Abstract
OBJECTIVE
Memory for odors is often associated with highly emotional experiences, and odors have long been noted by clinicians to be precipitants of trauma symptoms in PTSD. Primitive brain systems involved in fear responsivity and survival also mediate smell, including the olfactory cortex and amygdala. The purpose of this study was to measure neural correlates of olfaction in PTSD.
METHODS
We exposed male combat veterans with PTSD (N=8) and without PTSD (N=8) to a set of smells, including diesel (related to traumatic memories of combat), and three other types of smells: odorless air, vanilla/coconut and hydrogen sulfide (H2S) (resp. a neutral, positive, and negative hedonic non-traumatic smell) in conjunction with PET imaging of cerebral blood flow and assessment of psychophysiological and behavioral symptoms. All subjects also underwent a baseline of olfactory acuity.
RESULTS
PTSD patients rated diesel as unpleasant and distressing, resulting in increased PTSD symptoms and anxiety in PTSD versus combat controls. Exposure to diesel resulted in an increase in regional blood flow (rCBF) in amygdala, insula, medial prefrontal cortex (mPFC) and anterior cingulate cortex (ACC), and decreased rCBF in lateral prefrontal cortex (lPFC) in PTSD in comparison to combat controls. Combat controls showed less rCBF changes on any smell, and did not show amygdala activation upon diesel exposure.
CONCLUSIONS
These data support the hypothesis that in PTSD trauma-related smells can serve as strong emotional reminders. The findings indicate the involvement of a neural circuitry that shares olfactory elements and memory processing regions when exposed to trauma-related stimuli.
Keywords: PTSD, brain imaging techniques, olfaction, memory, amygdala
INTRODUCTION
It has been known for a long time that odors have the ability to serve as a reminder of a far and distant past. Odor perception can retrieve memories of life events with personal meaning and elicit strong affective experiences (1;2). Olfactory information is projected from the olfactory bulb to the primary olfactory cortex, which is composed of the anterior olfactory nucleus, the olfactory tubercle, the piriform cortex, and the amygdala, and projects to secondary olfactory regions, including the hippocampus, ventral striatum and pallidum, hypothalamus, thalamus, orbitofrontal cortex, insular cortex, and cingulate gyrus (Powell et al., 1965). The olfactory cortex is unique in having direct projections to the amygdala, which plays a critical role in the process of fear responding. Several studies provide evidence that the human amygdala also participates in the emotional processing of olfactory stimuli (3–11). The fact that the amygdala is closely connected to the hippocampus and enthorhinal cortex, leading to an emotional enhancement of odor memories, may explain their unique long-term retention.
Clinicians have long noted that specific trauma-associated smells, such as napalm or diesel in combat veterans can serve as precipitants of emotional memories and induce traumatic recall in patients with posttraumatic stress disorder (PTSD) (12–14). Several preclinical studies found that olfactory cues play a critical role in conditioned fear responses (15–18), which indicates that the neural circuitry of olfaction closely parallels the circuitry of the fear response. In these studies smell has been used successfully as a probe of the amygdala, and of the orbitofrontal and medial prefrontal cortex, all areas of interest in PTSD.
Neuroimaging studies have begun to map out a neural circuitry of PTSD (19;20). Specific for symptoms of traumatic recall in PTSD these studies found: decreased orbito-/prefrontal (21;22), parietal, hippocampal (23–26), and temporal cortical function (23;27–30), and increased function in posterior cingulate and motor cortex (24;30;31). Cerebral blood flow studies have also found evidence for alterations in middle/inferior frontal gyrus and cerebellum (24;26;31–41). Rauch et al found an increase in amygdala activation with exposure to masked fearful faces in PTSD (42). However, to date, and in spite of preclinical evidence of amygdala involvement in emotional processing and fear conditioning, clinical studies in PTSD have not consistently implicated the amygdala in the neural circuitry of Emotional memories (43). This may be due to different factors, including differences in research designs. Exteroceptive or internally generated emotional states (recall) as generated by traumatic scripts may or not correspond to exteroceptive events, such as external threat, and these differences may account for the variability in findings related to amygdala activation in PTSD. Smell memory represents a strong exteroceptive memory which is connected to neural circuits involving the amygdala and is therefore an excellent model for probing the neural circuitry of emotional processing in PTSD.
The purpose of this study was to use PET in the examination of neural correlates of olfactory induced emotional recall in combat-related PTSD. We hypothesized that the disposition of a smell to induce emotional memories would correlate with its emotional valence (44), and that specifically for patients with PTSD exposure of trauma-related smells would result in an increase in PTSD symptoms and increased activation of brain areas implicated in the disorder.
METHODS
Subjects
Sixteen male veterans with a history of combat in either Vietnam or the Gulf War participated in the study. Subjects included veterans with (N=8) and without PTSD (N=8) (see Table 1). All subjects were recruited through newspaper advertisement and through the National Center for PTSD, West Haven VA Medical Center. Diagnosis of PTSD was established with the Structured Clinical Interview for DSMIV (SCID), and consensus by three research psychiatrists (EV, SMS, JDB). All subjects gave written informed consent for participation, were free of major medical illness on the basis of history, physical examination and lab testing, were not actively abusing substances or alcohol (past three months). Subjects were excluded who were using neuroleptics or benzodiazepines. Subjects were not taken off of their medication for the purpose of the study. Subjects with a serious medical or neurological illness, organic mental disorders or co-morbid psychotic disorders, retained metal, a history of head trauma, loss of consciousness, cerebral infectious disease, or dyslexia were excluded. Subjects with a score <32 on the University of Pennsylvania Smell Identification Test (UPSIT) (45) were also excluded. The study was approved by the Yale University Investigational Review Board and was prepared in accordance with the ethical standards laid down in the Declaration of Helsinki. Subjects were told that the study involved how the brain processed different odors. Information was asked about memories they had for a wide array of smells. On a traumatic reminder list of olfactory cues for emotional memories the selected PTSD subjects all reported diesel as a smell that was strongly associated with traumatic experiences during their deployment.
Table 1.
Demogaphics and Clinical Characteristics of PTSD patients and combat controls
| PTSD (n=8) | TC (n=8) | |||||
|---|---|---|---|---|---|---|
| avg | stdev | avg | stdev | p | ||
| age | 47.5 | 10.7 | 41.3 | 10.8 | ns | |
|
| ||||||
| Race | caucasian | 8 | 8 | |||
|
| ||||||
| Combat | Vietnam | 6 | 3 | |||
| Gulf War | 2 | 5 | ||||
|
| ||||||
| CAPS | int | 21 | 7.5 | na | na | |
| ar | 31 | 15 | na | na | ||
| av | 23 | 8.6 | na | na | ||
| total | 75.5 | 27.7 | na | na | ||
|
| ||||||
| Comorbidity | MDD lifetime | 6 | 3 | |||
| MDD current | 2 | 0 | ||||
| Dysthymia | 1 | 0 | ||||
| Panic plus agora | 1 | 0 | ||||
| Gen Anx dis | 2 | 0 | ||||
| Soc Phobia | 1 | 0 | ||||
| Past Alc Dep | 3 | 4 | ||||
| Past Drug Dep | 5 | 3 | ||||
|
| ||||||
| UPSIT | 32.8 | 5.3 | 35.3 | 3.2 | ns | |
|
| ||||||
| smoking Hx | 5 | 3 | ||||
| current smoker | 0 | 1 | ||||
| years (avg) | 8.1 | 8.3 | ||||
There were no significant age differences in PTSD subjects (mean 47.5 years; SD=10.7) and the combat controls (mean 41.3 years; SD=10.8). The combat control population consisted of 5 Gulf War and 3 Vietnam veterans, 2 Gulf War veterans and 6 Vietnam veterans participated in the PTSD group. All PTSD patients were rated for baseline PTSD symptoms with the Clinician Administered PTSD Scale (CAPS) (46). Mean score on the CAPS in this group was 75.5 (27.7 SD). The CAPS was not assessed in the control group since they did not meet criteria for PTSD. Both groups did not differ in their olfactory acuity (PTSD mean UPSIT= 32.8; SD= 5.3 combat controls mean UPSIT=35.3; SD=3.2).
