Abstract
Bilateral temporomandibular joint (TMJ) luxation was diagnosed postmortem in a female, 6-mo-old CD rat (Rattus norvegicus) after probable head entrapment and subsequent disentanglement from a protective jacket. Clinical antemortem signs included inability to close her mouth, prehend food and drink water, anxiety, and linear skin erosions extending down the right and left commissures of the mouth. Radiography revealed rostral displacement of the mandible with concomitant malocclusion. The combination of clinical signs, acute nature of the presentation, and torn appearance of the protective jacket were strongly indicative of a traumatic etiology. To our knowledge, this is the first reported case of TMJ luxation in a rat.
The temporomandibular joint (TMJ) is a defining feature of mammals and separates them from other vertebrates.8 It is a condylar, synovial joint that shares many anatomic features among mammals but has also evolved specialized characteristics in the various mammalian species in response to biomechanical feeding forces.4,8,14 The TMJ functions to maintain alignment of the maxilla and mandible and allow movement of the jaws.6
The basic structure of the TMJ includes the condylar process of the head of the mandible, which articulates with the mandibular fossa in the squamous part of the temporal bone in most mammals.8,14 Both the condylar process and mandibular fossa are covered in hyaline cartilage and are separated by a biconcave, fibrocartilagenous disc that divides the joint into dorsal and ventral components.6,12,14 The joint is surrounded by a fibrous capsule with a synovial lining that is reinforced in some veterinary species with ligamentous attachments to the mandibular and temporal bones.6,8,13,14
Luxation of the TMJ occurs when the components of the joint disarticulate and move in an aberrant direction, usually as a result of some kind of head trauma.5,7,9 Temporomandibular joint luxation can be bilateral or unilateral. Sometimes mandibular or other cranial fractures will be present.5 The condylar process almost always luxates in a rostrodorsal direction with accompanying dental malocclusion.5-7,14, Luxations also can occur as a result of TMJ dysplasia.1,5,6,13
Several case reports in the veterinary literature describe traumatic or pathologic TMJ luxations and ensuing treatments in cats, dogs, horses, and guinea pigs.5-7,9,10 Here, we describe the first case of bilateral TMJ luxation in a rat (Rattus norvegicus) that resulted from a presumed traumatic incident.
Case Report
A female, 6-mo-old CD rat (Crl:CD/SD) acutely presented unable to close her mouth, as noted by a veterinary technician. While in her cage, the rat repeatedly wiped her open mouth with her front paws while standing on her hindlimbs. Ten minutes earlier, the veterinary technician had removed a protective canvass jacket from this animal and noticed no abnormalities of anatomy, posture, or behavior. The rat had been wearing the jacket for 2 wk to discourage ongoing self-trauma to the skin on her dorsum and flanks. During that time, no adverse effects had been noted.
The rat was individually housed in a polysulfone cage with corncob bedding in a conventional facility in accordance with the animal care policies and procedures of the Department of Animal Medicine (University of Massachusetts Medical School, Worcester, Massachusetts). She was provided with ad libitum, bottled, acidified water and standard rodent chow (Lab Diet, 5POO Prolab, RHI 3000, PMI Nutrition International, Brentwood, MO) consumed from a hopper. The housing room was on a 12:12-h light:dark cycle. Nestlets (Ancare, Bellmore, NY) and Shepherd Rat Shacks (Shepherd Paper Products, Watertown, TN) were provided for enrichment.
The rat was part of an IACUC-approved study that involved bilateral resection of the lattisimus dorsi muscles 2.5 mo prior to this clinical presentation. At the time of surgery, the rat weighed 334 g. Sterile procedures were followed, in accordance with the guidelines for survival rodent surgery at the facility. She was not intubated or masked, eliminating the possibility of iatrogenic trauma from either of those manipulations. During surgery, she was monitored with a veterinary pulse oximeter and kept warm with a recirculating water heating pad placed on top of the surgery table. The surgery was uneventful, and the rat recovered normally after the procedure. Postoperative pain management consisted of 2 doses of ketoprofen (5 mg/kg SC daily) and 2.5 d of buprenorphine (0.08 mg/kg SC) administered every 8 h.
Twelve days after the surgical procedure, the rat was seen scratching at the skin on her dorsum, initially near the incision site and then, in coming days, along her dorsum and flanks, leading to superficial, moist, inflamed, discrete lesions of various sizes. The treatment plan consisted of twice-a-week nail trimmings and topical antibiotics when necessary, but the lesions kept recurring, and the rat was seen licking at the wounds on several occasions. The cause of the self-trauma was not determined but included possible nerve damage from the surgery.
