Abstract
Our goal in this study was to identify objective criteria that could be used to predict an outcome of death in mice subjected to experimental inoculation with infectious organisms. We conducted a retrospective analysis of data collected from 4 independent studies that used several infectious agents (influenza virus strains A/HK/x31[H3N2] and A/Puerto Rico/8/34[H1N1], Streptococcus pneumoniae, and Candida albicans) and mouse strains (A/J, DBA/2J, C57BL/6J, BALB/cByJ). Postinoculation periods ranged from 5 to 21 d, with survival of 30% to 60% of the subjects. In all studies, mice were implanted with either a subcutaneous identification microchip or an intraabdominal radiofrequency transmitter to allow remote measurement of body temperature. After inoculation, mice were weighed and monitored regularly until death occurred or euthanasia was performed. Hypothermia was the most valuable characteristic for distinguishing mice that would survive or succumb to the infection. In addition, weight loss was useful in some of the models. In some cases, the derived measure of the product of temperature and body weight provided the best differentiation of mice in the 2 outcome categories. Therefore, the utility of these measures varied substantially depending on the specific model. This study demonstrates that specific endpoint markers are not uniformly applicable to different models. Rather, such markers should be developed and tested in the context of the model in which they will be used. The use of validated markers for eventual death can signal the need for preemptive euthanasia to alleviate terminal distress and permit timely collection of biologic samples.
Abbreviations: BW, body weight; PR8, influenza virus strain A/Puerto Rico/8/34 (H1N1); T, body temperature (°C); X31, influenza virus strain A/HK/X31 (H3N2)
Laboratory mice are the preferred species for many types of infectious disease research. However, infectious disease studies often involve mice becoming ill and perhaps dying as a result of the infectious challenges to which they are exposed. This likelihood has prompted interest in the identification of humane experimental endpoints that maintain the scientific integrity of the experiment or test.5,6 The development of accurate objective markers that could be used to predict eventual death and trigger preemptive euthanasia would therefore be useful in terms of both mitigating animal distress and permitting the performance of terminal procedures and antemortem sample collection.
Experimental endpoints in biomedical research should be determined based on a combination of scientific, ethical, legal, practical, and humane considerations. Our goal in this study was to determine whether criteria that have been proposed for prediction of imminent death in longevity studies11 can be applied to relatively acute studies of infectious disease. Data from studies performed for other reasons were collated and analyzed retrospectively to determine whether the measures of body weight (BW) and temperature could be used to differentiate mice that would live from those that would die in a variety of infectious disease models.
Materials and Methods
All mice were purchased from Jackson Laboratory and were housed in same-sex groups of 5 in 11 in. × 7 in. × 5 in. cages. Cages were solid-bottom shoebox-style open-top cages with woodchip bedding (Beta Chip, Northeastern Products, Warrensburg, NY). Mice were maintained by using conventional husbandry practices, with cages changed weekly. Room temperature was maintained at 70 ± 2 °F (21.1 ± 1.2 °C) and relative humidity at 40% to 60%. Most mice were fed LabDiet 5001 (PMI Nutrition International, St Louis, MO), although one group received a high-fat diet (D12492, 60 kcal% fat, Research Diets, New Brunswick, NJ). Food and tap water were available ad libitum. All mice were free of known infections with mouse hepatitis virus, minute virus of mice, mouse parvovirus, mouse norovirus, Theiler murine encephalomyelitis virus, epizootic diarrhea of infant mice virus, Sendai virus, Mycoplasma pulmonis, pneumonia virus of mice, reovirus 3, lymphocytic choriomeningitis virus, ectromelia virus, and endo- and ectoparasites, as monitored by using monthly testing of sentinel mice housed in the same room. The Laboratory Animal Care and Use Committee at Southern Illinois University School of Medicine approved all animals and experimental procedures used in this study.
Temperature measurement.
