7%) | 10 (6%) | 14 (7%) | 0.96 |
| Mean Body Mass Index (BMI), (SD)§ | 29.1 (5.9) | 29.3 (6.8) | 0.60 | 28.9 (5.7) | 29.3 (6.1) | 29.3 (6.8) | 0.67 |
| Smoker | 37 (9%) | 14 (7%) | 0.38 | 25 (10%) | 12 (8%) | 14 (7%) | 0.37 |
| Comorbidities - no.(%) | |||||||
| Hypertension | 179 (45%) | 96 (47%) | 0.71 | 114 (48%) | 65 (41%) | 96 (47%) | 0.44 |
| Diabetes | 60 (15%) | 20 (10%) | 0.091 | 33 (14%) | 27 (17%) | 20 (10%) | 0.12 |
| Osteoporosis | 46 (12%) | 23 (11%) | 0.98 | 25 (10%) | 21 (13%) | 23 (11%) | 0.66 |
| Heart Problem | 76 (19%) | 46 (23%) | 0.38 | 50 (21%) | 26 (17%) | 46 (23%) | 0.36 |
| Stomach Problem | 78 (20%) | 55 (27%) | 0.052 | 42 (18%) | 36 (23%) | 55 (27%) | 0.055 |
| Bowel or Intestinal Problem | 26 (7%) | 17 (8%) | 0.52 | 12 (5%) | 14 (9%) | 17 (8%) | 0.24 |
| Depression | 68 (17%) | 30 (15%) | 0.52 | 28 (12%) | 40 (25%) | 30 (15%) | <0.001 |
| Joint Problem | 241 (61%) | 103 (50%) | 0.021 | 149 (62%) | 92 (59%) | 103 (50%) | 0.045 |
| Other¶ | 143 (36%) | 91 (45%) | 0.05 | 75 (31%) | 68 (43%) | 91 (45%) | 0.007 |
| SF-36 scores, mean (SD)†† | |||||||
| Bodily Pain (BP) | 33 (20) | 33.8 (17.4) | 0.65 | 32.7 (20.4) | 33.5 (19.6) | 33.8 (17.4) | 0.83 |
| Physical Functioning (PF) | 33.9 (22.7) | 35.2 (21.8) | 0.48 | 33.2 (21.8) | 34.8 (24) | 35.2 (21.8) | 0.61 |
| Mental Component Summary (MCS) | 49.1 (11.6) | 52.1 (11) | 0.003 | 49.7 (11.8) | 48.1 (11.4) | 52.1 (11) | 0.004 |
| Oswestry (ODI) (SD)‡‡ | 41.3 (18.3) | 42 (17) | 0.65 | 41.1 (17.7) | 41.7 (19.2) | 42 (17) | 0.85 |
| Stenosis Frequency Index (0–24) (SD)§§ | 13.7 (5.6) | 14.4 (5.5) | 0.19 | 14.1 (5.6) | 13.2 (5.6) | 14.4 (5.5) | 0.14 |
| Stenosis Bothersome Index (0–24) (SD)§§ | 14.5 (5.8) | 15 (5.3) | 0.26 | 14.9 (5.8) | 13.8 (5.7) | 15 (5.3) | 0.087 |
| Low Back Pain Bothersomeness (0–6) (SD)¶¶ | 4.2 (1.9) | 4.5 (1.7) | 0.039 | 4.2 (1.8) | 4 (1.9) | 4.5 (1.7) | 0.072 |
| Leg Pain Bothersomeness (0–6) (SD)¶¶ | 4.5 (1.7) | 4.7 (1.6) | 0.17 | 4.5 (1.7) | 4.4 (1.7) | 4.7 (1.6) | 0.30 |
| Satisfaction with symptoms - very dissatisfied | 276 (70%) | 140 (69%) | 0.90 | 163 (68%) | 113 (72%) | 140 (69%) | 0.68 |
| Patient self-assessed health trend - no.(%) | 0.009 | 0.008 | |||||
| Getting better | 33 (8%) | 5 (2%) | 23 (10%) | 10 (6%) | 5 (2%) | ||
| Staying about the same | 131 (33%) | 63 (31%) | 85 (35%) | 46 (29%) | 63 (31%) | ||
| Getting worse | 226 (57%) | 135 (66%) | 127 (53%) | 99 (63%) | 135 (66%) | ||
| Treatment preference at baseline - no.(%) | 0.072 | 0.048 | |||||
| Preference for non-surg | 167 (42%) | 68 (33%) | 109 (45%) | 58 (37%) | 68 (33%) | ||
| Not sure | 90 (23%) | 47 (23%) | 47 (20%) | 43 (27%) | 47 (23%) | ||
| Preference for surgery | 139 (35%) | 89 (44%) | 83 (35%) | 56 (36%) | 89 (44%) | ||
| Pseudoclaudication - Any | 331 (83%) | 180 (88%) | 0.14 | 199 (83%) | 132 (84%) | 180 (88%) | 0.27 |
| SLR or Femoral Tension | 58 (15%) | 27 (13%) | 0.74 | 32 (13%) | 26 (17%) | 27 (13%) | 0.60 |
| Pain radiation - any | 313 (79%) | 155 (76%) | 0.49 | 193 (80%) | 120 (76%) | 155 (76%) | 0.47 |
| Any Neurological Deficit | 220 (55%) | 107 (52%) | 0.55 | 138 (57%) | 82 (52%) | 107 (52%) | 0.46 |
| Reflexes - Asymmetric Depressed | 88 (22%) | 62 (30%) | 0.035 | 51 (21%) | 37 (24%) | 62 (30%) | 0.077 |
| Sensory - Asymmetric Decrease | 115 (29%) | 54 (26%) | 0.58 | 73 (30%) | 42 (27%) | 54 (26%) | 0.59 |
| Motor - Asymmetric Weakness | 97 (24%) | 49 (24%) | 0.99 | 62 (26%) | 35 (22%) | 49 (24%) | 0.72 |
| Listhesis Level | 0.13 | 0.13 | |||||
| L3–L4 | 32 (8%) | 25 (12%) | 16 (7%) | 16 (10%) | 25 (12%) | ||
| L4–L5 | 365 (92%) | 179 (88%) | 224 (93%) | 141 (90%) | 179 (88%) | ||
| Stenosis Levels | |||||||
| L2–L3 | 32 (8%) | 21 (10%) | 0.45 | 18 (8%) | 14 (9%) | 21 (10%) | 0.58 |