To protect the skin from further trauma and allow the current lesions the opportunity to heal, the rat was dressed, about 1 mo after presenting with the excoriations, in a protective canvass jacket designed specifically for rats by a commercial vendor (Lomir Biomedical, Notre-Dame-de-I'lle Perrot, Canada). The jacket was 4 in. at its longest and 3 in. at its widest points, with 2 holes for the forearms and a hook-and-loop closure supplemented with eyehooks for ties, although ties were not used in this instance. Two weeks after the rat was dressed in the jacket, the skin lesions were almost healed. For the entire 2 wk, the rat was noted to be in good health, eating and drinking normally with good body condition. It was decided that the jacket would be removed.
At the time of jacket removal, the rat presented with an open mouth and appeared to be in distress, intermittently pawing at her mouth and running back and forth between the food hopper and water bottle. Her hydration status, mucous membrane color, and body condition were normal. Differential diagnoses in mammalian species for an inability to close the mouth include oral foreign body, TMJ luxation or subluxation, mandibular or zygomatic fractures, TMJ dysplasias with open-mouth locking, mandibular neoplasia, and trigeminal neurapraxia.1,5,6,9
The rat was lightly anesthetized with isoflurane gas. Examination revealed 2 fairly deep, linear, erosive skin lesions running down the rat's mandible from the right and left commissures of the mouth. A cursory oral examination did not reveal any laxity of the mandible or other cranial or jaw bones, and there were no other obvious abnormalities. Rostral luxation of the mandible was a differential diagnosis, but an attempt to reduce the mandible under the light plane of anesthesia was unsuccessful.
The principle investigator of the study was contacted and decided with the veterinarian to euthanize the rat with CO2 gas because she appeared to be in distress that could not easily be relieved and was near the endpoint of the study. Radiographs of the carcass were obtained with a mounted, portable radiograph machine (HF80+, MinXray, Northbrook, IL). The dorsoventral view of the head showed a significant rostral shifting of the mandible, with the right and left coronoid processes of the mandible visible toward the rostral third of the zygomatic bone (Figure 1). The condylar processes of the right and left mandibles were visible as radioopaque densities toward the caudal one-third of the zygomatic bone, and the right and left angular processes of the mandible could be observed jutting just past the bottom edge of the zygomatic bone (Figure 1). Skull radiographs from normal rats show the coronoid processes toward the caudal third of the zygomatic bones, the condylar processes superimposed with the caudal end of the zygomatic bone, and the angular processes jutting just below the level of the tympanic cavity.15
Figure 1.
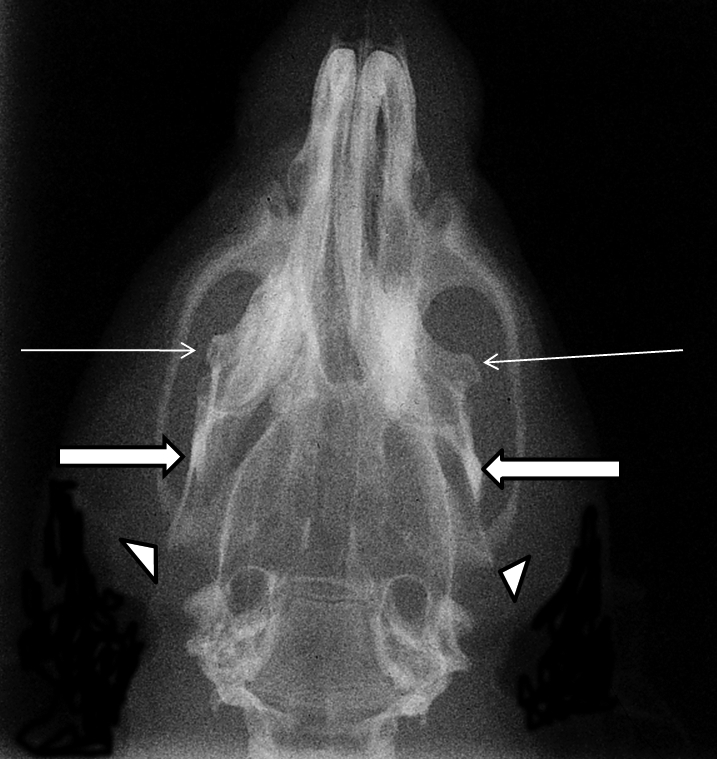
Dorsoventral plain-film radiographic view of rat skull. Thin arrows indicate the rostral shifting of the right and left coranoid processes of the mandible toward the upper third of the zygomatic bone region. Thick arrows show that the right and left condylar processes of the mandible are rostrally shifted toward the caudal area of the right and left zygomatic bones. Triangles indicate that the right and left angular processes of the mandible are visible just beyond the ends of the zygomatic bones. These radiographic signs indicate a rostral shifting of the mandible after bilateral luxation of the TMJ.