For most studies, mice were implanted under isoflurane anesthesia with a subcutaneous microchip that allows remote measurement of body temperature by using a wand-type reader (model IPTT300, BioMedic Data Systems, Seaford, DE). The microchip was implanted by using a delivery device involving 12-gauge needle, without an incision or wound closure. Individual chips were not tested for accuracy or otherwise calibrated prior to use but were used according to the manufacturer's recommendations. Mice inoculated with Candida albicans were implanted under ketamine–xylazine anesthesia with intraperitoneal transmitters to allow continuous measurement of body temperature via a receiver placed under the cage.15
The study was a retrospective assessment of mice exposed to various infectious challenges for use in other studies. Mice were evaluated at least daily both before and after inoculation. Temperatures and body weights were measured during the 2-h period after light onset. For mice with abdominal transmitters, the temperature values that were analyzed were the averages values obtained during the 2 h after light onset. Clinical endpoints for preemptive euthanasia included palpable hypothermia (in our experience of mice that were implanted with telemetric temperature transmitters during various infectious conditions, palpable hypothermia generally reflects a body temperature of less than 25 °C), inability or unwillingness to walk, lack of response to manipulation, and severe dyspnea or cyanosis. Mice that developed any of these signs were euthanized immediately and were considered to have died.
Infectious challenges.
Influenza strain A/HK/x31(H3N2).
Viral stocks of influenza strain A/HK/X31(H3N2) (X31) were prepared by inoculation of embryonated chicken eggs. Virus-infected allantoic fluid was harvested and aliquots frozen at −80 °C until use. The titer of the viral stock was determined by using a TCID50 assay. To create influenza infection, adult male A/J mice (n = 11) were anesthetized lightly and inoculated intranasally with 25 µL allantoic fluid containing between 5 × 101 and 5 × 105 TCID50 influenza virus strain X31, as described previously.16 In our experience using this virus, the highest dose used here in A/J mice is not lethal for C57BL/6J or BALB/cByJ mice. Euthanasia was performed by exsanguination under isoflurane anesthesia.
Streptococcus pneumoniae strain D39 (serotype 2).
S. pneumoniae that had been transformed with the lux operon2 was obtained from Dr Jonathan McCullers (St Jude Children's Research Hospital, Memphis, TN). To prepare stocks, bacterial colonies isolated from a tryptic soy agar supplemented with 3% defibrinated sheep blood (blood agar plates, Enova Medical, St Paul, MN) were inoculated into Todd Hewitt broth (Fisher Scientific, Pittsburgh, PA) and grown at 37 °C to a density of OD620 0.6 to 0.7. Sterile glycerol was added to a final concentration 16.7% prior to freezing aliquots at −80 °C. Serial dilutions of the bacterial stocks were plated in triplicate on blood agar plates to obtain the titer. For mouse inoculation, the bacterial stock was diluted in endotoxin-free sterile saline, and adult male BALB/cByJ mice (at least 4 mice per dose; total n = 36) were inoculated intranasally with 25 µL of 10-fold serial dilutions of the stock, with doses ranging between 1 × 102 and 1 × 107 cfu. The LD50 of lux-transformed S. pneumonia strain D39 for BALB/cByJ mice was 1 × 105.2 cfu, which is identical to the LD50 of the wild-type bacterial strain.12 Euthanasia was performed by exsanguination under isoflurane anesthesia.
Influenza strain A/Puerto Rico/8/34 (H1N1).
Viral stocks A/Puerto Rico/8/34 (H1N1) (P8) were kindly provided by Richard Webby (St Jude Children's Research Hospital) and prepared by inoculation of embryonated chicken eggs. Virus-infected allantoic fluid was harvested and aliquots frozen at −80 °C until use. The titer of the viral stock was determined by using a TCID50 assay.
In the study of BALB/cByJ mice, the viral stock was diluted in endotoxin-free sterile saline. Four adult male BALB/cByJ mice per dose (total, n = 28) were inoculated intranasally with 25 µL of 10-fold serial dilutions of the stock, with doses ranging between 1 × 101 and 1 × 107 TCID50 PR8. The LD50 of PR8 for BALB/cByJ mice was 1 × 103.5 TCID50.