Subjects with PTSD had a pattern of comorbidity that was similar to prior studies of PTSD from our group and others. In the PTSD group, 6 patients (75%) fulfilled criteria for a past history of major depression and 2 (25%) for current major depression, based on the SCID interview. One patient (13%) had a history of current and lifetime dysthymia. In all cases the onset of the affective disorder occurred after the onset of PTSD. One patient (13%) fulfilled criteria for current and lifetime history of panic disorder with agoraphobia. Two patients (25%) had current and lifetime generalized anxiety disorder, one patient (13%) had current and lifetime social phobia. Three patients (38%) fulfilled criteria for a past history of alcohol dependence, two (25%) for a past history of opiate dependence, two (25%) for a past history of marijuana dependence and two (25%) for a past history of cocaine dependence. No patients had a current history of alcohol or substance abuse or dependence. Four PTSD patients were taking antidepressant medication. In the control group two subjects were taking anti-inflammatory drugs.
The emotional valence of a smell is embedded in its hedonic judgment or hedonic tone, which can be assessed by asking questions about the ‘pleasantness’ of a smell. In order to assess The specificity of diesel as a trauma-related smell within the group of PTSD veterans, we compared this probe with three non-war related probes: a common positive hedonic smell, a negative hedonic smell and a neutral smell. Norm scores of hedonic tone of smells of the UPSIT were used to guide the choice of olfactory probe. Subjects were assessed at baseline and immediately after completion of each scan on:
Subjective Distress, using the Subjective Units of Distress Scale, ranging from 0 to 100%,
Rating of pleasantness of the smell, using a rating scale ranging from −10 to +10,
Emotional response ratings (e.g. talkative, happy, drowsy, nervous, sad, calm), 17 items ranging from 1 to 5 (23;28;30),
PTSD symptoms, using the PTSD Symptom Scale (e.g. flashback, startle, hypervigilant, out of body, distant from people, difficulty concentrating), 18 items on a scale ranging from 1 to 5,
Anxiety level, using the Panic Attack Symptoms Scale, PASS (e.g. nausea, sweating, numbness, dizziness, shortness of breath, headache), 16 items on a scale ranging from 1 to 4,
Dissociative Symptoms, using the Clinician Administered Dissociative States scale, CADSS (47), 20 items on a scale ranging from 1 to 4.
To assess if stress during the experiment was a confounder for regional cerebral blood flow, salivary cortisol samples were collected at baseline and after each scan (0, 10, 20, 30, 40, 50, 60, 70, and 80 minutes). Saliva samples were collected using Salivette collection and stored at 70C. Salivette tubes were centrifuged, and saliva was analyzed for cortisol using an 125 I immunoradiometric assay kit from Diagnostic Products Corporation (Los Angeles, CA).
Olfactory probes
Based on interviews with clinicians and Vietnam and Gulf war veterans with PTSD at the West Haven VA, and the well-known prominent role of diesel in these wars, this smell was selected as a traumatic smell (48). We balanced the trauma-related impact of diesel with a neutral, a common positive and a negative hedonic smell. These probes were selected on the basis of their hedonic tone as reported in the manual of the UPSIT; the common positive hedonic smell was vanilla (vanillin); the negative hedonic smell was sulfur (dimethyl disulphide or hydrogen sulfide, H2S). The neutral smell was odorless air (no smell). All smells were presented in odorous solutions, except for odorless air, which was presented as air in an empty bottle. For diesel we used diesel fuel. All smells were conserved in 15 ml glass flasks (opening diameter, 1.7 cm; height, 5.8 cm; filled with 5 ml of liquid), with a screw cap. Upon opening of the bottle the smells were presented birhinally by presenting the flasks for the duration of 60 seconds at a distance of around 5 cm from the nostrils. The order in which the smells were presented was randomized; the order of the diesel probe however was modified so that it was never presented as either the first or second smell. The subjects were not informed about any of the labels of the smells; they did not know what they smelled, or that each smell would be presented twice. After the experiment was completed and when they were outside the scanner, they were informed about the label of the smells.
Scanning Procedure
All subjects underwent positron emission tomographic (PET) measurement of cerebral blood flow as well as psychophysiological measurement of heart rate during smell exposure, on a single day. Psychometric assessment was assessed in between each scan. Heart rate was measured continuously every five seconds using a Polar Vantage heart rate recording device (Woodbury, NY). The Polar Vantage heart rate recording apparatus was moistened with water to facilitate recording and strapped around the subject’s chest for direct measurement of heart rate. Heart rate data was transmitted from the recording device to a Polar Vantage recording device worn on the subject’s wrist. Following the study session the data was downloaded to a PC for analysis. Heart rate over five second intervals was compared between a baseline period 10 minutes before the onset of the first session (after the subject had been positioned in the scanner) and over 5 second intervals during the period of smell exposure.
PET imaging was performed with a Posicam 6.5 PET Camera (Positron Corp., Houston, TX) (in plane resolution after filtering, 6-mm full width at half maximum (FWHM)). The subject was placed in the scanner with the head in a holder to minimize motion and positioned with the canthomeatal line parallel to an external laser light. An intravenous line was inserted for administration of [15O]H2O. Following positioning within the camera gantry, a transmission scan of the head was obtained by using an external 67Ga/68Ge rod source, in order to correct emission data for attenuation due to overlying bone and soft tissue. Baseline ratings were then collected. Subjects received a bolus of 30 mCi of [15O]H2O intravenously, for each of the eight scans. This was followed 10 seconds later by a PET scan acquisition and smell exposure, both at same time for one minute. The top lid of the bottle was unscrewed from the bottle at the time the instruction was given.
The onset of the PET scan acquisition was timed to correspond to the point of maximum rate of increase in uptake of tracer into the brain. With the bolus injection method of [15O]H2O (which has a half-life of 110 seconds), tracer peaks at 10 seconds, with 90% of counts obtained in the first 60 seconds after peak, which is the time when the smells were presented. During scanning subjects were exposed to one of the four olfactory probes. For the minute they were exposed subjects were asked ‘Close your eyes, breathe normally and try to sense the smell that is presented in front of your nose’. Each subject was then exposed to two sets of 4 smells. After each smell exposure the top was placed on the bottle and taken away from the subject. Subjects then underwent behavioral ratings with the abovementioned scales. Nowhere during the experiment were the subjects asked to identify the odorants, nor were they told what the odorants were. No information was given about the odorants that they were going to be exposed to prior to the study, except the number of smells they would be exposed to.
Image Processing and Statistical Analysis
Images were reconstructed and analyzed on a SunSparc workstation through use of statistical parametric mapping (spm96). Images for each patient set were realigned to the first scan of the study session. The mean concentration of radioactivity in each scan was obtained as an area-weighted sum of the concentration of each slice and was adjusted to a nominal value of 50 ml/min/100 g. The data underwent transformation into a common anatomical space and were smoothed with a three-dimensional Gaussian filter to 16-mm full FWHM before statistical analysis.
