Abstract
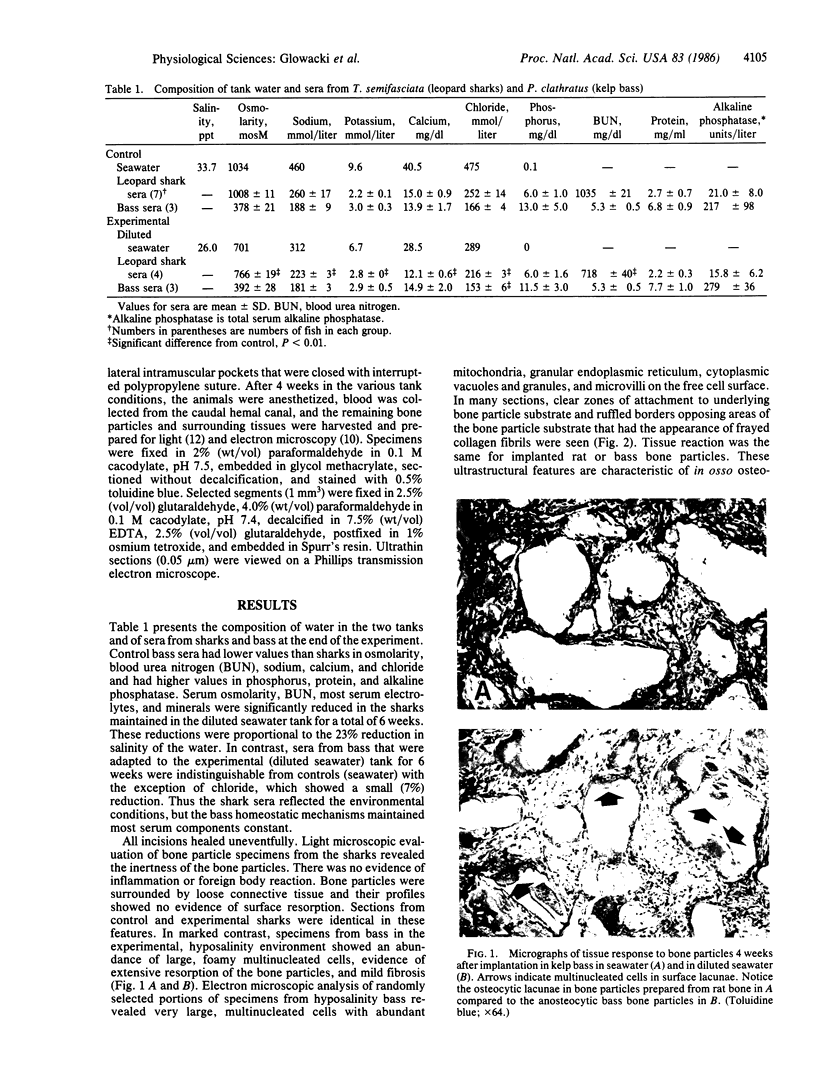
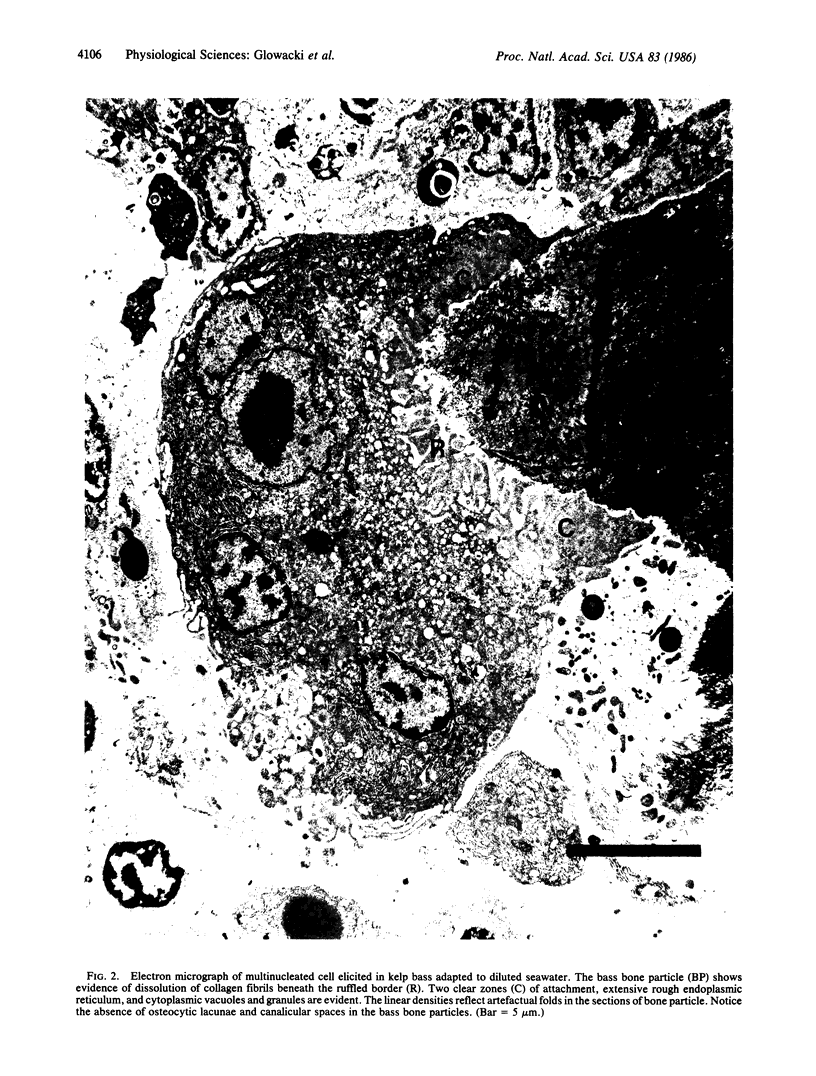
Kelp bass (Paralabrax clathratus) and leopard sharks (Triakis semifasciata) are characterized by an acellular (anosteocytic) bony skeleton and a focally calcified cartilaginous endoskeleton, respectively. These skeletal forms are not considered to function as mineral reservoirs. Previous studies showed that implanted bone particles are resorbed in rats by large multinucleated cells with ultrastructural features (ruffled borders) characteristic of osteoclasts. We tested the ability of fish to resorb bone matrix and to adapt to reduced salinity conditions. Bone particles were implanted in sharks and bass maintained in seawater (34 ppt, 40.5 mg of calcium per dl) or in diluted seawater (26 ppt, 28.5 mg of calcium per dl). Sera and elicited tissues were harvested 4 weeks later. In sharks, bone particles were not resorbed, and multinucleated cells were not evident under either normal or hyposalinity conditions. Shark sera were isoosmolar with the seawater or diluted seawater, with serum chemistries of the hyposalinity group reflecting the 23% reduction in environmental minerals and electrolytes, compared to sharks in normal seawater. In marked contrast, bass adapted to diluted seawater resorbed bone particles and maintained normal serum chemistries. Electron microcopy showed that the bone particles were surrounded by large, foamy multinucleated cells, many with membrane specializations typical of osteoclasts from higher vertebrates, i.e., extensive clear zones apposed to intact bone matrix and active ruffled borders overlying areas of matrix undergoing dissolution. Although osteoclasts had not been described in these fish, this study shows that bass have stem cells that can be stimulated to differentiate into bone-resorbing osteoclasts.
Keywords: osteoclast progenitors, differentiation, sharks, bass, minerals
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chartier M. M., Millet C., Martelly E., Lopez E., Warrot S. Stimulation par la vitamine D3 et le 1,25-dihydroxyvitamine D3 de l'absorption intestinale du calcium chez l'anguille (Anguilla anguilla L.). J Physiol (Paris) 1979;75(3):275–282. [PubMed] [Google Scholar]