| L3–L4 | 152 (38%) | 84 (41%) | 0.55 | 88 (37%) | 64 (41%) | 84 (41%) | 0.57 |
| L4–L5 | 385 (97%) | 195 (96%) | 0.52 | 234 (98%) | 151 (96%) | 195 (96%) | 0.53 |
| L5–S1 | 40 (10%) | 17 (8%) | 0.59 | 27 (11%) | 13 (8%) | 17 (8%) | 0.48 |
| Stenotic Levels (Mod/Severe) | 0.31 | 0.66 | |||||
| None | 18 (5%) | 5 (2%) | 11 (5%) | 7 (4%) | 5 (2%) | ||
| One | 239 (60%) | 131 (64%) | 146 (61%) | 93 (59%) | 131 (64%) | ||
| Two | 119 (30%) | 53 (26%) | 72 (30%) | 47 (30%) | 53 (26%) | ||
| Three+ | 21 (5%) | 15 (7%) | 11 (5%) | 10 (6%) | 15 (7%) | ||
| Stenosis Locations | |||||||
| Central | 353 (89%) | 196 (96%) | 0.005 | 212 (88%) | 141 (90%) | 196 (96%) | 0.011 |
| Lateral Recess | 355 (89%) | 191 (94%) | 0.12 | 215 (90%) | 140 (89%) | 191 (94%) | 0.24 |
| Neuroforamen | 159 (40%) | 84 (41%) | 0.86 | 101 (42%) | 58 (37%) | 84 (41%) | 0.57 |
| Stenosis Severity | 0.20 | 0.21 | |||||
| Mild | 18 (5%) | 5 (2%) | 11 (5%) | 7 (4%) | 5 (2%) | ||
| Moderate | 148 (37%) | 67 (33%) | 82 (34%) | 66 (42%) | 67 (33%) | ||
| Severe | 231 (58%) | 132 (65%) | 147 (61%) | 84 (54%) | 132 (65%) | ||
| Instability | 35 (9%) | 12 (6%) | 0.27 | 14 (6%) | 21 (13%) | 12 (6%) | 0.011 |
| Received surgery* | 254 (64%) | 137 (67%) | 0.49 | 151 (63%) | 103 (66%) | 137 (67%) | 0.64 |
Race or ethnic group was self-assessed. Whites and blacks could be either Hispanic or non-Hispanic.
This category includes patients who were receiving or had applications pending for workers compensation, Social Security compensation, or other compensation.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Other indicates problems related to stroke, cancer, lung, fibromyalgia, chronic fatigue syndrome, post traumatic stress disorder, alcohol, drug dependency, liver, kidney, blood vessel, nervous system, migraine, anxiety.
The SF-36 scores range from 0 to 100, with higher score indicating less severe symptoms.
The Oswestry Disability Index ranges from 0 to 100, with lower scores indicating less severe symptoms.
The Stenosis Bothersomeness index and the Stenosis Frequency index range from 0 to 24, with lower scores indicating less severe symptoms.
The Low Back Pain Bothersomeness and the Leg Pain Bothersomeness Scale range from 0 to 6, with lower scores indicating less severe symptoms.
Patients received surgery were classified according to whether they received surgical treatment during the first 4 years of enrollment
Table 6.
Operative treatments, complications and events.
| DS | One year or less (n=251) |
More than one year (n=138) |
p-value | 6 months or less (n=149) |
7 to 12 months (n=102) |
More than one year (n=138) |
p-value |
|---|---|---|---|---|---|---|---|
| Specific procedures† | 0.48 | 0.45 | |||||
| Decompression only | 17 (7%) | 7 (5%) | 9 (6%) | 8 (8%) | 7 (5%) | ||
| Non-instrumented fusion | 48 (20%) | 33 (24%) | 25 (17%) | 23 (23%) | 33 (24%) | ||
| Instrumented fusion | 181 (74%) | 96 (71%) | 114 (77%) | 67 (68%) | 96 (71%) | ||
| Multi-level fusion | 49 (20%) | 42 (30%) | 0.021 | 34 (23%) | 15 (15%) | 42 (30%) | 0.017 |
| Decompression level | |||||||
| L2–L3 | 25 (10%) | 20 (15%) | 0.23 | 14 (10%) | 11 (11%) | 20 (15%) | 0.37 |
| L3–L4 | 122 (50%) | 67 (50%) | 0.97 | 72 (49%) | 50 (50%) | 67 (50%) | 0.99 |
| L4–L5 | 240 (97%) | 134 (98%) | 0.79 | 144 (97%) | 96 (96%) | 134 (98%) | 0.70 |
| L5–S1 | 68 (28%) | 45 (33%) | 0.28 | 42 (29%) | 26 (26%) | 45 (33%) | 0.45 |
| Levels decompressed | 0.52 | 0.52 | |||||
| None | 3 (1%) | 1 (1%) | 1 (1%) | 2 (2%) | 1 (1%) | ||
| 1 | 105 (42%) | 54 (39%) | 59 (40%) | 46 (45%) | 54 (39%) | ||
| 2 | 92 (37%) | 46 (33%) | 60 (40%) | 32 (31%) | 46 (33%) | ||
| 3+ | 51 (20%) | 37 (27%) | 29 (19%) | 22 (22%) | 37 (27%) | ||
