Abstract
Selective intra-arterial chemotherapy (IAC) has been adopted by many ocular oncology centers to treat advanced intraocular retinoblastoma. In this report, we describe two patients with unilateral intraocular retinoblastoma and persistent vitreous seeding, who were treated with IAC after failed systemic chemotherapy. Despite multiple sessions and increasing dosage of drug delivery, vitreous seeding in these cases failed to respond to IAC, and ultimately both eyes were enucleated for tumor control. Based on the histopathologic findings in these two cases, IAC appears to have limitations in treating persistent vitreous seeding in eyes which have failed systemic chemotherapy. Possible causes for failure of IAC to treat persistent vitreous seeding include poor vitreous penetration, inactive state of tumor seeds within the avascular vitreous cavity, and chemotherapeutic drug resistance.
Keywords: chemotherapy, retinoblastoma, intra-arterial, failure, enucleation, eye
Introduction
Intra-arterial infusion of chemotherapy (IAC) has been recognized as a viable new method for treating advanced intraocular retinoblastoma.1–4 When compared to intravenous chemotherapy, potential advantages of selective IAC include the reduction of systemic effects such as myelosuppression, immunosuppression and the risk of secondary leukemia.5 Compared to external beam radiation, IAC can be used to treat children below age 12 months without concerns regarding local side effects such as orbital hypoplasia and the risk of secondary head and neck cancers later in life.6 Gobin et al have demonstrated globe salvage rates as high as 82% with IAC in primary cases with advanced intraocular tumors.2 In this report, we describe two patients with unilateral intraocular retinoblastoma and persistent vitreous seeding, who were treated with IAC after failed systemic chemotherapy. Despite multiple sessions and increasing dosage of drug delivery, vitreous seeding in these cases failed to respond to IAC, and ultimately both eyes were enucleated for tumor control.
Case description
Case one
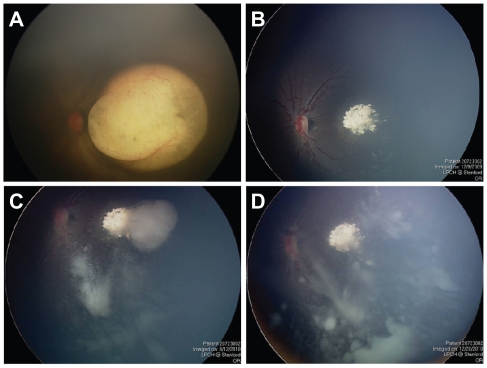
A one-month-old child born three weeks premature was diagnosed with a left retinal tumor during a screening examination. Fundus evaluation revealed a left macular tumor which was whitish in appearance with fine retinal vasculature ( Figure 1A). Based on her other clinical findings (symphalangism, pelviectasis, paired ribs, facial dysmorphic features), she was diagnosed with unilateral retinoblastoma in the setting of 13q deletion syndrome. The left eye was classified as having a Group C tumor based on the macular location and the presence of localized seeding. She received three sessions of systemic chemotherapy using a three drug regimen (carboplatin, etoposide, vincristine), with excellent clinical regression of the tumor (Figure 1B). There was complete calcification of the tumor, and it was decided to carefully observe the patient rather than perform laser treatment due to its macular location. The patient was then lost to follow-up when her family took her to India for approximately 9 months. When she finally returned to our institution, a tumor recurrence along the temporal edge of the macular scar was noted. There was also diffuse vitreous seeding arising from this area of tumor recurrence (Figure 1C). The patient was treated with two additional courses of systemic chemotherapy (topotecan and cyclophosphamide) with excellent regression of the retinal tumor recurrence, but there was no response by the vitreous seeding (Figure 1D). The patient was then treated with two sessions of IAC; the first with 5 mg of melphalan and the second (1 month later) with 8 mg of melphalan. After the second session of IAC, the patient developed diffuse periocular edema (Figure 2A), which resolved within 1 week. Unfortunately, the vitreous seeding appeared to increase in severity (Figure 2B), and the left eye was enucleated to prevent tumor spread, approximately 2 1/2 years after initial diagnosis.