Ratings of subjective distress, hedonic tone, and other behavioral measures (distress, hedonic tome, emotional responses, PTSD symptom scores, anxiety symptoms, and dissociative symptoms) were compared for PTSD and comparison subjects through use of paired samples T-tests. Repeated measures ANOVA with Duncan’s Multiple Range Test was used to compare the cortisol response during the experiment to smell exposure between the groups. Regional cerebral blood flow data were analyzed using spm96 (49–51) with global blood flow considered as a confounding covariate with image data sets in which the values assigned to individual voxels correspond to t statistic. Regional cerebral blood flow was compared in patients and controls between smell exposures. Analyses were also performed to examine the interaction of group by condition (e.g. greater increases with odorless air versus diesel in PTSD versus control subjects, or greater decreases with vanilla versus H2S in PTSD patients versus control subjects). Statistical images were displayed with values of Z score units >3.09 (p<0.001) and clusters of greater than 65 contingent significant voxels. Areas of activation were identified using standard stereotaxic coordinates based on the atlas of Talairach and Tournoux (52).
RESULTS
In 6 of the 8 PTSD patients, exposure to diesel resulted in a flashback (as measured with the PTSD Symptom Scale), causing distress, increased fearfulness and anxiety. After study completion, one of the participants reported after study completion that when he had a nightmare he could smell ‘the village’ where he was at that time. The smell of diesel, ‘brought back’ that same image. This also occurred in 1 of the control subjects. Diesel was rated as an unpleasant smell in the PTSD group; this differed significantly from the rating of the control group (p=.021), in which diesel was rated as slightly less unpleasant than vanilla. Both groups rated H2S as an equally distressing smell, causing more distress than exposure to any of the other conditions. A significant negative correlation (p = 0.00; r = −0.63) was found between the hedonic tone and distress for all smells in the PTSD group, this was not significant for the combat controls. Hedonic tone was significantly correlated with all behavioral assessments (PTSD symptoms, emotional symptoms, dissociation) (p = 0.001; r = 0.50); this was only the case for emotional symptoms in the control group (p = 0.001; r = 0.51). For the three non-neutral smells, PTSD Symptom Scale score, CADSS and PASS scores were higher in the PTSD group. There was a wide range in responses in the PTSD patients on all behavioral assessments. An overview of the scores on psychometric assessments is displayed in table 2. The PTSD group expressed high levels of subjective distress as measured by the SUDS, with significant differences on odorless air, diesel and H2S. The average maximum distress of H2S in the control group was half of the maximum distress in the PTSD group. PTSD subjects were very sensitive to exposure to any novel stimulus. Diesel was confirmed to have a significant different hedonic tone when both groups were compared.
Table 2.
Differences in distress, hedonics, and behavioral assessments after smell exposure between PTSD and Combat
| PTSD | Combat Controls | ||||
|---|---|---|---|---|---|
| Avg | Stdev | Avg | Stdev | p | |
| Distress (SUDS) | |||||
|
| |||||
| baseline | 23.1 | 25.5 | 6.3 | 7.4 | ns |
| neutral | 22.5 | 19.6 | 5.6 | 7.8 | 0.04 |
| vanilla | 22.5 | 22.5 | 10.0 | 9.3 | ns |
| diesel | 43.8 | 29.7 | 7.8 | 5.6 | 0.01 |
| sulfur | 52.2 | 28.2 | 23.8 | 20.1 | 0.04 |
|
| |||||
| Hedonic Tone (minus 10 – 10) | |||||
|
| |||||
| baseline | 0.5 | 5.0 | 0.0 | 0.0 | ns |
| neutral | 0.1 | 1.5 | 1.1 | 1.4 | ns |
| vanilla | −0.4 | 4.0 | −0.7 | 2.0 | ns |
| diesel | −4.6 | 2.8 | −0.9 | 2.9 | 0.02 |
| sulfur | −6.6 | 2.7 | −5.3 | 2.9 | ns |
|
| |||||
| Emotional response | |||||
|
| |||||
| baseline | 1.5 | 1.2 | 0.4 | 1.1 | ns |
| neutral | 1.3 | 1.8 | 0.7 | 1.3 | ns |
| vanilla | 5.6 | 10.7 | 0.5 | 0.8 | ns |
| diesel | 8.3 | 14.3 | 1.0 | 1.3 | ns |
| sulfur | 11.3 | 21.1 | 1.6 | 1.9 | ns |
|
| |||||
| PTSD symptom Scale | |||||
|
| |||||
| baseline | 18.8 | 7.9 | 0.8 | 1.5 | 0.00 |
| neutral | 8.7 | 7.7 | 1.5 | 2.5 | 0.03 |
| vanilla | 12.4 | 11.1 | 2.7 | 4.2 | 0.04 |
| diesel | 22.0 | 20.2 | 0.9 | 1.7 | 0.01 |
| sulfur | 30.6 | 18.1 | 2.7 | 3.0 | 0.00 |
|
| |||||
| Dissociative Symptoms (CADSS) | |||||
|
| |||||
| baseline | 10.4 | 13.9 | 0.4 | 0.7 | ns |
| neutral | 6.8 | 8.9 | 0.1 | 0.2 | ns |
| vanilla | 5.9 | 9.3 | 0.4 | 1.1 | ns |
| diesel | 9.4 | 11.9 | 0.1 | 0.2 | 0.04 |
|
| |||||
| sulfur | 9.5 | 16.4 | 0.9 | 2.3 | ns |
|
| |||||
| Panic Attack Symptom Scale (PASS) | |||||
|
| |||||
| baseline | 1.5 | 1.2 | 0.5 | 0.80 | ns |
| neutral | 0.8 | 1.5 | 1.0 | 1.28 | ns |
| vanilla | 5.6 | 10.7 | 1.6 | 1.83 | ns |
| diesel | 8.3 | 14.3 | 0.4 | 1.13 | ns |
| sulfur | 11.3 | 21.0 | 0.7 | 1.31 | ns |
Veterans with PTSD responded with significant increased intrusions and fearfulness following exposure to diesel. Both diesel and H2S caused intrusive memories and flashbacks in the PTSD group, whereas in the control group this was only the case in one patient, who reported a mild flashback with diesel. The rating of PTSD symptoms was in line with the other emotional rating scales. H2S caused the largest increase of PTSD symptoms. The control group did not show this (p < .05). They did not report an increase in PTSD related symptoms with any smell, irrespective of the hedonic tone. The same was found for the Panic Attack Symptoms Scale in this group. PTSD subjects scored significantly more panic symptoms than controls on vanilla, diesel and H2S. Their score on the CADDS was likewise (p = .044), there was a high score at baseline, and a slight drop of dissociative symptoms on vanilla exposure. The control group did not endorse items of dissociation.
The differences between diesel and odorless air for the two groups are listed in table 3, this table points to specific differences in fearfulness, anxiety and decrease of mellow feeling. This table demonstrates high PTSD symptoms, high anxiety symptoms, dissociation, Note the differences on the items of the PASS, CADDS, hedonic Tone and SUDS. The emotional items that are reported significantly between these smells significant items are fearfulness, anxiousness, and decrease in mellow feeling. None of these parameters were significant in the control group.
Table 3.