| Operation time, minutes (SD) | 201.7 (81.5) | 215.2 (86.8) | 0.13 | 199.2 (78.2) | 205.4 (86.6) | 215.2 (86.8) | 0.27 |
| Blood loss, cc (SD) | 550.2 (432.3) | 638.8 (525.8) | 0.075 | 516.9 (383.9) | 599.4 (493.2) | 638.8 (525.8) | 0.081 |
| Blood Replacement | |||||||
| Intraoperative replacement | 76 (31%) | 56 (41%) | 0.063 | 42 (28%) | 34 (34%) | 56 (41%) | 0.094 |
| Post-operative transfusion | 45 (18%) | 36 (26%) | 0.096 | 25 (17%) | 20 (20%) | 36 (26%) | 0.16 |
| Length of hospital stay, days (SD) | 4.8 (3.6) | 7.2 (31.3) | 0.25 | 4.9 (3.7) | 4.7 (3.5) | 7.2 (31.3) | 0.51 |
| Intraoperative complications§ | |||||||
| Dural tear/ spinal fluid leak | 22 (9%) | 19 (14%) | 0.17 | 12 (8%) | 10 (10%) | 19 (14%) | 0.28 |
| Vascular injury | 0 (0%) | 1 (1%) | 0.76 | 0 (0%) | 0 (0%) | 1 (1%) | 0.40 |
| Other | 6 (2%) | 3 (2%) | 0.83 | 3 (2%) | 3 (3%) | 3 (2%) | 0.88 |
| None | 223 (89%) | 117 (85%) | 0.32 | 134 (90%) | 89 (87%) | 117 (85%) | 0.42 |
| Postoperative complications/events¶ | |||||||
| Nerve root injury | 1 (0%) | 0 (0%) | 0.77 | 1 (1%) | 0 (0%) | 0 (0%) | 0.45 |
| Wound dehiscence | 0 (0%) | 1 (1%) | 0.77 | 0 (0%) | 0 (0%) | 1 (1%) | 0.41 |
| Wound hematoma | 0 (0%) | 1 (1%) | 0.77 | 0 (0%) | 0 (0%) | 1 (1%) | 0.41 |
| Wound infection | 4 (2%) | 7 (5%) | 0.10 | 1 (1%) | 3 (3%) | 7 (5%) | 0.083 |
| Other | 22 (9%) | 15 (11%) | 0.66 | 16 (11%) | 6 (6%) | 15 (11%) | 0.39 |
| None | 180 (73%) | 87 (63%) | 0.051 | 110 (74%) | 70 (71%) | 87 (63%) | 0.10 |
| Post-operative mortality (death within 6 weeks of surgery) |
1 (0.4%) | 0 (0%) | 0.76 | 0 (0%) | 1 (1%) | 0 (0%) | 0.25 |
| Post-operative mortality (death within 3 months of surgery) |
2 (0.8%) | 0 (0%) | 0.76 | 1 (0.7%) | 1 (1%) | 0 (0%) | 0.55 |
| Additional surgeries (1-year rate)|| | 17 (7%) | 8 (6%) | 0.73 | 11 (7%) | 6 (6%) | 8 (6%) | 0.83 |
| Additional surgeries (2-year rate)|| | 29 (11%) | 19 (14%) | 0.51 | 19 (13%) | 10 (10%) | 19 (14%) | 0.616 |
| Additional surgeries (3-year rate)|| | 33 (13%) | 21 (15%) | 0.55 | 21 (14%) | 12 (12%) | 21 (15%) | 0.716 |
| Additional surgeries (4-year rate)|| | 35 (14%) | 23 (17%) | 0.46 | 21 (14%) | 14 (14%) | 23 (17%) | 0.754 |
| Recurrent stenosis / progressive listhesis |
11 (5%) | 8 (6%) | 6 (4%) | 5 (5%) | 8 (6%) | ||
| Pseudarthrosis / fusion exploration | 2 (0.8%) | 2 (1.5%) | 0 | 2 (2.1%) | 2 (1.5%) | ||
| Complication or Other | 16 (6.5%) | 9 (6.6%) | 10 (6.8%) | 6 (6.1%) | 9 (6.6%) | ||
| New condition | 5 (2.1%) | 4 (2.9%) | 4 (2.7%) | 1 | 4 (2.9%) |
Surgical information was available for 149 patients with duration of symptoms 6 months or less, 102 patients with duration of symptoms 7 to 12 months and 138 patients with duration of symptoms one year or more.
Specific procedure data were available for 148 patients with duration of symptoms 6 months or less, 98 patients with duration of symptoms 7 to 12 months and 136 patients with duration of smyptoms more than one year.
No cases were reported of aspiration into the respiratory tract or operation at wrong level.
Complications or events occurring up to 8 weeks after surgery are listed. There were no reported cases of bone-graft complication, cerebrospinal fluid leak, paralysis, cauda equina injury or pseudarthrosis.
Rates of repeated surgery at 1, 2,3,and 4 years are Kaplan-Meier estimates. P values were calculated with the use of the log-rank test. Numbers and percentages are based on the first additional surgery if more than one additional surgery.
Table 7.
Subgroup results from adjusted* as-treated outcome analysis by two groups duration of symptoms (One year or less vs. More than one year) for the randomized and observational cohorts combined patients with lumbar degenerative spondylolisthesis.