- FISCHMAN D. A., HAY E. D. Origin of osteoclasts from mononuclear leucocytes in regenerating newt limbs. Anat Rec. 1962 Aug;143:329–337. doi: 10.1002/ar.1091430402. [DOI] [PubMed] [Google Scholar]
- Fenwick J. C., Smith K., Smith J., Flik G. Effect of various vitamin D analogs on plasma calcium and phosphorus and intestinal calcium absorption in fed and unfed American eels, Anguilla rostrata. Gen Comp Endocrinol. 1984 Sep;55(3):398–404. doi: 10.1016/0016-6480(84)90010-8. [DOI] [PubMed] [Google Scholar]
- Fenwick J. C., So Y. P. A perfusion study of the effect of stanniectomy on the net influx of calcium 45 across an isolated eel gill (1). J Exp Zool. 1974 Apr;188(1):125–131. doi: 10.1002/jez.1401880112. [DOI] [PubMed] [Google Scholar]
- Fleming W. R. Calcium metabolism of teleosts. Am Zool. 1967 Nov;7(4):835–842. doi: 10.1093/icb/7.4.835. [DOI] [PubMed] [Google Scholar]
- Glowacki J., Altobelli D., Mulliken J. B. Fate of mineralized and demineralized osseous implants in cranial defects. Calcif Tissue Int. 1981;33(1):71–76. doi: 10.1007/BF02409414. [DOI] [PubMed] [Google Scholar]
- Glowacki J., O'Sullivan J., Miller M., Wilkie D. W., Deftos L. J. Calcitonin produces hypercalcemia in leopard sharks. Endocrinology. 1985 Feb;116(2):827–829. doi: 10.1210/endo-116-2-827. [DOI] [PubMed] [Google Scholar]
- Glowacki J. The effects of heparin and protamine on resorption of bone particles. Life Sci. 1983 Sep 12;33(11):1019–1024. doi: 10.1016/0024-3205(83)90655-0. [DOI] [PubMed] [Google Scholar]
- Göthlin G., Ericsson J. L. On the histogenesis of the cells in fracture callus. Electron microscopic autoradiographic observations in parabiotic rats and studies on labeled monocytes. Virchows Arch B Cell Pathol. 1973 Mar 30;12(4):318–329. [PubMed] [Google Scholar]
- Lopez E., Peignoux-Deville J., Lallier F., Martelly E., Milet C. Effects of calcitonin and ultimobranchialectomy (UBX) on calcium and bone metabolism in the eel, Anguilla anguilla L. Calcif Tissue Res. 1976 Apr 20;(2):173–186. doi: 10.1007/BF02546406. [DOI] [PubMed] [Google Scholar]
- Lopez E., Tisserand-Jochem E. M., Eyquem A., Milet C., Hillyard C., Lallier F., Vidal B., Mac Intyre I. Immunocytochemical detection in eel corpuscles of Stannius of a mammalian parathyroid-like hormone. Gen Comp Endocrinol. 1984 Jan;53(1):28–36. doi: 10.1016/0016-6480(84)90221-1. [DOI] [PubMed] [Google Scholar]