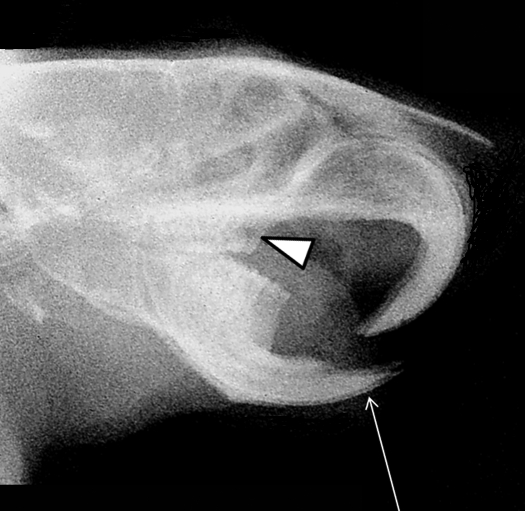
The lateral radiographic view of the head revealed rostral protrusion of the mandibular incisors that incorrectly overlapped the upper incisors (Figure 2). In addition, the upper and lower molar arches did not articulate appropriately, with the mandibular third molar nearly apposed to the first maxillary molar (Figure 2). In normal, resting rodent occlusion, the upper incisors overlap the lower incisors, and the molar arcades are aligned, with the third maxillary molar meeting the third mandibular molar, and so on.16
Figure 2.
Lateral plain-film radiographic view of rat skull. The thin arrow shows that the mandibular incisors are malpositioned under the maxillary incisors. Triangles show that the upper and lower molar arcades are misaligned, with the maxillary first molar nearly apposed to the mandibular third molar. These radiographic signs reveal the dental malocclusion after bilateral luxation of the temporomandibular joint due to presumed traumatic injury.
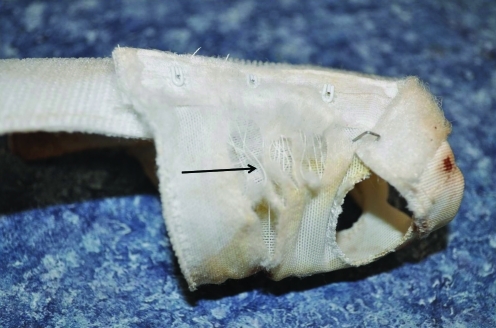
Additional details of the TMJ anatomy could not be discerned with the imaging technique used. However, the radiographic anomalies indicate bilateral rostrodorsal dislocation of the TMJ with concomitant malocclusion. These radiographic abnormalities combined with the linear skin erosions along the right and left sides of the chin suggest a traumatic etiology, with string entrapment from the jacket being the most likely scenario. The rat may have tried to remove the jacket or was biting at the jacket at some point over the weekend, with her lower jaw becoming ensnared in the fabric. She then could have struggled to break free, luxating her mandible in the process. The removed jacket had several frayed areas in the fabric (Figure 3), although when these torn areas first appeared is unknown.
Figure 3.
Protective canvass jacket that was removed from the rat before clinical signs were observed. The black arrow indicates the frayed areas of fabric where the rat's head could have been ensnared. The rat may have struggled to break free, potentially bilaterally luxating the TMJ.
Discussion
To our knowledge, this is the first report of a bilateral TMJ luxation in a rat (Rattus norvegicus). The injury most likely occurred as a result of a traumatic incident involving head entrapment and subsequent disentanglement from a protective canvass jacket. The rat had been dressed in this jacket to discourage self-trauma after repeated incidents of self-excoriation on her dorsum and flanks after experimental surgery was performed on the back muscles several weeks prior to the appearance of the skin lesions. Few references in the veterinary literature describe cases of injuries involving protective jackets or other coverings designed to promote healing in animals. These include anecdotal reports of injuries to animals dressed in Elizabethan collars,2,3 and one report of 2 dogs accidentally asphyxiated when their Elizabethan collars became ensnared in plastic bags.17 One experimental study described depression of feed intake and weight loss in rats wearing neck collars.11
Case reports and references to cases discuss unilateral or bilateral TMJ luxations in cats, dogs, horses, and guinea pigs, usually as a result of head trauma but sometimes due to TMJ dysplasia.1,5-7,9,10 One publication describes unilateral, right TMJ luxation in a 20-y-old American Saddlebred gelding that presented unable to close his mouth after his head had been caught in a fence.7 Radiographs revealed rostral displacement of the right condylar process and lower molar arcade with no associated fractures. The TMJ luxation in this horse was repaired by closed reduction under anesthesia.7 Closed reduction is recommended as a conservative treatment for dogs, cats, and horses, but would be impractical for most laboratory rodents involved in studies. For cats and dogs, one recommended treatment involves placing a dowel transversely between the maxillary and mandibular molars to distract the condyle distally while the rostral mandible and maxillary bones are squeezed together.5 This method of reduction can be unstable, and often a tape muzzle or interarcade wiring is applied for 1 to 2 wk after the reduction to allow time for healing.5,6 Although the horse case cited7 involved unilateral luxation, the scenario of head entrapment and subsequent escape, along with the traumatic and acute nature of the incident, shares many details with that of the rat in the current report.