| Outcome |
Duration of Symptoms |
1-Year |
2-Year |
3-Year |
4-Year |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DS | Surgical | Non- operative |
Treatment Effect† (95% CI) |
Surgical | Non- operative |
Treatment Effect† (95% CI) |
Surgical | Non- operative |
Treatment Effect† (95% CI) |
Surgical | Non- perative |
Treatment Effect† (95% CI) |
|
|
Primary Outcomes |
|||||||||||||
| SF-36 Bodily Pain (BP) (0–100) (SE) |
One year or less |
34.8 (1.8) | 14 (1.7) | 20.8 (16, 25.5) |
32.4 (1.8) | 14 (1.9) | 18.4 (13.4, 23.5) |
34.3 (1.8) | 16.1 (2) | 18.2 (12.9, 23.5) |
32.2 (1.9) | 16.2 (2.2) | 16 (10.3, 21.7) |
| More than 1 year |
30.3 (1.7) | 13.3 (2.5) | 17 (11.3, 22.6) |
31.2 (1.6) | 11.3 (2.6) | 19.9 (14.1, 25.7) |
30.7 (1.6) | 13.3 (2.9) | 17.4 (11.1, 23.7) |
29.9 (1.8) | 15 (3) | 14.9 (8.3, 21.5) |
|
| pvalue | 0.065 | 0.81 | 0.31 | 0.59 | 0.40 | 0.71 | 0.12 | 0.42 | 0.84 | 0.35 | 0.73 | 0.80 | |
| SF-36 Physical Function (PF) (0–100) (SE) |
One year or less |
29.3 (1.7) | 11.6 (1.6) | 17.7 (13.2, 22.3) |
25.7 (1.7) | 10 (1.8) | 15.7 (10.9, 20.5) |
24.9 (1.8) | 9.9 (1.9) | 15.1 (10, 20.1) |
26.6 (1.9) | 8.9 (2.1) | 17.6 (12.2, 23.1) |
| More than 1 year |
27.2 (1.7) | 9 (2.4) | 18.1 (12.8, 23.5) |
25.9 (1.6) | 8.6 (2.5) | 17.3 (11.8, 22.8) |
25 (1.6) | 7.3 (2.8) | 17.7 (11.7, 23.7) |
25.8 (1.7) | 6.9 (2.9) | 18.8 (12.5, 25.1) |
|
| pvalue | 0.37 | 0.36 | 0.90 | 0.93 | 0.64 | 0.66 | 0.97 | 0.45 | 0.51 | 0.74 | 0.57 | 0.78 | |
| Mental Component Summary (MCS) (0–100) (SE) |
One year or Less |
3.4 (0.7) | 1.7 (0.7) | 1.8 (−0.2, 3.8) |
2.8 (0.7) | 1.3 (0.8) | 1.5 (−0.6, 3.6) |
2.1 (0.8) | 0.3 (0.8) | 1.7 (−0.5, 4) |
2.5 (0.8) | 1 (1) | 1.5 (−1, 4) |
| More than 1 year |
2.5 (0.7) | 1 (1) | 1.5 (−0.9, 3.9) |
2.4 (0.7) | 0 (1.1) | 2.4 (−0.1, 4.9) |
2.9 (0.7) | −0.2 (1.2) | 3.1 (0.4, 5.8) |
2.1 (0.8) | −1.6 (1.3) | 3.7 (0.7, 6.6) |
|
| pvalue | 0.36 | 0.61 | 0.85 | 0.73 | 0.35 | 0.57 | 0.39 | 0.73 | 0.44 | 0.76 | 0.12 | 0.26 | |
| Oswestry Disability Index (ODI) (0–100) (SE) |
One year or less |
−26.8 (1.3) | −8.1 (1.3) | −18.7 (−22.3, −15.2) |
−24.5 (1.3) | −8.6 (1.4) | −16 (−19.7, −12.2) |
−23.1 (1.4) | −10.5 (1.5) | −12.6 (−16.5, −8.7) |
−23.6 (1.4) | −9.8 (1.6) | −13.8 (−18, −9.6) |
| More than 1 year |
−23.3 (1.3) | −6.6 (1.9) | −16.7 (−20.9, −12.6) |
−23.5 (1.2) | −6 (2) | −17.5 (−21.8, −13.1) |
−20.9 (1.2) | −5.6 (2.2) | −15.3 (−20, −10.6) |
−22.1 (1.3) | −6 (2.2) | −16.2 (−21, −11.3) |
|
| pvalue | 0.055 | 0.48 | 0.48 | 0.54 | 0.29 | 0.60 | 0.20 | 0.063 | 0.39 | 0.44 | 0.16 | 0.46 | |
|
Secondary Outcomes |
|||||||||||||
| Stenosis Bothersomeness Index (0–24) (SE) |
One year or less |
−9.5 (0.5) | −3.9 (0.5) | −5.6 (−7, −4.2) | −9 (0.5) | −4.2 (0.5) | −4.8 (−6.2, −3.3) |
−9.4 (0.5) | −4.6 (0.6) | −4.9 (−6.4, −3.3) |
−9.1 (0.5) | −4.2 (0.6) | −4.8 (−6.5, −3.2) |
| More than 1 year |
−9.5 (0.5) | −4 (0.7) | −5.5 (−7, −4) | −9.1 (0.4) | −3.3 (0.7) | −5.8 (−7.4, −4.2) |
−8.8 (0.5) | −3.7 (0.8) | −5.1 (−6.8, −3.3) |
−9.1 (0.5) | −2.7 (0.8) | −6.4 (−8.2, −4.6) |
|
| pvalue | 0.98 | 0.90 | 0.91 | 0.89 | 0.30 | 0.36 | 0.35 | 0.40 | 0.87 | 0.90 | 0.15 | 0.20 | |
| Low Back Pain Bothersomeness (0–6) (SE) |
One year or less |
−2.3 (0.1) | −1.1 (0.1) | −1.2 (−1.6, −0.8) |
−2.1 (0.1) | −1.4 (0.1) | −0.8 (−1.2, −0.4) |
−2.2 (0.1) | −1.5 (0.2) | −0.7 (−1.1, −0.3) |
−2.1 (0.2) | −1.2 (0.2) | −0.9 (−1.4, −0.4) |
| More than 1 year |
−2.4 (0.1) | −1.1 (0.2) | −1.3 (−1.7, −0.9) |
−2.1 (0.1) | −0.9 (0.2) | −1.3 (−1.8, −0.8) |