Differences between exposure to diesel and odorless air in PTSD and combat controls
| Neutral Air | Diesel | p | ||||
|---|---|---|---|---|---|---|
| Avg | Stdev | Avg | Stdev | |||
| PTSD | PTSD Symptoms Scale | 19.7 | 13.6 | 39.3 | 41.9 | ns |
|
| ||||||
| PASS | 1.3 | 1.79 | 8.31 | 14.2 | ns | |
|
| ||||||
| Emotional Response | ||||||
|
| ||||||
| fearful | 0.62 | 1.09 | 1.81 | 1.6 | 0.049 | |
| anxious | 0.18 | 0.37 | 1.75 | 1.66 | 0.044 | |
| mellow | 0.93 | 0.82 | 0.12 | 0.35 | 0.035 | |
|
| ||||||
| CADDS | 6.81 | 8.9 | 9.37 | 11.9 | ns | |
|
| ||||||
| Hedonic Tone | 10.1 | 1.45 | 5.43 | 2.77 | 0.006 | |
|
| ||||||
| SUDS | 22.5 | 19.6 | 43.1 | 29.7 | 0.002 | |
|
| ||||||
| Combat Controls | PTSD Symptoms Scale | 3 | 5.1 | 1.8 | 3.5 | ns |
|
| ||||||
| PASS | 0.68 | 1.25 | 1 | 1.28 | ns | |
|
| ||||||
| Emotional Response | ||||||
|
| ||||||
| fearful | 0 | 0 | 0 | 0 | ns | |
| anxious | 0 | 0 | 0.06 | 0.17 | ns | |
| mellow | 0.12 | 0.35 | 0.12 | 0.35 | ns | |
|
| ||||||
| CADDS | 0.12 | 0.23 | 0.06 | 0.17 | ns | |
| Hedonic Tone | 11.06 | 1.4 | 9.12 | 2.91 | ns | |
| SUDS | 5.62 | 7.76 | 7.81 | 5.5 | ns | |
After study completion subjects were asked to retrospectively report which smells they had been exposed to. Odorless air was identified by 14 subjects, as ‘I smelled nothing’, in 7 PTSD and 7 combat controls). Vanilla was identified by 8 subjects (4 PTSD, 4 combat controls). H2S was correctly identified as ‘rotten eggs’ in all cases. Diesel was correctly labeled by 6 of the PTSD patients and 5 of the control subjects. Six of the subjects reported correctly that they had smelled all smells twice (3 PTSD and 3 combat controls).
Changes in heart rate (HR) compared to baseline were not significant across smells, except for diesel exposure (p = 0.044; r = 0.721). There also was a trend to an increase in systolic blood pressure in both groups comparing vanilla to diesel and H2S. The combat controls showed the largest increase in systolic blood pressure, when changes were compared to baseline. The same trend was found for PTSD subjects. Diastolic blood pressure was also higher in PTSD subjects, and changed most when compared to baseline in the control group. Diastolic blood pressure showed the largest increase in the diesel condition in the PTSD subjects (data not shown).
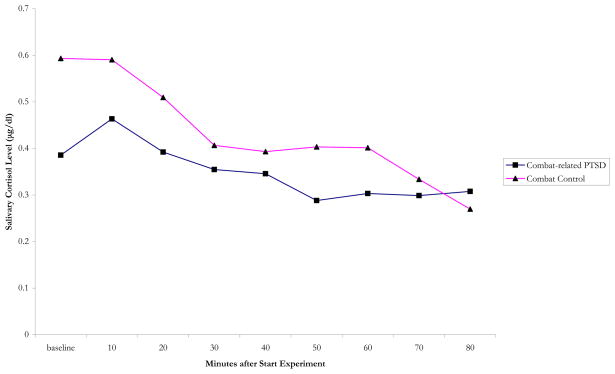
Cortisol was assessed every 10 minutes in between scans. Since the experiment was stressful, this served as an index of stress. The experimental procedure did not result in increased cortisol levels. Salivary cortisol dropped by an average 20% in PTSD patients (t = 1.69; df = 7; ns), compared to 54% in combat controls (t = 2.54; df = 7; p = 0.038) (no significant time by diagnosis interaction) (figure 1).
Figure 1.
Salivary Cortisol during the PET olfaction experiment in combat-related PTSD and combat controls.
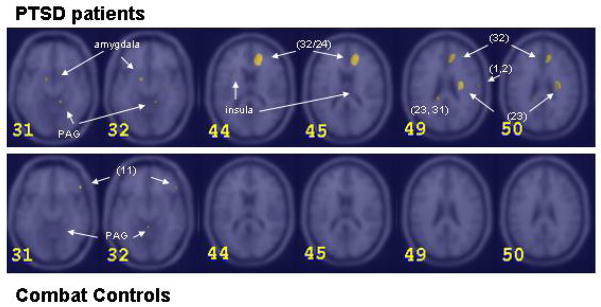
When analyzing rCBF changes on smell exposure in the two groups, a robust difference in general increase was found especially for diesel and H2S. Combat controls however showed less rCBF increases in comparison to PTSD patients upon exposure to these two smells. PTSD patients showed activation of left limbic structures during exposure to all three smells: vanilla, diesel and H2S. They demonstrated increased blood flow in the left hippocampus with vanilla, in left amygdala with diesel and in both left amygdala and hippocampus and right hippocampus and insula with H2S. In H2S the regional increase was most widespread. Left amygdala/insula involvement was strongest for H2S, less for diesel and least strong for vanilla.
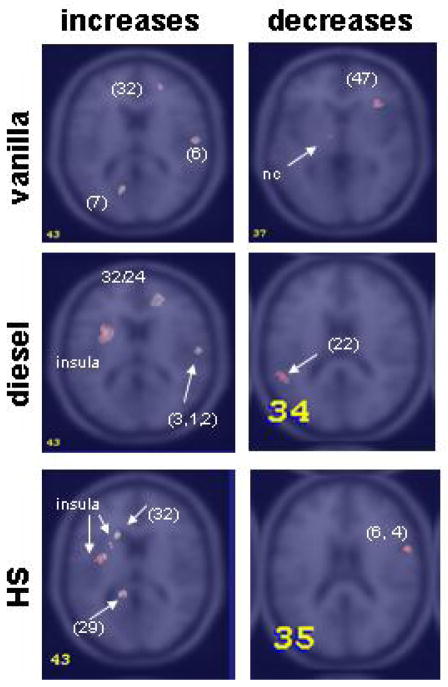
When combat controls were analyzed for differences in regional activation for each smell and were compared with the PTSD group, vanilla showed main involvement in three areas: left amygdala, right visual association cortex, and right lateral PFC. Limbic involvement in the combat control group with vanilla was found more anterior than it was with this smell in the PTSD group. Vanilla in this group showed no medial PFC involvement. Diesel did not involve any limbic activity in the combat controls, no PFC activity; only a left posterior cingulate area of activation was seen here. H2S exposure in this group resulted in large areas of activation in insula, similar to the PTSD group, which included hippocampus and amygdala. Similar to vanilla, H2S also increased rCBF in lateral frontal cortex in combat controls, with similar intensity as vanilla. Figure 2 and 3 are a summation of PET scans for comparisons of diesel in the two groups (figure 2), and the greater increases between the groups respectively (figure 3). The strength of the alterations in rCBF upon smell exposure are tabulated in table 4. Insular activity was paralleled with activity in posterior cingulate with H2S and diesel. Visual association cortex showed an increase for vanilla, less for diesel, no increase was found for H2S. Diesel exposure elicited a large increase in rCBF in right medial (orbito-/)prefrontal cortex (PFC), as was found for vanilla, but in a smaller area. H2S exposure did not lead to an increase in medial PFC, here we observed rCBF increase in the lateral PFC. The z scores and coordinates for activation patterns in PTSD and combat controls are listed in table 5.
Figure 2.