In the study of C57BL/6J mice, mice were fed either a normal diet (4.5% fat; n = 10) or high-fat diet (60% fat; n = 9) from 6 through 34 wk of age. Mice then were inoculated with 25 µL allantoic fluid containing 1 × 103 TCID50 PR8 (1/3 the LD50 dose for BALB/cByJ mice). Euthanasia was performed by exsanguination under isoflurane anesthesia.
C. albicans (strain 10231).
C. albicans was grown overnight on Sabouraud dextrose agar. For intravenous inoculation of mice, colonies were suspended in sterile, pyrogen-free saline. The titer of the culture was estimated by using a hemocytometer, and the suspension was diluted to achieve approximately 5 × 105 cfu in 0.2 mL. The actual inoculated doses were determined subsequently by culture of serial dilutions of the inoculum plated on Sabouraud Dextrose agar and revealed an average dose of 5.6 ± 0.6 × 105 cfu (n = 24). For inoculation, adult male DBA/2J mice (n = 24) were anesthetized with isoflurane and injected intravenously through the retroorbital sinus. The retroorbital route was used for 2 reasons: 1) the injection is performed under general anesthesia and therefore does not require animal restraint, and 2) because the sinus is large relative to a vein, administration of substances by this route is extremely easy to perform and gives high assurance that the injected material has entered the circulation rather than the perivascular space. The study was terminated by design at 5 d after inoculation. Euthanasia was performed by exsanguination under isoflurane anesthesia.
Statistics.
Descriptive measures consisted of means and standard error of the means. Statistical analysis was conducted with independent t tests with statistical significance set at the 5% level.
Results
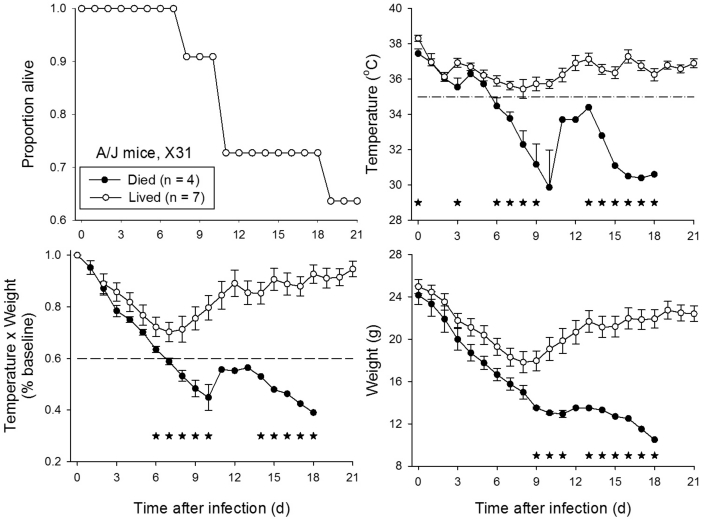
A/J mice (n = 11) were inoculated intranasally with a range of doses of influenza virus strain X31 (1 × 101.65 to 1 × 105.65 TCID50; n = 2 to 3 per dose). Among these mice, 63% (7 of 11) survived through day 21 after infection (Figure 1). Values for temperature (T) and the product of temperature and body weight (T × BW) of mice that lived compared with died showed sustained significant (P < 0.05) differences beginning on day 6 after infection, as compared with day 9 for body weight (Figure 1). Use of the threshold value of a fall in core temperature to below 35 °C on day 7 after infection would have detected all 4 mice that eventually died, with no incorrect detection of mice that survived. In comparison, a reduction in T × BW to less than 60% of baseline values on day 7 after infection would have detected 3 of 4 mice that eventually died and incorrectly detected 1 of 7 mice that survived.
Figure 1.
A/J mice infected with influenza virus strain X31. A/J mice (n = 11) were inoculated intranasally with 25 µL allantoic fluid containing between 5 × 101 and 5 × 105 TCID50 influenza virus strain A/HKX31(H3N2). Mice were monitored for 21 d after inoculation. *, P < 0.05 for comparison of mice that survived compared with those that eventually died.