Figure 1.
(A) One-month-old child with unilateral retinoblastoma and a left macular tumor. (B) Excellent regression of the tumor after three sessions of systemic chemotherapy, causing complete calcification of the macular lesion. (C) Tumor recurrence along temporal edge of the tumor, with associated vitreous seeding. (D) Excellent regression of the retinal tumor recurrence but no response by vitreous seeding after systemic chemotherapy.
Figure 2.
(A) Diffuse periocular edema following intra-arterial infusion of melphalan, which resolved without sequelae. (B) Fundus photograph showing progression of vitreous seeding despite two sessions of intra-arterial chemotherapy.
Gross examination of the sectioned enucleated eye showed extensive tumor seeding within the vitreous cavity (Figure 3A). Histopathologic examination showed complete regression and calcification of the macular tumor. (Figure 3B). The tumor was replaced by a scar comprising glial cells and retinal pigment epithelial proliferation. Bruch’s membrane was broken and a portion of the retina had shifted through this break into the choroid. Numerous tumor seeds were seen lying on the internal limiting membrane (Figure 3C), but the retina away from the macular scar was otherwise normal in appearance. The vitreous contained numerous tumor nodules of small, basophilic cells with a high nuclear to cytoplasmic ratio. The diffuse vitreous seeding demonstrated 80% positive staining with Ki67 (Figure 3D), an immunohistochemistry marker for cellular proliferation.7 No histopathologic abnormalities were noted within the cornea, iris, choroid, sclera or optic nerve (data not shown).
Figure 3.
(A) Gross photograph of enucleated eye showing a calcific scar of the regressed macular retinoblastoma and extensive vitreous seeding. (B) Section through the macula with the optic nerve to the right of the photograph. Macular scar in area without calcification; the scar consists of glial cell proliferation and retinal pigment epithelium hyperplasia. In addition, a portion of the retina has shifted into the choroid through a break in Bruch’s membrane (hematoxylin-eosin, original magnification 100×). (C) Temporal, equatorial retina with numerous tumor seeds (basophilic cells, see arrow) in the overlying vitreous (hematoxylin-eosin, original magnification 100×). (D) Vitreous seeds with markedly positive nuclear reaction to Ki67 (original magnification 200×).
Case two
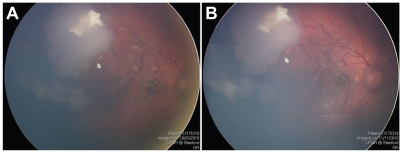
A four-year-old boy was referred with persistent unilateral retinoblastoma involving the right eye after systemic chemotherapy. The patient had received five total courses of systemic chemotherapy at another institution; the first three courses with vincristine, etoposide, and carboplatin, and the final two courses with topotecan and cyclophosphamide. The patient had no family history of retinoblastoma and his past medical history was otherwise negative. The patient’s vision measured 20/30 OD and 20/15 OS. Fundus examination revealed a partially regressed supero-temporal tumor, with multiple vitreous seeds overlying the macula (Figure 4A). He was treated with three sessions of IAC; the first with 5 mg of melphalan, the second with 8 mg of melphalan, and the third with 4 mg of melphalan and 30 mg of carboplatin. After the first IAC infusion, his white blood cell count decreased from 5600 to 3700 but he did not require admission. Visual acuity after the IAC infusions ranged between 20/30–20/50 in the right eye. Examination under anesthesia after the third session of IAC showed a slight increase in the vitreous seeding, and no response by the main tumor mass (Figure 4B). Five months following the initial IAC infusion, enucleation was performed due to concerns regarding the persistently active tumor.
Figure 4.
(A) Fundus photograph of the right eye showing partially regressed superotemporal tumor and vitreous seeds overlying the macula. (B) Same view of right fundus showing no regression of the vitreous seeds despite three sessions of intra-arterial chemotherapy.