Areas of increased blood flow in diesel and PTSD patients (top row) and combat controls (bottom row) in different horizontal sections through the brain (slice 31 and 32: z= −12; slice 44 and 45: z=6; slice 49 and 50: z=26). Brain sections were chosen to illustrate the relevant activations. Between brackets are Brodman areas. PAG= periaquaductal grey. (Z Score >3.09, p<0.001)
Figure 3.
Areas of greater increases (left column) and greater decreases (right column) in blood flow in vanilla (top), diesel (middle) and hydrogen sulfide (bottom) in PTSD compared to combat controls (z=0 and z=14). Brain sections were chosen to illustrate the relevant activations. Precise locations of the peaks for the activity shown are given in the corresponding table (Table 5). Between brackets are Brodman areas. nc=nucleus caudatus (Z Score >3.09, p<0.001)
Table 4.
Regional changes (increase and decrease) in activation in smell between PTSD and combat controls*
| VANILLA | DIESEL | SULFUR | ||||
|---|---|---|---|---|---|---|
| PTSD | CC | PTSD | CC | PTSD | CC | |
| Olfactory regions | ||||||
|
| ||||||
| piriform cortex | – | – | – | – | – | – |
| amygdala | – | ↑ | ↑↑ | – | ↑↑ | ↑ |
| hippocampus | ↑ | ↓ | – | – | ↑ | – |
| insula | ↓ | ↓↓ | ↑↑/↓ | – | ↑↑↑ | ↑ |
|
| ||||||
| (Pre-/orbito-) frontal regions | ||||||
|
| ||||||
| medial PFC (24, 25, 32, 10) | ↑↑ | – | ↑↑↑ | – | ↑↑↑ | ↑ |
| lateral PFC (11, 45, 47/12) | – | ↑↑ | ↓↓ | ↑/↓ | ↑ | ↑ |
|
| ||||||
| Posterior cingulate (23, 29, 30) | ↑ | ↓↓ | ↑↑ | – | ↑↑ | – |
|
| ||||||
| Temporal regions (22, 39, 42) | ↓ | ↓ | – | – | ↑/↓↓ | ↓↓ |
|
| ||||||
| Parietal regions | ||||||
| precentral gyrus (4,6) | – | ↓ | – | – | – | – |
| postcentral gyrus (1, 2, 3) | – | ↓ | ↑/↓ | – | – | – |
| other (40) | – | ↑/↓ | – | – | – | – |
|
| ||||||
| Occipital regions | ||||||
|
| ||||||
| lingual/(pre)cuneus (7, 17, 18, 19, 31) | ↑ | ↑/↓↓ | – | ↑ | ↑ | ↑/↓ |
|
| ||||||
| Cerebellum | ↓ | ↓↓ | ↓ | ↑/↓↓ | ↓↓ | ↓↓ |
arrows indicate increases, decreases, or no activation (-).
Number of arrows indicates strength of activation
Table 5.
Areas of Greater Increased and Decreased Blood Flow in Smell Exposure versus Neutral in PTSD compared to Combat Controls
| Increased Blood Flow | Decreased Blood Flow | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Z | Talairach Coordinates | Z | Talairach Coordinates | ||||||
| Score* | x | y | z | Brain Region | Score* | x | y | z | Brain Region |
| a. Vanilla | |||||||||
|
| |||||||||
| 2.95 | 4 | −78 | 38 | R Cuneus (7) | 2.94 | −18 | −84 | −28 | L Cerebellum |
| 2.9 | 56 | −14 | 14 | R Precentral Gyrus (6) | 2.89 | 34 | 28 | −2 | R Lat Prefrontal Cortex (47) |
| 2.4 | 32 | −12 | 28 | 2.44 | 46 | 30 | −10 | ||
| 2.86 | −32 | −14 | −18 | L Hippocampus | 2.83 | −46 | −64 | 36 | L Supramarginal Gyrus (39) |
| 2.73 | −12 | −68 | 6 | L Lingual Gyrus (18) | 2.77 | 28 | 10 | 32 | R Gyrus Cinguli (24) |
| 2.5 | 22 | 48 | 4 | R Gyrus Cinguli (32) | 2.5 | −12 | −10 | 0 | L Caudate |
| 2.5 | −28 | −56 | 20 | L Medial Temporal Gyrus (39) | |||||
| 2.45 | −22 | −66 | −50 | L Cerebellum | |||||
|
| |||||||||
| b. Diesel | |||||||||
|
| |||||||||
| 3.51 | −30 | 8 | 8 | L Insula | 3.28 | −16 | −84 | −26 | L Cerebellum |
| 2.76 | 18 | 42 | 12 | R Gyrus Cinguli (32, 24) | 3.26 | 2 | −60 | 50 | R Cuneus (17, 19, 31) |
| 2.41 | 30 | −10 | 26 | R Postcentral Gyrus (1, 2, 3) | 2.88 | 0 | −86 | 8 | |
| 2.7 | 58 | −14 | 14 | R Insula | 2.82 | −14 | −82 | −2 | |
| 2.64 | 16 | −44 | −18 | R Cerebellum | 2.4 | 8 | −78 | 28 | |
| 2.63 | 0 | 10 | 2 | Fornix | 2.82 | −2 | −44 | −10 | L Cerebellum |
| 2.37 | −34 | −42 | 30 | L Gyrus Supramarginalis | 2.82 | −52 | −46 | 14 | L Sup Temporal Gyrus (22) |
| 2.49 | −66 | −20 | 30 | L Postcentral Gyrus (1, 2, 3) | |||||
|
| |||||||||
| c. Hydrogen Sulfide (H-2 S) | |||||||||
|
| |||||||||
| 3.11 | −20 | 16 | 14 | L Anterior Cingulate (32, 24) | 3.03 | −38 | −82 | −32 | L Cerebellum |
| 3.09 | −14 | −40 | 18 | L Posterior Cingulate (29) | 3.00 | 56 | 6 | 16 | R Precentral Gyrus (6) |
| 2.95 | 16 | −50 | −22 | R Cerebellum | 2.69 | 0 | −84 | 10 | Cuneus (17) |
| 2.94 | −34 | −4 | 8 | L Insula | |||||
| 2.78 | 0 | 6 | 2 | Fornix | |||||
| 2.67 | 32 | −16 | 18 | R Insula | |||||
| 2.66 | −24 | 12 | 12 | ||||||
| 2.54 | 34 | −8 | −2 | ||||||
| 2.61 | 48 | −16 | −40 | R Inferior Temporal Gyrus (20) | |||||
| 2.50 | 54 | −10 | −48 | ||||||
| 2.51 | −18 | −50 | −26 | L Cerebellum | |||||
| 2.49 | −32 | −10 | −18 | L Amygdala/Hippocampus | |||||
| 2.4 | −34 | −48 | 26 | L Inferior Parietal Lobule (40) | |||||
Z Score>3.09, p<.001
DISCUSSION
The present study demonstrated that diesel served as a strong reminder of emotional experiences in veterans with combat -related PTSD. The patients rated diesel as significantly more unpleasant and distressing, resulting in increased PTSD symptoms and anxiety in PTSD patients compared to combat controls. PTSD patients (but not non-PTSD veterans) demonstrated more rCBF alterations to any smell. Both hedonic tone and reported distress were different in comparison between odorless air and diesel in PTSD, but not in combat controls, which confirmed the specific nature of diesel as a probe for emotional responsivity in PTSD. The responses varied in range, perhaps due to secondary consequences of labeling and other attributional difficulties. Diesel exposure in PTSD patients resulted in activity in left amygdala, insula and right PFC and ACC (BA 24, 25, 32, 10), which was absent in the combat controls. Exposure to H2S showed similar regional changes like diesel, although the differences between patients and controls were less pronounced. Vanilla and diesel showed similar increases in brain blood flow in the right anterior and posterior cingulate gyrus and medial PFC, although activity was stronger with diesel. Diesel differed in inducing a decrease in rCBF in lateral PFC (BA) 22, 47) in the PTSD group only. Vanilla response additionally differed from diesel in an increase in lingual and visual cortex, and in left hippocampus, whereas diesel was correlated with reduced visual cortical blood flow (BA 31, 7, 17, 18). H2S exposure induced an increase in the lateral PFC (BA 10, 32, 24), which was similar in PTSD and controls.