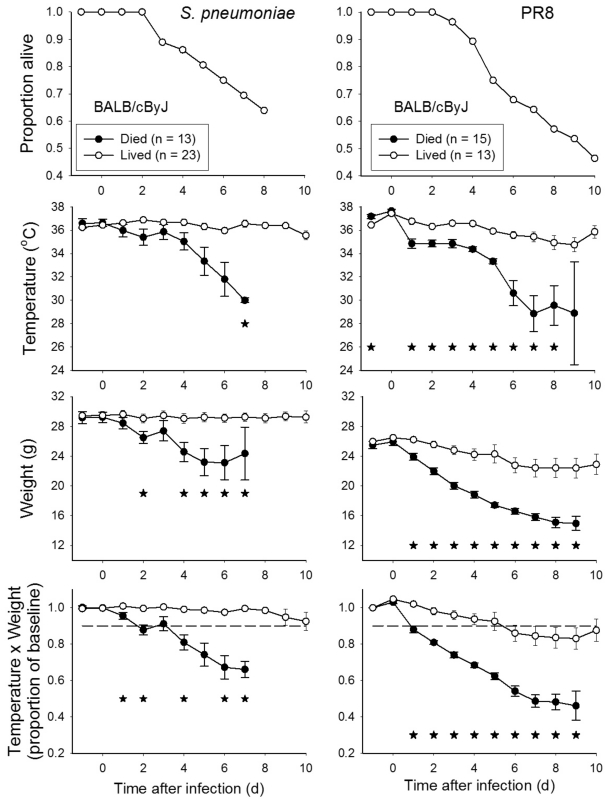
Among BALB/cByJ mice inoculated with a range of doses of Streptococcus pneumoniae (1 × 102 to 1 × 107 CFU), 64% (23 of 36) survived through day 10 after infection (Figure 2). Body weight and T × BW values of mice that lived compared with died differed significantly (P < 0.05) beginning on day 2 after infection (Figure 2). Use of the threshold value of a reduction in T × BW to less than 90% of baseline values on day 2 after infection would have detected 9 of 15 mice that eventually died and incorrectly detected 1 of 23 mice that survived.
Figure 2.
BALB/cByJ infected with Streptococcus pneumoniae or influenza virus strain PR8. BALB/cByJ mice anesthetized with isoflurane were inoculated intranasally with Streptococcus pneumoniae in doses ranging between 1 × 102 and 1 × 107 cfu (n = 36) or with influenza virus strain PR8 in doses ranging between 1 × 101 and 1 × 107 TCID50 (n = 28). *, P < 0.05 for comparison of mice that survived compared with those that eventually died.
Among BALB/cByJ mice inoculated with a range of doses of influenza virus strain PR8 (1 × 101 to 1 × 107 TCID50), 46% (13 of 28) survived through day 10 after infection (Figure 2). Temperature, BW, and T × BW were all informative with regard to differentiation of mice that lived and died, with all 3 measures revealing significant (P < 0.05) differences between groups on day 2 after inoculation (Figure 2). A reduction in T × BW to less than 90% of baseline values on day 2 after infection would have detected 10 of 15 mice that eventually died and incorrectly detected 1 of 13 mice that survived.