Gross evaluation of the sectioned enucleated eye revealed a 5 × 5 mm mass superotemporally on the retina with calcifications (Figure 5A). The mass was differentiated, containing cells with small bland nuclei and relatively abundant eosinophilic cytoplasm (Figure 5B). No fleurettes, rosettes or mitotic figures were seen. Scattered areas of glial cells were seen adjacent to the tumor and within the tumor. Occasional vitreous seeds were found in the vitreous near the retina. The retina otherwise appeared normal in all quadrants. The immunohistochemical stain for Ki-67, a cellular proliferation marker, revealed no positive reactions in either the main tumor or the vitreous seeds, consistent with their differentiated appearance (Figure 5C).
Figure 5.
(A) Gross photograph of enucleated eye showing main superotemporal tumor and vitreous seeds overlying the macula (arrow). (B) The main tumor consisting of well-differentiated cells with abundant cytoplasm (hematoxylin-eosin, original magnification 400×). (C) A vitreous seed containing differentiated cells, with negative staining with Ki67 (hematoxylin-eosin, original magnification 400×).
Comment
Selective intra-arterial chemotherapy (IAC) has been adopted by many ocular oncology centers to treat advanced intraocular retinoblastoma.1–4 In some centers, IAC has become the primary modality for treating advanced tumors, while in other institutions IAC is used mainly as salvage therapy after failed systemic chemotherapy and/or radiation.2,3 The ocular effects of directly infusing the ophthalmic artery with chemotherapy agents are still being studied. Local transient side effects have been reported including peri-orbital swelling and erythema, temporary eyelash loss and more rarely, retinal arterial occlusion.8–10 Brodie and colleagues have demonstrated through electrophysiologic testing that some eyes after IAC have preserved and even improved ERG readings.11 However, Vajzovic et al reported retinopathy and vitreous hemorrhage in three of their first eleven eyes treated at the University of Miami.3,9 There has also been a recent report of choroidal artery occlusion and retinal emboli formation following IAC.10
We report two children with intraocular retinoblastoma who underwent enucleation following failed systemic chemotherapy and multiple infusions with IAC. In the original series of nine patients reported by Abramson, the two eyes enucleated after IAC did not show evidence of ocular toxicity. 1 A histopathologic report from Vajzovic and colleagues showed no evidence of toxicity to the retina, optic nerve, choroid or vascular structures.12 Graeber and colleagues recently reported pathologic findings in ten eyes that were enucleated for tumor growth after IAC.13 They also did not identify any specific evidence of ocular toxicity attributed to IAC, although nine of the ten eyes had received prior multimodal treatment such as external beam radiotherapy and laser therapy. In our two cases, there was no previous history of any local therapy being delivered to the globes. The two eyes were treated with doses of melphalan as high as 8 mg, utilizing an infusion technique identical to the one described by Abramson and colleagues.1 There was no evidence of ocular toxicity on histopathologic examination, as evidenced by the lack of degeneration of the retina and optic nerve in areas uninvolved by tumor. Although electrophysiologic testing was not performed, the older patient in our case series demonstrated fairly well-preserved visual acuity after all three of his IAC infusions. Based on the limited evidence to date, it does not appear that IAC causes anatomic damage to the globe in the absence of a retinal occlusive event.
In both of our cases, vitreous seeding did not respond to treatment with IAC, despite escalating doses of melphalan (cases one and two) and even multiple drug combinations (case two). We used doses of melphalan as high as 8 mg per treatment in our two cases, which is slightly higher than the 7.5 mg dose reported by both Gobin et and Vajzovic.2,12 Both patients developed temporary side effects following IAC (periocular edema, neutropenia), but the vitreous seeding persisted and even continued to progress. It is noteworthy that all three of the eyes reported by Vajzovic et al which were removed for poor response after systemic chemotherapy and salvage IAC also had vitreous seeding.12 Based on a limited number of cases to date, IAC appears to have limitations in treating persistent vitreous seeding in eyes which have failed systemic chemotherapy. Possible causes for failure of IAC to treat persistent vitreous seeding include poor vitreous penetration, inactive state of tumor seeds within the avascular vitreous cavity, and chemotherapeutic drug resistance. The decision to use IAC for any eye with retinoblastoma must be individualized based on several factors including unilateral or bilateral tumor involvement, the availability of other treatment options, and whether the eye has significant visual potential. For a patient with unilateral retinoblastoma and recalcitrant vitreous seeding after systemic chemotherapy, multiple sessions of IAC with escalating doses of melphalan may not be advisable given that enucleation is typically curative in these cases.