Our data are in line with the notion that PTSD patients in general are more sensitive to any novel stimulus in comparison with a control population (stress sensitized), and are specifically responsive to traumatic reminders (e.g. diesel) (fear-conditioned) (53). This is in line with other studies showing a relation between distress, hedonic tone, and the induction of emotional memories (44). Unpleasant odors have psychophysiological effects, e.g. in inducing heart-rate acceleration during both a smelling trask and a pleasantness judgment (54), or biological effects, e.g. in a study with newborns exposed to smells it was seen that they responded positively to vanilla, as shown by an increase in hemoglobin oxygenation (55). Studies also report on the capacity of hedonic tone to induce mood-related cognitions (56). We found vanilla to have a positive (reducing) effect on distress, heart rate, and dissociative response in the PTSD group. Vanilla has also been associated with involvement in positive/approach-related emotion and is related to relative left frontal activation (57). When healthy females were exposed to vanillin, bilateral amygdala and piriform cortex activity was found (58). We could not find this in either of our two groups of interest.
Several PET studies in healthy patients have yielded different levels of activity during passive smelling; in the piriform cortex, varying from no activity (4) to strong bilateral activity (8), amygdala and insula (11;59–62), unilateral right orbitofrontal (6;8;63), prefrontal cortical regions, and the cerebellum (64).
The piriform cortex has long been considered to be the primary olfactory cortex because it is the largest area that receives direct input from the olfactory bulb, the structure that monosynaptically relays input from olfactory receptor neurons. However, physiological and anatomical studies suggest that this cortical area is organized in a fundamentally different way than the primary cortical areas for nonchemical senses. Previous morphological studies have also shown that the piriform cortex projects to areas that are thought to play a role in mediating functions related to behavior (prefrontal cortex), assessing the emotional or motivational significance of sensory cues (amygdala), multisensory association, and memory (enthorhinal and perirhinal cortex) (65). The piriform cortex plays a role in linking representations of odorant structure that constitute the olfactory code, and in associating olfactory and other forms of information. In our study, we did not observe blood flow alterations in the piriform cortex. This may be explained by the passive smelling instruction, where no demand for recognition was given. In addition it may be explained by the engagement of limbic structures in particular at an early stage in the signal processing (66). Our finding of increased rCBF in the amygdala is in line with its role in the processing of emotional salient stimuli. Earlier studies in olfaction demonstrated that the amygdala participates in both hedonic and emotional processing of olfactory stimuli (Zald and Pardo, 1997). Absence of rCBF changes in the amygdala with diesel in the combat controls was in line with the notion that it did not cause PTSD symptoms with associated fear and distress. Diesel only had a slightly negative hedonic tone, failed to lead to emotional responses, and did not induce dissociative symptoms or anxiety in this group. Scores in the PTSD group were significantly different on all these assessments. We found similar alterations in rCBF in the insula as well as right orbitofrontal cortex with both vanilla and diesel exposure. While the hedonic tone and behavioral data were different for vanilla and diesel, rCBF alterations in right medial PFC involvement was still seen with diesel. Involvement of this region has been described in relation to ‘rightness of a stimulus’ or familiarity (67).
Few studies have looked into the neocortical projections of the human olfactory pathway and its functional organization (identification, hedonicity and familiarity), and a precise functional organisation has not been characterized. In this respect at best it can be stated that brain regions mediating emotional processing are differentially activated by odor valence, providing evidence for a close anatomical coupling between olfactory and emotional processes (Gottfried et al., 2002). The OFC and mPFC both play a role in cognitive processing (e.g. judgement of hedonicity vs detection itself and judgement of intensity and familiarity); emotional tasks with cognitive demands have been demonstrated to involve the ACC and insula. The processing of ‘(un)pleasantness’ as a basic emotion is regulated by the ACC. Both OFC and regions of mPFC contribute to affective behavior. In addition, aspects of odor processing are lateralized depending on the type of olfactory task. Right OFC activity was highest during familiarity judgments, whereas left OFC increased significantly during hedonic judgments (68). Our results showed an increase in right mPFC (BA 10, 24, 32) in vanilla and diesel, which was not seen in H2S exposure in PTSD. H2S exposure showed increased left insular activity in this condition. A decrease in blood flow was found in the lateral prefrontal cortex (BA 45, 47) in PTSD, but not in the control group, except for a small increase in BA 47 with diesel exposure. Our data also show enhanced involvement of the left anterior cingulate cortex (BA 32, 24) in concordance with distress for diesel and H2S. Different patterns of activations are seen in our two experimental groups, predominantly in the insula and cingulate gyrus (BA 32, 24). The involvement of this area on increased blood flow can best be understood in relation to emotional processing (43).
Interestingly, we found decreased left cerebellar rCBF in all smells for all subjects. This is different with what has been found in other studies (64);(6;69) (63) and needs further exploration in reference to the model in which the cerebellum coordinates acquisition of sensory information (70).
Yet, our data as well as other recent neuroimaging studies of olfactory induction (59;71–74) show discrete overlap with data from studies using script driven imagery (28;75–78), and combat slides and sounds (79) with PTSD.
Some limitations have to be considered in interpreting our results. These results are based on a small sample of 8 patients and 8 controls and are the first study to report on olfaction as a traumatic reminder. Exposing the participants to two sets of smells, and presenting each smell twice in random order may have introduced an error, since the second exposure participants may remember the smell from an earlier run, thereby introducing a familiarity process (‘I have smelled this odor before’), that may induce brain activity that did not occur when first exposed to the smell when all smells were novel. In our results brain activation was summed for each smell and then averaged over the group. It may have been better to differentially look at the brain images in first and second run. Another limitation is that the procedure of odor exposure was not optimally standardized. We do not know whether differences in concentration and intensity (‘how much’) of the smells subjects were exposed to could have contributed to changes in rCBF. The use of devices such as squeeze bottles or sophisticated olfactometers (80) could have eliminated this confound. A caveat in smell experimentation is the bimodality of aversive odorants. Vanilla is considered to be a unimodal odorant, meaning that it induces the olfactory cortex solely, without trigeminal nerve involvement (Savic et al., 2002). Both diesel and H2S can be considered to induce trigeminal activity, and have implications for recruitment of different pathways in relation to the signal transducing cranial nerves. However, the differences in our two populations cannot be fully explained by this. Lastly, in this study the data were not co-registered with a magnetic resonance image (MRI) of the subjects. PET-MRI coregistration would have enabled quantification of rCBF changes to the different smells. Moreover, this would have allowed correlating PET activation patterns with psychometric and behavioral data. Overcoming these limitations can enhance the specificity of rCBF changes in olfactory induced traumatic recall.
Acknowledgments
We thank Charles Greer, PhD for expert advice, Sajid Siddiq, MD and Heather Douglas Palumberi, MS for assistance in data collection and Helen Sayward, MS for image processing. This study was supported NIMH 1R01MH56120-01A1 and a Veterans Administration Career Development Award to Dr. Bremner, and the VA National Center for Posttraumatic Stress Disorder.