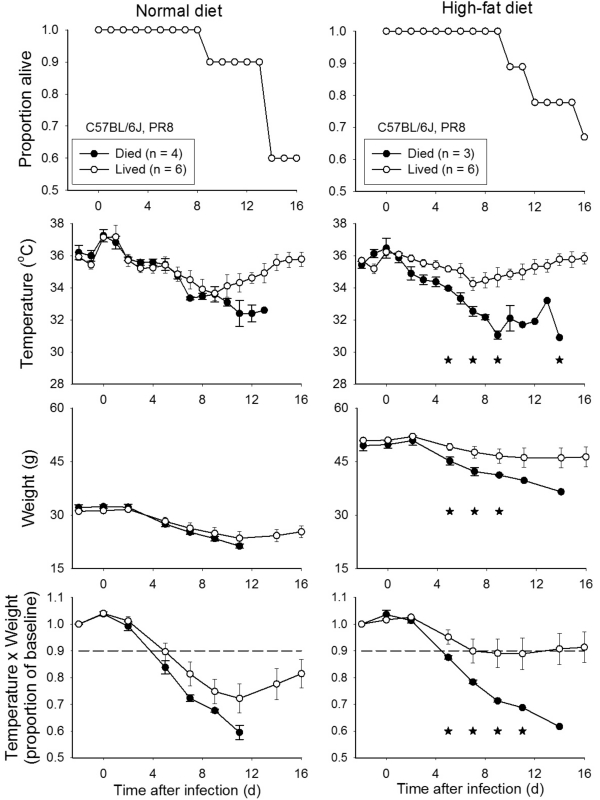
C57BL/6J mice were maintained on a normal-fat (4.5% fat) or high-fat (60% fat) diet for 34 wk beginning at 6 wk of age. All mice then were inoculated intranasally under isoflurane anesthesia with 1000 TCID50 influenza PR8. In this study, temperatures were measured daily, but body weights were measured only on Monday, Wednesday, and Friday. Among the mice maintained on normal diet, 60% (6 of 10) survived, whereas 66% of mice on high-fat diet (6 of 9) survived (Figure 3). In mice fed normal diet, none of the measured variables (temperature, BW, and T × BW) differed significantly between mice that lived compared with died. In mice fed high-fat diet, all 3 measures were informative with regard to differentiation of mice that lived and died, revealing significant (P < 0.05) differences between groups on day 2 after inoculation (Figure 3). A reduction in T × BW to less than 90% of baseline values on day 5 after infection would have detected all 3 mice that eventually died and incorrectly detected 2 of 6 mice that survived.
Figure 3.
Obese and normal C57BL/6J mice infected with influenza virus strain PR8. C57BL/6J mice were fed either a normal diet (4.5% fat; n = 10) or a high-fat diet (60% fat; n = 9) from 6 through 34 wk of age. Mice then were inoculated with 25 µL allantoic fluid containing 1 × 103 TCID50 influenza virus strain PR8 (1/3 the MLD50 dose for BALB/cByJ mice). *, P < 0.05 for comparison of mice that survived compared with those that eventually died.
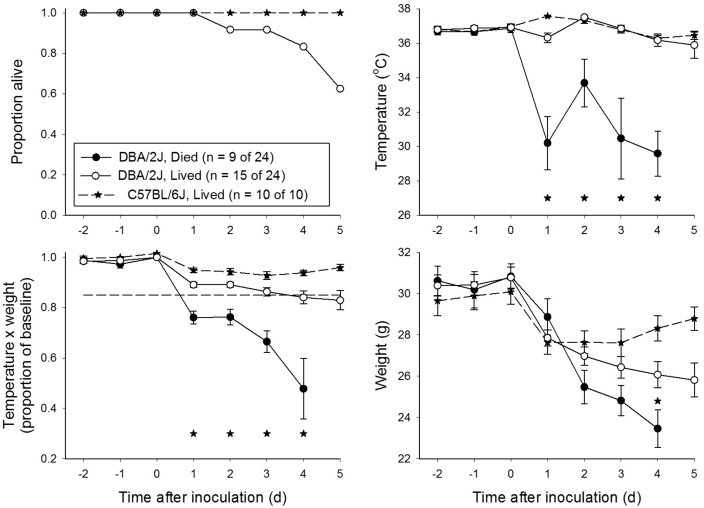
Among DBA/2J mice inoculated with C. albicans, 63% (15 of 24) survived through day 5 after infection (Figure 4). Temperature and T × BW were informative with regard to differentiation of mice that lived and died, whereas body weight was not (Figure 4). A reduction in T × BW to 85% or less of baseline values on day 1 after infection would have detected all 9 mice that eventually died and incorrectly detected 3 of 15 mice that survived.