Footnotes
Disclosure
The authors have no financial or other conflict of interest which may arise from the publication of this manuscript.
References
- 1.Abramson DH, Dunkel IJ, Brodie SE, Kim JW, Gobin YP. A phase I/II study of direct intraarterial (ophthalmic artery) chemotherapy with melphalan for intraocular retinoblastoma initial results. O–hthalmology. 2008;115(8):1398–1404. doi: 10.1016/j.ophtha.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: Four-year experience. Arch Ophthalmol. 2011;129(6):732–737. doi: 10.1001/archophthalmol.2011.5. [DOI] [PubMed] [Google Scholar]
- 3.Peterson EC, Elhammady MS, Quintero-Wolfe S, Murray TG, Aziz-Sultan MA. Selective ophthalmic artery infusion of chemotherapy for advanced intraocular retinoblastoma: initial experience with 17 tumors. J Neurosurg. 2011;114(6):1603–1608. doi: 10.3171/2011.1.JNS10466. [DOI] [PubMed] [Google Scholar]
- 4.Shields CL, Ramasubramanian A, Rosenwasser R, Shields JA. Superselective catheterization of the ophthalmic artery for intraarterial chemotherapy for retinoblastoma. Retina. 2009;29(8):1207–1209. doi: 10.1097/IAE.0b013e3181b4ce39. [DOI] [PubMed] [Google Scholar]
- 5.Gombos DS, Hungerford J, Abramson DH, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: Is chemotherapy a factor? Ophthalmology. 2007;114(7):1378–1383. doi: 10.1016/j.ophtha.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 6.Abramson DH, Frank CM. Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk. Ophthalmology. 1998;105(4):573–579. doi: 10.1016/S0161-6420(98)94006-4. [DOI] [PubMed] [Google Scholar]
- 7.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 8.Marr B, Gobin PY, Dunkel IJ, Brodie SE, Abramson DH. Spontaneously resolving periocular erythema and ciliary madarosis following intraarterial chemotherapy for retinoblastoma. Middle East Afr J Ophthalmol. 2010;17(3):207–209. doi: 10.4103/0974-9233.65492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vajzovic LM, Murray TG, Aziz-Sultan MA, et al. Supraselective intraarterial chemotherapy: Evaluation of treatment-related complications in advanced retinoblastoma. Clin Ophthalmol. 2011;5:171–176. doi: 10.2147/OPTH.S12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munier FL, Beck-Popovic M, Balmer A, Gaillard MC, Bovey E, Binaghi S. Occurrence of sectoral choroidal occlusive vasculopathy and retinal arteriolar embolization after superselective ophthalmic artery chemotherapy for advanced intraocular retinoblastoma. Retina. 2011;31(3):566–573. doi: 10.1097/IAE.0b013e318203c101. [DOI] [PubMed] [Google Scholar]
- 11.Brodie SE, Pierre Gobin Y, Dunkel IJ, Kim JW, Abramson DH. Persistence of retinal function after selective ophthalmic artery chemotherapy infusion for retinoblastoma. Doc Ophthalmol. 2009;119(1):13–22. doi: 10.1007/s10633-008-9164-3. [DOI] [PubMed] [Google Scholar]
- 12.Vajzovic LM, Murray TG, Aziz-Sultan MA, et al. Clinicopathologic review of enucleated eyes after intra-arterial chemotherapy with melphalan for advanced retinoblastoma. Arch Ophthalmol. 2010;128(12):1619–1623. doi: 10.1001/archophthalmol.2010.296. [DOI] [PubMed] [Google Scholar]
- 13.Graeber CP, Gobin YP, Marr BP, et al. Histopathologic findings of eyes enucleated after treatment with chemosurgery for retinoblastoma. Open Ophthalmol J. 2011;5:1–5. doi: 10.2174/1874364101105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]