−2 (0.1) | −1.1 (0.2) | −0.9 (−1.4, −0.4) |
−2 (0.1) | −1 (0.2) | −1.1 (−1.6, −0.6) |
|
| pvalue | 0.80 | 0.93 | 0.91 | 0.93 | 0.032 | 0.11 | 0.28 | 0.16 | 0.64 | 0.66 | 0.39 | 0.67 | |
| Leg pain (0–6) (SE) |
One year or less |
−3 (0.2) | −1.5 (0.1) | −1.5 (−1.9, −1.1) |
−2.8 (0.2) | −1.6 (0.2) | −1.3 (−1.8, −0.8) |
−3 (0.2) | −1.7 (0.2) | −1.3 (−1.8, −0.8) |
−3.1 (0.2) | −1.7 (0.2) | −1.4 (−1.9, −0.9) |
| More than one year |
−3.1 (0.1) | −1.4 (0.2) | −1.6 (−2.1, −1.1) |
−3 (0.1) | −1.2 (0.2) | −1.8 (−2.3, −1.3) |
−2.9 (0.1) | −1.5 (0.2) | −1.4 (−2, −0.8) | −3 (0.2) | −1.2 (0.3) | −1.8 (−2.4, −1.2) |
|
| pvalue | 0.86 | 0.77 | 0.72 | 0.48 | 0.087 | 0.074 | 0.61 | 0.55 | 0.83 | 0.63 | 0.15 | 0.34 | |
| Very/somewhat satisfied with symptoms (%) |
One year or less |
76.4 | 31.8 | 44.6 (34.6, 54.7) |
73.3 | 35.9 | 37.5 (26.3, 48.6) |
69.5 | 39.5 | 30 (17.7, 42.3) |
66.6 | 29.7 | 36.9 (24.2, 49.6) |
| More than 1 year |
69.3 | 16.5 | 52.8 (42, 63.7) |
65.7 | 24.5 | 41.2 (28.7, 53.7) |
63 | 28.5 | 34.5 (20.4, 48.6) |
60.3 | 28.4 | 32 (16.6, 47.3) |
|
| pvalue | 0.19 | 0.024 | 0.29 | 0.15 | 0.15 | 0.69 | 0.25 | 0.21 | 0.67 | 0.31 | 0.89 | 0.70 | |
| Self-rated pogress major mprovement (%) |
One year or less |
78.2 | 28.2 | 50 (40.2, 59.8) |
72 | 26.8 | 45.2 (34.4, 56) |
73.9 | 28.4 | 45.5 (34.1, 56.8) |
68.3 | 27.9 | 40.5 (27.8, 53.1) |
| More than 1 year |
72.6 | 19.8 | 52.8 (41.4, 64.2) |
73.8 | 17.8 | 56.1 (44.8, 67.3) |
67.5 | 18.5 | 49.1 (36.5, 61.7) |
63.7 | 9.1 | 54.7 (43.4, 66) |
|
| pvalue | 0.29 | 0.23 | 0.73 | 0.72 | 0.20 | 0.20 | 0.25 | 0.25 | 0.64 | 0.45 | 0.052 | 0.12 | |
Adjusted for age, gender, BMI, race, smoking status, compensation, joint, stomach, bowel, osteoporosis, number of moderate/severe stenotic levels, self-assessed health trend at baseline, treatment preference, baseline stenosis bothersomeness, other ** comorbiditiy, baseline score and center.
Other comorbidities include: stroke, cancer, fibromyalgia, cfs, PTSD, alcohol, drug dependency, lung, liver, kidney, blood vessel, nervous system, migraine, anxiety
Treatment effect is the difference between the surgical and non-operative mean change from baseline. Analysis is done using a mixed model with a random subject intercept term. Treatment is a time-varying covariate where a patients' experience prior to surgery is attributed to the non-operative arm and time is measured from enrollment and his/her post-surgery outcomes are attributed to the surgical arm and time is measured from time of surgery.
Table 8.
Subgroup results from adjusted* as-treated outcome analysis by three groups duration of symptoms for the randomized and observational cohorts combined patients with lumbar degenerative spondylolisthesis.
| Outcome |
Duration of Symptoms |
1-Year |
2-Year |
3-Year |
4-Year |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DS | Surgical | Non- operative |
Treatment Effect† (95% CI) |
Surgical | Non- operative |
Treatment Effect† (95% CI) |
Surgical | Non- operative |
Treatment Effect† (95% CI) |
Surgical | Non- operative |
Treatment Effect† (95% CI) |
|
|
Primary Outcomes |
|||||||||||||
| SF-36 Bodily Pain (BP) (0–100) (SE) |
6 months or less |
36.5 (3.3) | 17.1 (2.1) | 19.4 (11.6, 27.2) |
30.7 (3.3) | 16 (2.4) | 14.7 (6.6, 22.8) |
31.4 (3.6) | 18 (2.6) | 13.5 (4.8, 22.1) |
28.4 (3.6) | 16.8 (2.8) | 11.6 (2.6, 20.5) |
| 7 to 12 months |
34.1 (2.1) | 9 (2.7) | 25.2 (18.5, 31.8) |
33.2 (2.1) | 10.7 (2.9) | 22.5 (15.5, 29.5) |