Figure 4.
DBA/2J mice infected with Candida albicans. DBA/2J mice (n = 24) were anesthetized with isoflurane and injected intravenously through the retroorbital sinus with 5.6 ± 0.6 × 105 cfu Candida albicans. *, P < 0.05 for comparison of mice that survived compared with those that eventually died.
Discussion
The data presented here indicate that reductions in temperature, body weight, and the product of both, as recently applied to the prediction of imminent death in mice used in longevity studies,11 can predict the eventual death of mice used in some infectious disease models. The current data were collected in a number of independent experiments that used different infectious organisms. We analyzed these data retrospectively in an attempt to identify markers that would be useful for prediction of the eventual death of mice with experimentally-induced infectious disease. The assessment revealed that specific measures may be useful in some models but not in others. For example, among A/J mice inoculated with influenza virus and DBA/2J mice inoculated with C. albicans, temperature and T × BW were more useful than BW. However, useful T × BW benchmark values for predicting eventual death or performing preemptive euthanasia were 60% and 85% of baseline for influenza-infected A/J and C. albicans-infected mice, respectively. Among BALB/cByJ mice inoculated with influenza virus, temperature, body weight, and T × BW were all useful predictors of eventual death. In contrast, these markers were not useful for normal C57BL/6J mice that received similar inoculations but were informative for obese C57BL/6J mice with influenza infection.
Several of the experiments that produced the data analyzed here were preliminary dose-finding studies that evaluated outcomes among mice that received different doses of the same agent. Numbers tested at each dose in these studies were not sufficient to allow comparison within doses. However, given that infectious dose is undoubtedly the best predictor of death after infectious challenge, the ideal test for verification of these makers would be a follow-up study using an LD50 dose. Such studies would be important for validation of both the dose-finding and the endpoint markers if the model was intended for repeated use (for example, for screening therapeutics).
Previous work indicates that temperature and BW can be used independently as markers for disease severity, a moribund state, imminent death, and the need for euthanasia in a variety of infectious disease models.1,7-10,13,14,17,18 However, application and interpretation of these measures can be complex. For example, in a study of bacterial endotoxemia in mice, weight loss was not found to be an accurate predictor of death,7 whereas in a study of endotoxemic shock induced by cecal ligation and puncture in mice, weight gain was a predictor of imminent death but weight loss was not.9 Some authorities recommend using weight loss to assess wellbeing but do not offer guidelines or benchmarks for amount of weight loss that would be acceptable.8,10 Important considerations in using temperature as a benchmark are that 1) body temperatures of mice are influenced by numerous factors, including the time of day, ambient temperature, the presence and type of bedding, and the number of cage mates,3,4 and 2) different strains of mice can show widely different temperature responses to the same experimental challenge.14,15 These complexities are further illustrated by the present analysis, which demonstrates that the informative markers and their relevant thresholds vary substantially depending on the specific model used (that is, mouse strain, mouse condition, and infectious challenge). Other factors (for example, route of administration and virulence of the challenge organism) also are likely to influence these determinations.
Taken together with previous work, our findings underscore the importance of carefully tailoring the selection of endpoint markers to the specific experimental model and validating those markers for that model. Determination of humane endpoints for laboratory animals is increasingly important to IACUC and regulatory agencies. The present study and our previous work11 provide a simple strategy for the identification of markers of eventual death in mice. In light of our data, we suggest that investigators who conduct similar studies using other mouse models of infectious disease assess our approach in their ongoing studies and report their findings to the scientific community.
Acknowledgments
This work was supported in part by NIH grants K26-RR17543 and R01-NS40220 and by the Southern Illinois University School of Medicine. We thank Christine Bosgraaf and Lisa Cox for providing excellent technical assistance.