- MOSS M. L. STUDIES OF THE ACELLULAR BONE OF TELEOST FISH. V. HISTOLOGY AND MINERAL HOMEOSTASIS OF FRESH-WATER SPECIES. Acta Anat (Basel) 1965;60:262–276. [PubMed] [Google Scholar]
- MOSS M. L. Studies of the acelluar bone of teleost fish. I. Morphological and systematic variations. Acta Anat (Basel) 1961;46:343–362. [PubMed] [Google Scholar]
- MOSS M. L. Studies of the acellular bone of teleost fish. II. Response to fracture under normal and acalcemic conditions. Acta Anat (Basel) 1962;48:46–60. doi: 10.1159/000141826. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Imai M. Control of renal function in freshwater and marine teleosts. Fed Proc. 1982 Jun;41(8):2355–2360. [PubMed] [Google Scholar]
- Pang P. K., Griffith R. W., Schreibman M. P. The pituitary gland and calcium metabolism in Fundulus diaphanus (teleosteii). Gen Comp Endocrinol. 1973 Apr;20(2):358–361. doi: 10.1016/0016-6480(73)90189-5. [DOI] [PubMed] [Google Scholar]
- Pang P. K. Hypercalcemic effects of ovine prolactin on intact killifish, Fundulus heteroclitus, subjected to different environmental calcium challenges. Gen Comp Endocrinol. 1981 Jun;44(2):252–255. doi: 10.1016/0016-6480(81)90256-2. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part I of IV parts: mechanisms of calcium transfer between blood and bone and their cellular basis: morphological and kinetic approaches to bone turnover. Metabolism. 1976 Jul;25(7):809–844. doi: 10.1016/0026-0495(76)90151-7. [DOI] [PubMed] [Google Scholar]
- Simmons D. J. Calcium and skeletal tissue physiology in teleost fishes. Clin Orthop Relat Res. 1971 May;76:244–280. doi: 10.1097/00003086-197105000-00031. [DOI] [PubMed] [Google Scholar]
- Watts E. G., Copp D. H., Deftos L. J. Changes in plasma calcitonin and calcium during the migration of salmon. Endocrinology. 1975 Jan;96(1):214–218. doi: 10.1210/endo-96-1-214. [DOI] [PubMed] [Google Scholar]
- Weiss R. E., Watabe N. Studies on the biology of fish bone. III. Ultrastructure of osteogenesis and resorption in osteocytic (cellular) and anosteocytic (acellular) bones. Calcif Tissue Int. 1979 Aug 24;28(1):43–56. doi: 10.1007/BF02441217. [DOI] [PubMed] [Google Scholar]