TMJ luxations are rare in horses due to the inherent stability of the joint, which is strengthened with an overlay of musculature and strong attachments of the joint capsule and mandibular ligaments.9 These abnormalities are also relatively rare in dogs and cats for similar reasons,5,14 although TMJ dysplasia with associated open-jaw locking or subluxation occurs more frequently in brachycephalic and chondrodyplastic breeds of dogs and cats than in other breeds.1,6 One report describes the use of computed tomography for diagnosis of and treatment planning for open mouth locking due to TMJ dysplasia in a 2.5-y-old, female Persian cat.1 The cat presented pawing at her open mouth, as did the rat in our report. The use of a tomographic scanner and 3D reconstruction of the joint images eliminated many of the problems, such as the superimposition of the complex structures of the joint, associated with conventional plain-film radiographic imaging of the TMJ.1,6,14
Caudal luxation of the condylar process from the mandibular fossa is rare in cats and dogs because of the bony tubercle called the retroarticular process, which is a caudoventral extension of the mandibular fossa.6,14 Rostral luxation of the condylar process in those species is limited by the articular tubercle in the rostral mandibular fossa.6 This structure is relatively small in dogs, slightly more pronounced in cats, and also exists in herbivore and omnivore species.14 However, the mandibular fossa in male, adult Wistar rats is reported in one study12 to be flat with no bony tubercles. The authors12 speculated that the absence of articular tubercles in rats may make the movement of their mandibles highly specialized for extensive protrusive movements.12 The lack of articular tubercles may have contributed to the forward bilateral TMJ luxation in the rat in our report. The previously cited report12 did not remark on the presence of ligamentous structures in the rat TMJ, but absence of these structures might increase the risk of traumatic TMJ luxation in rats. Extensive rostral-caudal gliding movements have been noted in the temporomandibular articulations of rats;4 in addition, the temporomandibular articulations subluxate during normal gnawing motions in rats, resulting in transient mandibular prograthism while feeding.4 All of these features of the rat TMJ apparatus may predispose rats to rostral temporomandibular luxations in certain circumstances.
Although the current report is the first of a rat with traumatic, bilateral TMJ luxation, chronic molar malocclusion in guinea pigs due to molar overgrowth may lead to overextension of the masticatory muscles, concomitant jaw laxity, and possible TMJ luxation.10 These animals may present with anorexia, weight loss, inability to prehend food or masticate, drooling, facial swelling, and sometimes exopthalmos.4,10
Clinical signs of unilateral or bilateral TMJ luxation include inability to close the mouth or prehend and chew food or drink water and dental malocclusion. There may also be signs of discomfort (pawing at the mouth), swelling of the jaw muscles, asymmetry of the jaw bones with possible abnormal bony protrusions on palpation, weight loss in chronic cases, pain on palpation of the jaw bones, exopthalmos, and drooling. Radiographic imaging is the most useful diagnostic tool, with the dorsoventral view deemed the most informative, although lateral, oblique, and open-mouth views should be included if possible.5,6,14 Radiographic signs of note include rostrodorsal (in most instances) displacement of the mandible, widened joint space width, dental malocclusion, and any accompanying mandibular or cranial fractures . Unilateral luxation causes the mandible to shift to the opposite side of the mouth5 and is associated with more subtle dental malocclusion than that seen with bilateral luxation. Suspected fractures should be confirmed by at least 2 views. Computed tomography is more sensitive than plain-film radiography for detection of TMJ abnormalities, allowing enhanced visualization and the ability to manipulate the images1,6,14 but would be impractical for most laboratory and even companion animals due to high costs and less availability. However, conventional digital radiography has become more available to veterinarians in academic, institutional, and private practice settings and likely would provide improved resolution of the temporomandibular joint over plain film radiography without the expense of tomographic scanners. Although, superimposition of the structures of the joint would still be a limiting factor on digital films.