References
- 1.Douce G, Goudling D. 2010. Refinement of the hamster model of Clostridium difficile disease. Methods Mol Biol 646:215–227 [DOI] [PubMed] [Google Scholar]
- 2.Francis KP, Yu J, Bellinger-Kawahara C, Joh D, Hawkinson MJ, Xiao C, Purchio TE, Caparon MG, Lipsitch M, Contag PR. 2001. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infect Immun 69:3350–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon CJ. 2004. Effect of cage bedding on temperature regulation and metabolism of group-housed female mice. Comp Med 54:63–68 [PubMed] [Google Scholar]
- 4.Gordon CJ, Becker P, Ali JS. 1998. Behavioral thermoregulatory responses of single- and group-housed mice. Physiol Behav 65:255–262 [DOI] [PubMed] [Google Scholar]
- 5.Hendriksen CF. 2009. Replacement, reduction, and refinement alternatives to animal use in vaccine potency measurement. Expert Rev Vaccines 8:313–322 [DOI] [PubMed] [Google Scholar]
- 6.Jennings M, Morton DB, Charton E, Cooper J, Hendriksen C, Martin S, Pearce MC, Price S, Redhead K, Reed N, Simmons H, Spencer S, Willingale H. 2010. Application of the three Rs to challenge assays used in vaccine testing. Biologicals 38:684–695 [DOI] [PubMed] [Google Scholar]
- 7.Krarup A, Chattopadhyay P, Bhattacharjee AK, Burge JR, Ruble GR. 1999. Evaluation of surrogate markers of impending death in the galactosamine-sensitized murine model of bacterial endotoxemia. Lab Anim Sci 49:545–550 [PubMed] [Google Scholar]
- 8.Morton DB. 2000. A systematic approach for establishing humane endpoints. ILAR J 41:80–86 [DOI] [PubMed] [Google Scholar]
- 9.Nemzek JA, Xiao HY, Minard AE, Bolgos GL, Remick DG. 2004. Humane endpoints in shock research. Shock 21:17–25 [DOI] [PubMed] [Google Scholar]
- 10.Olfert ED, Goodson DL. 2000. Humane endpoints for infectious disease animal models. ILAR J 41:99–104 [DOI] [PubMed] [Google Scholar]
- 11.Ray MA, Johnston NA, Verhulst SJ, Toth LA. 2010. Determination of humane endpoints in longevity research. J Am Assoc Lab Anim Sci 49:282–288 [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MW, Schmidt JE, Rehg JE, Orihuela CJ, McCullers JA. 2007. Induction of pro- and antiinflammatory molecules in a mouse model of pneumococcal pneumonia after influenza. Comp Med 57:82–89 [PMC free article] [PubMed] [Google Scholar]
- 13.Soothill JS, Morton DB, Ahmad A. 1992. The HID50 (hypothermia-inducing dose 50): an alternative to the LD50 for measurement of bacterial virulence. Int J Exp Pathol 73:95–98 [PMC free article] [PubMed] [Google Scholar]
- 14.Stiles BG, Campbell YG, Castle RM, Grove SA. 1999. Correlation of temperature and toxicity in murine studies of staphylococcal enterotoxins and toxic shock syndrome toxin 1. Infect Immun 67:1521–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toth LA, Hughes LF. 2006. Sleep and temperature responses of inbred mice with Candida albicans-induced pyelonephritis. Comp Med 56:252–261 [PubMed] [Google Scholar]
- 16.Toth LA, Rehg JE, Webster RG. 1995. Strain differences in sleep and other pathophysiological sequelae of influenza virus infection in naive and immunized mice. J Neuroimmunol 58:89–99 [DOI] [PubMed] [Google Scholar]
- 17.Vlach KD, Boles JW, Stiles BG. 2000. Telemetric evaluation of body temperature and physical activity as predictors of mortality in a murine model of staphylococcal enterotoxic shock. Comp Med 50:160–166 [PubMed] [Google Scholar]
- 18.Warn PA, Brampton MW, Sharp A, Morrissey G, Steel N, Denning DW, Priest T. 2003. Infrared body temperature measurement of mice as an early predictor of death in experimental fungal infections. Lab Anim 37:126–131 [DOI] [PubMed] [Google Scholar]