References
- 1.Herz RS. Emotion experienced during encoding enhances odor retrieval cue effectiveness. Am J Psychol. 1997;110:489–505. [PubMed] [Google Scholar]
- 2.Maylor EA, Carter SM, Hallett EL. Preserved olfactory cuing of autobiographical memories in old age. J Gerontol B Psychol Sci Soc Sci. 2002;57:P41–6. doi: 10.1093/geronb/57.1.p41. [DOI] [PubMed] [Google Scholar]
- 3.Vasterling JJ, Brailey K, Sutker PB. Olfactory identification in combat-related posttraumatic stress disorder. J Trauma Stress. 2000;13:241–53. doi: 10.1023/A:1007754611030. [DOI] [PubMed] [Google Scholar]
- 4.Dade LA, Zatorre RJ, Jones-Gotman M. Olfactory learning: convergent findings from lesion and brain imaging studies in humans. Brain. 2002;125:86–101. doi: 10.1093/brain/awf003. [DOI] [PubMed] [Google Scholar]
- 5.Zald DH, Pardo JV. Functional neuroimaging of the olfactory system in humans. Int J Psychophysiol. 2000;36:165–81. doi: 10.1016/s0167-8760(99)00110-5. [DOI] [PubMed] [Google Scholar]
- 6.Zatorre RJ, Jones-Gotman M, Rouby C. Neural mechanisms involved in odor pleasantness and intensity judgments. Neuroreport. 2000;11:2711–6. doi: 10.1097/00001756-200008210-00021. [DOI] [PubMed] [Google Scholar]
- 7.Royet JP, Koenig O, Gregoire MC, Cinotti L, Lavenne F, Le Bars D, Costes N, Vigouroux M, Farget V, Sicard G, Holley A, Mauguiere F, Comar D, Froment JC. Functional anatomy of perceptual and semantic processing for odors. J Cogn Neurosci. 1999;11:94–109. doi: 10.1162/089892999563166. [DOI] [PubMed] [Google Scholar]
- 8.Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339–40. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]
- 9.Otto T, Cousens G, Herzog C. Behavioral and neuropsychological foundations of olfactory fear conditioning. Behav Brain Res. 2000;110:119–28. doi: 10.1016/s0166-4328(99)00190-4. [DOI] [PubMed] [Google Scholar]
- 10.Zald DH, Donndelinger MJ, Pardo JV. Elucidating dynamic brain interactions with across-subjects correlational analyses of positron emission tomographic data: the functional connectivity of the amygdala and orbitofrontal cortex during olfactory tasks. J Cereb Blood Flow Metab. 1998;18:896–905. doi: 10.1097/00004647-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci U S A. 1997;94:4119–24. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kline NA, Rausch JL. Olfactory precipitants of flashbacks in posttraumatic stress disorder: case reports. J Clin Psychiatry. 1985;46:383–4. [PubMed] [Google Scholar]
- 13.Pointer SC, Bond NW. Context-dependent memory: colour versus odour. Chem Senses. 1998;23:359–62. doi: 10.1093/chemse/23.3.359. [DOI] [PubMed] [Google Scholar]
- 14.Vermetten E, Bremner JD. Olfaction as a traumatic reminder in posttraumatic stress disorder: case reports and review. J Clin Psychiatry. 2003;64:202–7. doi: 10.4088/jcp.v64n0214. [DOI] [PubMed] [Google Scholar]
- 15.Otto T, Cousens G, Rajewski K. Odor-guided fear conditioning in rats: 1. Acquisition, retention, and latent inhibition. Behav Neurosci. 1997;111:1257–64. [PubMed] [Google Scholar]
- 16.Richardson R, Vishney A, Lee J. Conditioned odor potentiation of startle in rats. Behav Neurosci. 1999;113:787–94. doi: 10.1037//0735-7044.113.4.787. [DOI] [PubMed] [Google Scholar]
- 17.Richardson R, Tronson N, Bailey GK, Parnas AS. Extinction of conditioned odor potentiation of startle. Neurobiol Learn Mem. 2002;78:426–40. doi: 10.1006/nlme.2002.4074. [DOI] [PubMed] [Google Scholar]
- 18.Paschall GY, Davis M. Olfactory-mediated fear-potentiated startle. Behav Neurosci. 2002;116:4–12. doi: 10.1037//0735-7044.116.1.4. [DOI] [PubMed] [Google Scholar]
- 19.Vermetten E, Bremner JD. Circuits and systems in stress. I. Preclinical studies. Depress Anxiety. 2002;15:126–47. doi: 10.1002/da.10016. [DOI] [PubMed] [Google Scholar]
- 20.Vermetten E, Bremner JD. Circuits and systems in stress. II. Applications to neurobiology and treatment in posttraumatic stress disorder. Depress Anxiety. 2002;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- 21.Bremner JD, Innis RB, Ng CK, Staib LH, Salomon RM, Bronen RA, Duncan J, Southwick SM, Krystal JH, Rich D, Zubal G, Dey H, Soufer R, Charney DS. Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:246–54. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- 22.Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, Koeppe RA, Fig LM. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–26. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 23.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–16. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–95. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY. Longitudinal mri study of hippocampal volume in trauma survivors with ptsd. Am J Psychiatry. 2001;158:1248–51. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey AM, Thomas RK. The effects of nucleus basalis magnocellularis lesions in Long-Evans hooded rats on two learning set formation tasks, delayed matching-to- sample learning, and open-field activity. Behav Neurosci. 2001;115:328–40. [PubMed] [Google Scholar]
- 27.Bremner JD, Innis RB, Salomon RM, Staib LH, Ng CK, Miller HL, Bronen RA, Krystal JH, Duncan J, Rich D, Price LH, Malison R, Dey H, Soufer R, Charney DS. Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch Gen Psychiatry. 1997;54:364–74. doi: 10.1001/archpsyc.1997.01830160092012. [DOI] [PubMed] [Google Scholar]
- 28.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–95. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carboni AA, Lavelle WG. Ultrastructural characterizations of olfactory pathway neurons in layer II of the entorhinal cortex in monkey. Acta Otolaryngol. 2000;120:424–31. doi: 10.1080/000164800750000685. [DOI] [PubMed] [Google Scholar]
- 30.Polak EH. Mutiple profile-multiple receptor site model for vertebrate olfaction. J Theor Biol. 1973;40:469–84. doi: 10.1016/0022-5193(73)90005-2. [DOI] [PubMed] [Google Scholar]
- 31.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–16. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Macklin ML, Pitman RK. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Arch Gen Psychiatry. 1997;54:233–41. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- 33.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 34.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999;156:575–84. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 35.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 36.Osuch EA, Benson B, Geraci M, Podell D, Herscovitch P, McCann UD, Post RM. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol Psychiatry. 2001;50:246–53. doi: 10.1016/s0006-3223(01)01107-6. [DOI] [PubMed] [Google Scholar]
- 37.Post RM, Weiss SR, Li H, Smith MA, Zhang LX, Xing G, Osuch EA, McCann UD. Neural plasticity and emotional memory. Dev Psychopathol. 1998;10:829–55. doi: 10.1017/s0954579498001898. [DOI] [PubMed] [Google Scholar]
- 38.Hebets EA, Chapman RF. Electrophysiological studies of olfaction in the whip spider Phrynus parvulus (Arachnida, Amblypygi) J Insect Physiol. 2000;46:1441–1448. doi: 10.1016/s0022-1910(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 39.Sakuma K, Kakigi R, Kaneoke Y, Hoshiyama M, Koyama S, Nagata O, Takeshima Y, Ito Y, Nakashima K. Odorant evoked magnetic fields in humans. Neurosci Res. 1997;27:115–22. doi: 10.1016/s0168-0102(96)01138-8. [DOI] [PubMed] [Google Scholar]