35.5 (2.1) | 13.2 (3.2) | 22.4 (14.9, 29.9) |
34.1 (2.3) | 15.3 (3.5) | 18.8 (10.6, 27) |
|
| More than 1 year |
30.3 (1.7) | 13.3 (2.5) | 17 (11.4, 22.7) |
31.2 (1.6) | 11.2 (2.6) | 19.9 (14.1, 25.7) |
30.7 (1.6) | 13.2 (2.9) | 17.4 (11.1, 23.8) |
29.9 (1.8) | 14.9 (3) | 15 (8.3, 21.6) |
|
| pvalue | 0.16 | 0.052 | 0.18 | 0.66 | 0.26 | 0.34 | 0.16 | 0.36 | 0.29 | 0.22 | 0.88 | 0.49 | |
| SF-36 Physical Function PF) (0–100) (SE) |
6 months or less |
32 (3.3) | 13.9 (2) | 18.1 (10.5, 25.7) |
27.1 (3.3) | 10.1 (2.3) | 17 (9.1, 24.9) |
30.4 (3.4) | 11.3 (2.5) | 19 (10.7, 27.4) |
27.4 (3.5) | 6.4 (2.7) | 21 (12.3, 29.6) |
| 7 to 12 months |
28.8 (2) | 7.3 (2.6) | 21.5 (15.1, 27.8) |
25.8 (2) | 9.4 (2.8) | 16.4 (9.7, 23.1) |
23.5 (2) | 7.1 (3.1) | 16.4 (9.2, 23.6) |
27 (2.2) | 12.7 (3.4) | 14.3 (6.4, 22.2) |
|
| More than 1 year |
27 (1.7) | 8.8 (2.4) | 18.2 (12.8, 23.6) |
25.7 (1.6) | 8.4 (2.5) | 17.2 (11.7, 22.8) |
24.7 (1.6) | 7.2 (2.8) | 17.6 (11.5, 23.6) |
25.5 (1.7) | 6.8 (2.9) | 18.8 (12.5, 25.1) |
|
| pvalue | 0.38 | 0.083 | 0.70 | 0.93 | 0.89 | 0.98 | 0.22 | 0.42 | 0.89 | 0.81 | 0.29 | 0.50 | |
| Mental Component Summary (MCS) (0–100) (SE) |
6 months or less |
3.6 (1.3) | 2.3 (0.9) | 1.3 (−1.9, 4.5) |
3.5 (1.4) | 1.4 (1) | 2.1 (−1.2, 5.5) |
2.1 (1.5) | 1.8 (1.1) | 0.3 (−3.3, 3.9) |
2.4 (1.5) | 2.3 (1.2) | 0.1 (−3.7, 3.8) |
| 7 to 12 months |
3.5 (0.9) | 0.6 (1.1) | 2.8 (0.1, 5.6) |
2.5 (0.9) | 1.1 (1.2) | 1.5 (−1.5, 4.4) |
2.1 (0.9) | −2 (1.4) | 4.2 (1, 7.4) | 2.6 (1) | −1.2 (1.5) | 3.8 (0.3, 7.3) |
|
| More than 1 year |
2.5 (0.7) | 1 (1) | 1.5 (−0.9, 3.9) |
2.4 (0.7) | 0 (1.1) | 2.4 (−0.1, 4.8) |
2.9 (0.7) | −0.2 (1.2) | 3.1 (0.4, 5.7) |
2.1 (0.8) | −1.6 (1.3) | 3.7 (0.7, 6.6) |
|
| pvalue | 0.61 | 0.43 | 0.69 | 0.75 | 0.65 | 0.90 | 0.74 | 0.074 | 0.25 | 0.91 | 0.054 | 0.25 | |
| Oswestry Disability Index (ODI) (0–100) (SE) |
6 months or less |
−25.6 (2.6) | −10.1 (1.6) | −15.5 (−21.4, −9.5) |
−21.2 (2.6) | −10.9 (1.8) | −10.3 (−16.4, −4.1) |
−21.6 (2.7) | −12.9 (1.9) | −8.7 (−15.2, −2.2) |
−20.2 (2.7) | −10.7 (2) | −9.6 (−16.3, −2.9) |
| 7 to 12 months |
−27.5 (1.6) | −5.3 (2) | −22.2 (−27.1, −17.3) |
−26.1 (1.5) | −5.2 (2.2) | −20.8 (−26.1, −15.6) |
−23.9 (1.6) | −7 (2.4) | −16.9 (−22.5, −11.4) |
−25.2 (1.7) | −8.8 (2.6) | −16.4 (−22.5, −10.3) |
|
| More than 1 year |
−23.2 (1.3) | −6.5 (1.9) | −16.7 (−20.9, −12.6) |
−23.4 (1.2) | −6 (2) | −17.4 (−21.7, −13.1) |
−20.7 (1.2) | −5.5 (2.2) | −15.2 (−19.9, −10.5) |
−22 (1.3) | −5.9 (2.2) | −16.1 (−21, −11.2) |
|
| pvalue | 0.11 | 0.11 | 0.14 | 0.17 | 0.063 | 0.031 | 0.24 | 0.022 | 0.14 | 0.17 | 0.29 | 0.23 | |
|
Secondary Outcomes |
|||||||||||||
| Stenosis Bothersomeness Index (0–24) (SE) |
6 months or less |
−10.3 (0.9) | −4.1 (0.6) | −6.2 (−8.4, −4) |
−9 (0.9) | −4.5 (0.7) | −4.5 (−6.8, −2.3) |
−9.3 (1) | −5.3 (0.7) | −4 (−6.4, −1.6) |
−9.1 (1) | −4.4 (0.8) | −4.7 (−7.2, −2.2) |
| 7 to 12 months |
−9.1 (0.6) | −3.4 (0.7) | −5.7 (−7.6, −3.8) |
−9 (0.6) | −3.8 (0.8) | −5.2 (−7.2, −3.2) |
−9.5 (0.6) | −3.4 (0.9) | −6.1 (−8.2, −4) |
−9 (0.6) | −3.9 (1) | −5.2 (−7.5, −2.9) |
|
| More than 1 year |
−9.5 (0.5) | −4 (0.7) | −5.5 (−7, −4) | −9.1 (0.4) | −3.3 (0.7) | −5.8 (−7.4, −4.2) |
−8.8 (0.5) | −3.7 (0.8) | −5.1 (−6.8, −3.4) |
−9.2 (0.5) | −2.7 (0.8) | −6.4 (−8.3, −4.6) |
|
| pvalue | 0.52 | 0.71 | 0.88 | 0.99 | 0.49 | 0.65 | 0.66 | 0.18 | 0.42 | 0.99 | 0.32 | 0.49 | |
| Low Back Pain Bothersomeness (0–6) (SE) |
6 months or less |
