Abstract
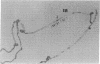
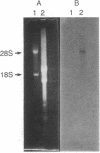
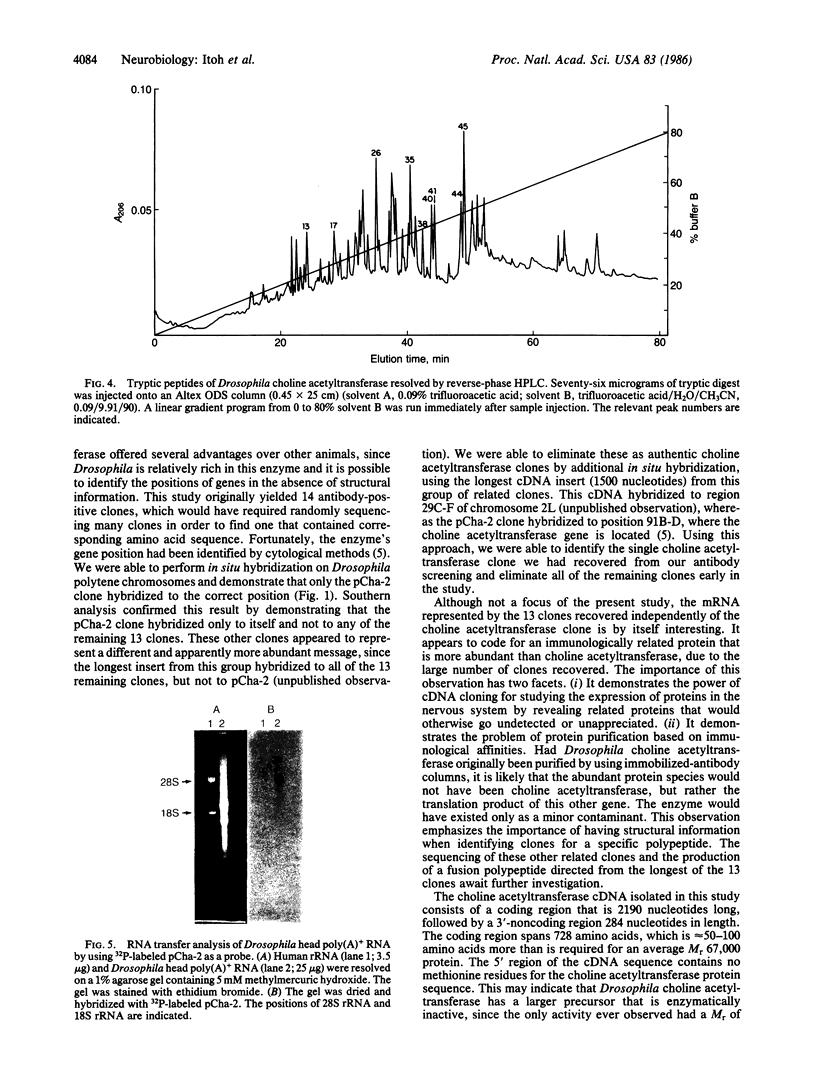
Choline acetyltransferase (EC 2.3.1.6) is the biosynthetic enzyme for the neurotransmitter acetylcholine. To isolate choline acetyltransferase cDNA clones, a cDNA library was constructed from poly(A)+ RNA of Drosophila melanogaster heads, these being one of the richest known sources of the enzyme. By screening the cDNA library with a mixture of three different monoclonal antibodies to Drosophila choline acetyltransferase, we isolated 14 positive clones. Only 1 of these clones was identified to be a Drosophila choline acetyltransferase cDNA clone based on the following evidence. (i) The amino acid sequence deduced from the nucleotide sequence of the cDNA insert completely corresponded to that of several tryptic peptides from choline acetyltransferase. (ii) The cDNA insert hybridized specifically to only the region on Drosophila polytene chromosomes that had been identified as the site of the choline acetyltransferase (Cha) gene by cytogenetic analysis. The cDNA insert consisted of a coding region 2190 nucleotides long, a 3'-noncoding region 284 nucleotides long, and EcoRI linkers. RNA analysis of Drosophila head poly(A)+ RNA with the cDNA insert as a probe showed the choline acetyltransferase mRNA to be approximately equal to 4700 nucleotides long.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Crawford G., Slemmon J. R., Salvaterra P. M. Monoclonal antibodies selective for Drosophila melanogaster choline acetyltransferase. J Biol Chem. 1982 Apr 10;257(7):3853–3856. [PubMed] [Google Scholar]
- Dewhurst S. A., McCaman R. E., Kaplan W. D. The time course of development of acetylcholinesterase and choline acetyltransferase in Drosophila melanogaster. Biochem Genet. 1970 Aug;4(4):499–508. doi: 10.1007/BF00486600. [DOI] [PubMed] [Google Scholar]
- Dewhurst S. A., Seecof R. L. Development of acetylcholine metabolizing enzymes in Drosophila embryos and in cultures of embryonic drosophila cells. Comp Biochem Physiol C. 1975 Jan 1;50(1):53–58. [PubMed] [Google Scholar]
- Hawke D. H., Harris D. C., Shively J. E. Microsequence analysis of peptides and proteins. V. Design and performance of a novel gas-liquid-solid phase instrument. Anal Biochem. 1985 Jun;147(2):315–330. doi: 10.1016/0003-2697(85)90278-7. [DOI] [PubMed] [Google Scholar]
- Hawke D., Yuan P. M., Shively J. E. Microsequence analysis of peptides and proteins. II. Separation of amino acid phenylthiohydantoin derivatives by high-performance liquid chromatography on octadecylsilane supports. Anal Biochem. 1982 Mar 1;120(2):302–311. doi: 10.1016/0003-2697(82)90351-7. [DOI] [PubMed] [Google Scholar]
- Klemm N. Histochemistry of putative transmitter substances in the insect brain. Prog Neurobiol. 1976;7(2):99–169. doi: 10.1016/0301-0082(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Landis S. C., Keefe D. Evidence for neurotransmitter plasticity in vivo: developmental changes in properties of cholinergic sympathetic neurons. Dev Biol. 1983 Aug;98(2):349–372. doi: 10.1016/0012-1606(83)90365-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Patterson P. H. Environmental determination of autonomic neurotransmitter functions. Annu Rev Neurosci. 1978;1:1–17. doi: 10.1146/annurev.ne.01.030178.000245. [DOI] [PubMed] [Google Scholar]
- Salvaterra P. M., McCaman R. E. Choline acetyltransferase and acetylcholine levels in Drosophila melanogaster: a study using two temperature-sensitive mutants. J Neurosci. 1985 Apr;5(4):903–910. doi: 10.1523/JNEUROSCI.05-04-00903.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slemmon J. R., Salvaterra P. M., Crawford G. D., Roberts E. Purification of choline acetyltransferase from Drosophila melanogaster. J Biol Chem. 1982 Apr 10;257(7):3847–3852. [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Yuan P. M., Pande H., Clark B. R., Shively J. E. Microsequence analysis of peptides and proteins. I. Preparation of samples by reverse-phase liquid chromatography. Anal Biochem. 1982 Mar 1;120(2):289–301. doi: 10.1016/0003-2697(82)90350-5. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Fukushima H., Dewji N. N., Wilcox E., O'Brien J. S., Helinski D. R. Chromogenic immunodetection of human serum albumin and alpha-L-fucosidase clones in a human hepatoma cDNA expression library. DNA. 1984 Dec;3(6):437–447. doi: 10.1089/dna.1.1984.3.437. [DOI] [PubMed] [Google Scholar]