- 40.Stellar E, Corbit JD. Neural control of motivated behavior. Neurosci Res Program Bull. 1973;11:296–410. [PubMed] [Google Scholar]
- 41.Dimov D, Popov V. Determination and interdependance of thresholds of perception and identification of the olfactory analyser. JFORL J Fr Otorhinolaryngol Audiophonol Chir Maxillofac. 1973;22:807–13. [PubMed] [Google Scholar]
- 42.Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53:380–7. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 43.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 44.Vernet-Maury E, Alaoui-Ismaili O, Dittmar A, Delhomme G, Chanel J. Basic emotions induced by odorants: a new approach based on autonomic pattern results. J Auton Nerv Syst. 1999;75:176–83. doi: 10.1016/s0165-1838(98)00168-4. [DOI] [PubMed] [Google Scholar]
- 45.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- 46.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 47.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–36. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 48.Stuart JA, Murray KM, Ursano RJ, Wright KM. The Department of Defense’s Persian Gulf War registry year 2000: an examination of veterans’ health status. Mil Med. 2002;167:121–8. [PubMed] [Google Scholar]
- 49.Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 50.Friston KJ. In: Statistical Parametric Mapping in Neuroimaging: Technical Foundations. Thatcher R, editor. San Diego: Academic Press; 1994. [Google Scholar]
- 51.Bourgeois M, Paty J. Autodysosmophobia and the psychopathology of smell (a propos of 7 cases) Bord Med. 1972;5:2269–86. [PubMed] [Google Scholar]
- 52.Talairach JT. Co-Planar Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 53.Charney DS, Deutch AY, Krystal JH, Southwick SM, Davis M. Psychobiologic mechanisms of posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:295–305. doi: 10.1001/archpsyc.1993.01820160064008. [DOI] [PubMed] [Google Scholar]
- 54.Bensafi M, Rouby C, Farget V, Bertrand B, Vigouroux M, Holley A. Influence of affective and cognitive judgments on autonomic parameters during inhalation of pleasant and unpleasant odors in humans. Neurosci Lett. 2002;319:162–6. doi: 10.1016/s0304-3940(01)02572-1. [DOI] [PubMed] [Google Scholar]
- 55.Bartocci M, Winberg J, Ruggiero C, Bergqvist LL, Serra G, Lagercrantz H. Activation of olfactory cortex in newborn infants after odor stimulation: a functional near-infrared spectroscopy study. Pediatr Res. 2000;48:18–23. doi: 10.1203/00006450-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Ehrlichman H, Halpern JN. Affect and memory: effects of pleasant and unpleasant odors on retrieval of happy and unhappy memories. J Pers Soc Psychol. 1988;55:769–79. doi: 10.1037//0022-3514.55.5.769. [DOI] [PubMed] [Google Scholar]
- 57.Kline JP, Blackhart GC, Woodward KM, Williams SR, Schwartz GE. Anterior electroencephalographic asymmetry changes in elderly women in response to a pleasant and an unpleasant odor. Biol Psychol. 2000;52:241–50. doi: 10.1016/s0301-0511(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 58.Savic I. Imaging of brain activation by odorants in humans. Curr Opin Neurobiol. 2002;12:455–61. doi: 10.1016/s0959-4388(02)00346-x. [DOI] [PubMed] [Google Scholar]
- 59.Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–45. doi: 10.1016/s0896-6273(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 60.Fulbright RK, Skudlarski P, Lacadie CM, Warrenburg S, Bowers AA, Gore JC, Wexler BE. Functional MR Imaging of Regional Brain Responses to Pleasant and Unpleasant Odors. AJNR. 1998;19:1721–1726. [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards DA, Thompson ML, Burge KG. Olfactory bulb removal vs peripherally induced anosmia: differential effects on the aggressive behavior of male mice. Behav Biol. 1972;7:823–8. doi: 10.1016/s0091-6773(72)80174-3. [DOI] [PubMed] [Google Scholar]
- 62.Rottman SJ, Snowdon CT. Demonstration and analysis of an alarm pheromone in mice. J Comp Physiol Psychol. 1972;81:483–90. doi: 10.1037/h0033703. [DOI] [PubMed] [Google Scholar]
- 63.Berglund B, Berglund U, Engen T, Ekman G. Multidimensional analysis of twenty- one odors. Scand J Psychol. 1973;14:131–7. doi: 10.1111/j.1467-9450.1973.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 64.Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, Gabrieli JD, Sullivan EV. Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci. 1998;18:8990–9001. doi: 10.1523/JNEUROSCI.18-21-08990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luskin MB, Price JL. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J Comp Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- 66.Savic I. Brain imaging studies of the functional organization of human olfaction. Neuroscientist. 2002;8:204–211. doi: 10.1177/1073858402008003006. [DOI] [PubMed] [Google Scholar]
- 67.Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- 68.Royet JP, Hudry J, Zald DH, Godinot D, Gregoire MC, Lavenne F, Costes N, Holley A. Functional neuroanatomy of different olfactory judgments. Neuroimage. 2001;13:506–19. doi: 10.1006/nimg.2000.0704. [DOI] [PubMed] [Google Scholar]
- 69.Savic I, Gulyas B, Berglund H. Odorant differentiated pattern of cerebral activation: comparison of acetone and vanillin. Hum Brain Mapp. 2002;17:17–27. doi: 10.1002/hbm.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bower JM, Beermann DH, Gibson JM, Shambes GM, Welker W. Principles of organization of a cerebro-cerebellar circuit. Micromapping the projections from cerebral (SI) to cerebellar (granule cell layer) tactile areas of rats. Brain Behav Evol. 1981;18:1–18. doi: 10.1159/000121772. [DOI] [PubMed] [Google Scholar]
- 71.Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature. 1998;392:282–6. doi: 10.1038/32654. [DOI] [PubMed] [Google Scholar]
- 72.Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, Gervais R. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci. 2000;20:7752–9. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davis RG, Tapp JT. Odor artifacts of an olfactometer evoke a CER in the rat. Percept Mot Skills. 1972;35:931–6. doi: 10.2466/pms.1972.35.3.931. [DOI] [PubMed] [Google Scholar]
- 74.von Bekesy G. Sense organs and their sensitivity. Adv Otorhinolaryngol. 1973;19:1– 30. [PubMed] [Google Scholar]
- 75.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am J Psychiatry. 1999;156:575–84. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 76.Lanius R, Williamson P, Boksman K, Densmore M, Gupta M, Neufeld R, Gati J, Menon R. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52:305. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 77.Labayle J, Hadengue A. Post-traumatic anosmia and its medico-legal implications: occupational consequences. Med Leg Dommage Corpor. 1973;6:343–55. [PubMed] [Google Scholar]
- 78.Payne AP, Swanson HH. The effects of neonatal androgen administration on the aggression and related behavior of male golden hamsters during interactions with females. J Endocrinol. 1973;58:627–36. doi: 10.1677/joe.0.0580627. [DOI] [PubMed] [Google Scholar]
- 79.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–16. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sobel N, Prabhakaran V, Desmond JE, Glover GH, Sullivan EV, Gabrieli JD. A method for functional magnetic resonance imaging of olfaction. J Neurosci Methods. 1997;78:115–23. doi: 10.1016/s0165-0270(97)00140-4. [DOI] [PubMed] [Google Scholar]