−2.4 (0.2) | −1.1 (0.2) | −1.3 (−1.9, −0.7) |
−2.2 (0.3) | −1.5 (0.2) | −0.6 (−1.2, 0) |
−2 (0.3) | −1.6 (0.2) | −0.5 (−1.2, 0.2) |
−2.1 (0.3) | −1.2 (0.2) | −0.9 (−1.6, −0.2) |
| 7 to 12 months |
−2.3 (0.2) | −1.1 (0.2) | −1.2 (−1.7, −0.7) |
−2.1 (0.2) | −1.1 (0.2) | −1 (−1.6, −0.4) |
−2.2 (0.2) | −1.3 (0.3) | −0.9 (−1.5, −0.3) |
−2.2 (0.2) | −1.3 (0.3) | −0.9 (−1.6, −0.2) |
|
| More than 1 year |
−2.4 (0.1) | −1.1 (0.2) | −1.3 (−1.7, −0.9) |
−2.1 (0.1) | −0.9 (0.2) | −1.3 (−1.8, −0.8) |
−2 (0.1) | −1.1 (0.2) | −0.9 (−1.4, −0.4) |
−2.1 (0.1) | −1 (0.2) | −1.1 (−1.6, −0.6) |
|
| pvalue | 0.86 | 0.99 | 0.91 | 1 | 0.043 | 0.25 | 0.48 | 0.25 | 0.53 | 0.86 | 0.68 | 0.87 | |
| Leg pain (0–6) (SE) |
6 months or less |
−3.2 (0.3) | −1.8 (0.2) | −1.4 (−2.1, −0.7) |
−2.8 (0.3) | −1.9 (0.2) | −0.9 (−1.6, −0.2) |
−3 (0.3) | −2 (0.2) | −1 (−1.7, −0.3) |
−3.3 (0.3) | −1.8 (0.2) | −1.5 (−2.3, −0.7) |
| 7 to 12 months |
−3 (0.2) | −1 (0.2) | −1.9 (−2.5, −1.3) |
−2.9 (0.2) | −1.1 (0.3) | −1.8 (−2.4, −1.2) |
−3 (0.2) | −1.2 (0.3) | −1.8 (−2.5, −1.1) |
−3 (0.2) | −1.5 (0.3) | −1.5 (−2.2, −0.8) |
|
| More than 1 year |
−3.1 (0.1) | −1.4 (0.2) | −1.6 (−2.1, −1.1) |
−3 (0.1) | −1.1 (0.2) | −1.8 (−2.3, −1.3) |
−2.9 (0.1) | −1.5 (0.2) | −1.4 (−2, −0.8) |
−3 (0.2) | −1.2 (0.3) | −1.8 (−2.4, −1.2) |
|
| pvalue | 0.74 | 0.096 | 0.55 | 0.74 | 0.018 | 0.056 | 0.86 | 0.099 | 0.26 | 0.46 | 0.23 | 0.79 | |
| Very/somewhat satisfied with symptoms (%) |
6 months or less |
81.2 | 35 | 46.3 (31.3, 61.2) |
76 | 41.8 | 34.3 (17.1, 51.4) |
78.7 | 45.2 | 33.5 (15.5, 51.5) |
69.3 | 27.3 | 42 (23.1, 60.9) |
| 7 to 12 months |
74.6 | 26.4 | 48.2 (34.7, 61.8) |
72.5 | 27 | 45.4 (30.9, 60) |
66.2 | 30.6 | 35.6 (19.2, 52) |
65.8 | 32.6 | 33.2 (14.9, 51.5) |
|
| More than 1 year |
69.4 | 16.5 | 52.9 (42.1, 63.7) |
65.7 | 24.5 | 41.2 (28.7, 53.7) |
62.9 | 28.4 | 34.5 (20.4, 48.6) |
60.4 | 28.4 | 32 (16.7, 47.3) |
|
| pvalue | 0.31 | 0.038 | 0.78 | 0.31 | 0.083 | 0.72 | 0.18 | 0.12 | 1 | 0.53 | 0.88 | 0.75 | |
| Self-rated progress major improvement (%) |
6 months or less |
72.4 | 32 | 40.4 (23.6, 57.2) |
73.2 | 28.9 | 44.3 (27.1, 61.4) |
70.9 | 37.7 | 33.2 (13.9, 52.4) |
60.1 | 30.6 | 29.6 (8.9, 50.2) |
| 7 to 12 months |
80.5 | 22.7 | 57.8 (45.2, 70.3) |
71.7 | 23.5 | 48.3 (34, 62.5) |
75.2 | 15.9 | 59.3 (46.1, 72.5) |
71.8 | 23.6 | 48.2 (31.6, 64.8) |
|
| More than 1 year |
72.4 | 19.6 | 52.8 (41.5, 64.1) |
73.5 | 17.6 | 55.9 (44.7, 67.1) |
67.3 | 18.2 | 49.1 (36.6, 61.7) |
63.6 | 8.9 | 54.7 (43.4, 65.9) |
|
| pvalue | 0.32 | 0.22 | 0.25 | 0.95 | 0.36 | 0.50 | 0.41 | 0.029 | 0.10 | 0.32 | 0.12 | 0.12 | |
Adjusted for age, gender, BMI, race, smoking status, compensation, joint, stomach, bowel, osteoporosis, number of moderate/severe stenotic levels, self-assessed health trend at baseline, treatment preference, baseline stenosis bothersomeness, other *
Other comorbidities include: stroke, cancer, fibromyalgia, cfs, PTSD, alcohol, drug dependency, lung, liver, kidney, blood vessel, nervous system, migraine, anxiety
Treatment effect is the difference between the surgical and non-operative mean change from baseline. Analysis is done using a mixed model with a random subject intercept term. Treatment is a time-varying covariate where a patients' experience prior to surgery is attributed to the non-operative arm and time is measured from enrollment and his/her post-surgery outcomes are attributed to the surgical arm and time is measured from time of surgery.