Treatment for TMJ luxation can be either conservative, with closed reduction methods, as discussed earlier, or more radical, involving surgical approaches in selected cases that require stabilization of fractures or do not initially respond to conservative treatments. Some surgical treatments for this condition include open reduction of the TMJ joint, condylectomy, and partial excision of the zygomatic arches.5,6 For guinea pigs, trimming of any overgrown molars should be curative.4,10
The rat discussed in this case report probably was injured after becoming entangled in a protective jacket. This explanation is the most plausible, given the evidence of torn fabric on the jacket and the animal's clinical signs, which included linear skin erosions extending down the sides of her mouth, normal body condition, and acute presentation. In this instance, the benefit of the jacket was overcome by the devastating, but unusual, injury that it caused. More frequent monitoring of the rat may have resulted in the detection of any entrapment, although the time frame of this incident could not be determined.
TMJ luxations are rare in veterinary species, but clinical signs can be identified, and diagnostic and treatment options are available. Similar to the rat reported in our case, diagnostic and treatment options can be extrapolated across species.
References
- 1.Beam RC, Kunz DA, Cook CR, Carson RL, Briscoe P, Cook JL. 2007. Use of 3-dimensional computer tomography for diagnosis and treatment planning for open-mouth jaw locking in a cat. J Am Vet Med Assoc 230:59–63 [DOI] [PubMed] [Google Scholar]
- 2.Brown C. 2006. Restraint collars. Part I: Elizabethan collars and other types of restraint collars. Lab Anim (NY) 35:23–25 [DOI] [PubMed] [Google Scholar]
- 3.Brown C. 2006. Restraint collars. Part II: specific issues with restraint collars. Lab Anim (NY) 35:25–27 [DOI] [PubMed] [Google Scholar]
- 4.Crossley DA. 1995. Clinical aspects of rodent dental anatomy. J Vet Dent 12:131–135 [PubMed] [Google Scholar]
- 5.Fossum TW. 1997. Temporomandibular joint luxation, p 898–903. : Small animal surgery. St Louis (MO): Mosby [Google Scholar]
- 6.Gemmill T. 2008. Conditions of the temporomandibular joint in dogs and cats. In Pract 30:36–43 [Google Scholar]
- 7.Hardy J, Shiroma JT. 1991. What is your diagnosis? J Am Vet Med Assoc 198:1663–1664 [PubMed] [Google Scholar]
- 8.Herring SW. 2003. TMJ anatomy and animal models. J Musculoskelet Neuronal Interact 3:391–394 [PMC free article] [PubMed] [Google Scholar]
- 9.Hurtig MB, Barber SM, Farrow CS. 1984. Temporomandibular joint luxation in a horse. J Am Vet Med Assoc 185:78–80 [PubMed] [Google Scholar]
- 10.Legendre LFJ. 2002. Malocclusions in guinea pigs, chinchillas, and rabbits. Can Vet J 43:385–390 [PMC free article] [PubMed] [Google Scholar]
- 11.Neale RJ. 1984. Coprophagy in iron-deficient rats: II. Two novel methods of prevention. Lab Anim 18:119–124 [DOI] [PubMed] [Google Scholar]
- 12.Porto GG, Vasconcelos BCE, Andrade ESS, Silva-Junior VA. 2010. Comparison between human and rat TMJ: anatomic and histopathologic features. Acta Cir Bras 25:290–293 [DOI] [PubMed] [Google Scholar]
- 13.Scapino RP. 1965. The third joint of the canine jaw. J Morphol 116:23–50 [DOI] [PubMed] [Google Scholar]
- 14.Schwarz T, Weller R, Dickie AM, Konar M, Sullivan M. 2002. Imaging of the canine and feline temporomandibular joint: a review. Vet Radiol Ultrasound 43:85–97 [DOI] [PubMed] [Google Scholar]
- 15.Silverman S, Tell LA. 2005. Chapter 3: Norway rat, p 31. : Radiology of rodents, rabbits, and ferrets. St Louis (MO): Elsevier [Google Scholar]
- 16.Silverman S, Tell LA. 2005. Chapter 3: Norway rat, p 29. : Radiology of rodents, rabbits, and ferrets. St Louis (MO): Elsevier [Google Scholar]
- 17.Wilson S. 1993. Elizabethan collars and plastic bags. Vet Rec 132:664. [DOI] [PubMed] [Google Scholar]