Acknowledgments
NIH funds were received in support of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The manuscript submitted does not contain information about medical device(s)/drug(s). No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Amundsen T, Weber H, Nordal HJ, et al. Lumbar spinal stenosis: conservative or surgical management? A prospective 10-year study. Spine. 2000;25:1424–1435. doi: 10.1097/00007632-200006010-00016. discussion 35–6. [DOI] [PubMed] [Google Scholar]
- 2.Chao T, Pham K, Steward O, Gupta R. Chronic nerve compression injury induces a phenotypic switch of neurons within the dorsal root ganglia. J Comp Neurol. 2008 Jan 10;506(2):180–193. doi: 10.1002/cne.21537. [DOI] [PubMed] [Google Scholar]
- 3.Gupta R, Rowshan K, Chao T, Mozaffar T, Steward O. Chronic nerve compression induces local demyelination and remyelination in a rat model of carpal tunnel syndrome. Exp Neurol. 2004 Jun;187(2):500–508. doi: 10.1016/j.expneurol.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Herno A, Airaksinen O, Saari T, Luukkonen M. Lumbar spinal stenosis: a matched-pair study of operated and non-operated patients. Br J Neurosurg. 1996 Oct;10(5):461–465. doi: 10.1080/02688699647087. [DOI] [PubMed] [Google Scholar]
- 5.Herno A, Partanen K, Talaslahti T, Kaukanen E, Turunen V, Suomalainen O, Airaksinen O. Long-term clinical and magnetic resonance imaging follow-up assessment of patients with lumbar spinal stenosis after laminectomy. Spine (Phila Pa 1976) 1999 Aug 1;24(15):1533–1537. doi: 10.1097/00007632-199908010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Jönsson B, Annertz M, Sjöberg C, Strömqvist B. A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part I: Clinical features related to radiographic findings. Spine (Phila Pa 1976) 1997 Dec 15;22(24):2932–2937. doi: 10.1097/00007632-199712150-00016. [DOI] [PubMed] [Google Scholar]
- 7.Jönsson B, Annertz M, Sjöberg C, Strömqvist B. A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part II: Five-year follow-up by an independent observer. Spine (Phila Pa 1976) 1997 Dec 15;22(24):2938–2944. doi: 10.1097/00007632-199712150-00017. [DOI] [PubMed] [Google Scholar]
- 8.Jönsson B, Stromqvist B. Motor affliction of the L5 nerve root in lumbar nerve root compression syndromes. Spine. 1995;20:2012–2015. doi: 10.1097/00007632-199509150-00012. [DOI] [PubMed] [Google Scholar]
- 9.Jönsson B, Stromqvist B. Repeat decompression of lumbar nerve roots. A prospective two-year evaluation. J Bone Joint Surg Br. 1993a;75:894–897. doi: 10.1302/0301-620X.75B6.8245078. [DOI] [PubMed] [Google Scholar]
- 10.Jönsson B. Patient-related factors predicting the outcome of decompressive surgery. Acta Orthop Scand Suppl. 1993;251:69–70. doi: 10.3109/17453679309160123. [DOI] [PubMed] [Google Scholar]
- 11.Katz JN, Lipson SJ, Larson MG, McInnes JM, Fossel AH, Liang MH. The outcome of decompressive laminectomy for degenerative lumbar stenosis. J Bone Joint Surg Am. 1991 Jul;73(6):809–816. [PubMed] [Google Scholar]
- 12.Katz JN, Stucki G, Lipson SJ, Fossel AH, Grobler LJ, Weinstein JN. Predictors of surgical outcome in degenerative lumbar spinal stenosis. Spine (Phila Pa 1976) 1999 Nov 1;24(21):2229–2233. doi: 10.1097/00007632-199911010-00010. [DOI] [PubMed] [Google Scholar]
- 13.Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976) 2004 Apr 1;29(7):726–733. doi: 10.1097/01.brs.0000119398.22620.92. discussion 733–4. [DOI] [PubMed] [Google Scholar]
- 14.McGregor AH, Hughes SP. The evaluation of the surgical management of nerve root compression in patients with low back pain: Part 1: the assessment of outcome. Spine. 2002a;27:1465–1470. doi: 10.1097/00007632-200207010-00018. [DOI] [PubMed] [Google Scholar]
- 15.McGregor AH, Hughes SP. The evaluation of the surgical management of nerve root compression in patients with low back pain: Part 2: patient expectations and satisfaction. Spine. 2002b;27:1471–1476. doi: 10.1097/00007632-200207010-00019. [DOI] [PubMed] [Google Scholar]
- 16.Spratt KF, Keller TS, Szpalski M, et al. A predictive model for outcome after conservative decompression surgery for lumbar spinal stenosis. Eur Spine J. 2004;13:14–21. doi: 10.1007/s00586-003-0583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis. Attempted meta-analysis of the literature. Spine (Phila Pa 1976) 1992 Jan;17(1):1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, Blood EA, Birkmeyer NJ, Hilibrand AS, Herkowitz H, Cammisa FP, Albert TJ, Emery SE, Lenke LG, Abdu WA, Longley M, Errico TJ, Hu SS. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007 May 31;356(22):2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein JN, Tosteson TD, Lurie JD, Tosteson A, Blood E, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H. Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine (Phila Pa 1976) 2010 Jun 15;35(14):1329–1338. doi: 10.1097/BRS.0b013e3181e0f04d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamashita K, Hayashi J, Ohzono K, Hiroshima K. Correlation of patient satisfaction with symptom severity and walking ability after surgical treatment for degenerative lumbar spinal stenosis. Spine (Phila Pa 1976) 2003 Nov 1;28(21):2477–2481. doi: 10.1097/01.BRS.0000090888.63860.4F. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita K, Ohzono K, Hiroshima K. Five-year outcomes of surgical treatment for degenerative lumbar spinal stenosis: a prospective observational study of symptom severity at standard intervals after surgery. Spine (Phila Pa 1976) 2006 Jun 1;31(13):1484–1490. doi: 10.1097/01.brs.0000219940.26390.26. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita K, Ohzono K, Hiroshima K. Patient satisfaction as an outcome measure after surgical treatment for lumbar spinal stenosis: testing the validity and discriminative ability in terms of symptoms and functional status. Spine (Phila Pa 1976) 2006 Oct 15;31(22):2602–2608. doi: 10.1097/01.brs.0000240717.25787.7d. [DOI] [PubMed] [Google Scholar]
- 23.Yaşar B, Simşek S, Er U, Yiğitkanli K, Ekşioğlu E, Altuğ T, Belen D, Kars ZH, Bavbek M. Functional and clinical evaluation for the surgical treatment of degenerative stenosis of the lumbar spinal canal. J Neurosurg Spine. 2009 Sep;11(3):347–352. doi: 10.3171/2009.3.SPINE08692. [DOI] [PubMed] [Google Scholar]
- 24.Parke W, Gammell K, Rothman R. Arterial vascularization of the cauda equina. The Journal of Bone and Joint Surgery. 1981;63(1):53. [PubMed] [Google Scholar]
- 25.Olmarker K, et al. Effects of experimental graded compression on blood flow in spinal nerve roots. A vital microscopic study on the porcine cauda equina. Journal of Orthopaedic Research. 1989;7(6):817–823. doi: 10.1002/jor.1100070607. [DOI] [PubMed] [Google Scholar]