Abstract
Objective
The aim of the current study was to investigate which is the most suitable classification for colorectal cancer, log odds of positive lymph nodes (LODDS) classification or the classifications based on the number of positive lymph nodes (pN) and positive lymph node ratio(LNR) in a Chinese single institutional population.
Design
Clinicopathologic and prognostic data of 1297 patients with colorectal cancer were retrospectively studied. The log-rank statistics, Cox's proportional hazards model, the Nagelkerke R2 index and a Harrell's C statistic were used.
Results
Univariate and three-step multivariate analyses identified that LNR was a significant prognostic factor and LNR classification was superior to both the pN and LODDS classifications. Moreover, the results of the Nagelkerke R2 index (0.130) and a Harrell's C statistic (0.707) of LNR showed that LNR and LODDS classifications were similar and LNR was a little better than the other two classifications. Furthermore, for patients in each LNR classification, prognosis was homologous between those in different pN or LODDS classifications. However, for patients in pN1a, pN1b, LODDS2 and LODDS3 classifications, significant differences in survival were observed among patients in different LNR classifications.
Conclusions
For patients with colorectal cancer, the LNR classification is more suitable than pN and LODDS classifications for prognostic assessment in a Chinese single institutional population.
Introduction
Colorectal cancer is the third most common cancer for both males and females, as well as the second leading cause of cancer-related death in the western world [1]. In China, with improvements in living standards and changes in diet, the incidence of colorectal cancer is gradually increasing [2]. Recently, the incidence of colorectal cancer and its cancer-related mortality have become the fourth highest of all cancers in China [3]. As is well known, lymph node (LN) metastasis is one of the most important prognostic factors in patients with colorectal cancers [4]–[10].
In the 7th edition of the UICC/AJCC TNM staging system, based on the number of tumor-infiltrated lymph nodes, the pN category was stratified into pN1 (1–3 positive LNs) and pN2 (≥4 positive LNs) [4]. The lymph node ratio (LNR), namely, the ratio of positive LNs divided by the total number of retrieved LNs, reflects the probability of positive LNs in the retrieved LNs [5]. Recently, the LNR has been reported to represent a powerful independent prognostic value in colorectal cancer 5–10. Interestingly, another novel prognostic indicator, log odds of positive lymph nodes (LODDS), has been proposed in recent years. LODDS is defined as the log of the quotient of the number of positive lymph nodes and the number of negative lymph nodes and has been introduced as a new prognostic factor in breast cancer research [11], [12]. Moreover, Wang et al. studied 24,477 patients with stage III colon cancer who were registered in the Surveillance Epidemiology and End Results (SEER) database and revealed that LODDS was a better prognostic factor than LNR [13]. However, to date, no study comparing the prognostic value among pN, LNR and LODDS classifications for colorectal cancer in Chinese patients has been reported.
In light of these considerations, the aim of the current study was to investigate which is the most suitable classification among pN, LNR and LODDS classifications in prognostic assessment for colorectal cancer patients with R0 resection in a Chinese single institutional population.
Materials and Methods
Patients
From our prospective database, clinical information on all patients with colorectal cancer that underwent surgery at the Department of Surgical Oncology at the First Hospital of China Medical University from April 1994 to December 2007 were retrospectively collected, reviewed, and analyzed. No previous local or systemic treatment had been conducted for these patients before operation. Specimens which were fixed in formalin and stained with hematoxylin-eosin (H&E) were used for histopathological evaluation. This study consisted of stage I–III colorecal cancers. Patients (i) who died in the postoperative period (within 30 days), (ii) with multiple adenocarcinomas of the colon and rectum, (iii) with synchronous or metachronous tumors, (iv) who underwent neoadjuvant treatment due to presumed treatment-related changes in the TNM classification, (v) with incomplete pathological data entries, (vi) who were lost to follow-up, (vii) with tumor deposits, and (viii) distant metastasis were excluded in this study. Follow-up was completed for the entire study population until November 2008.
Of the remaining 1297 patients, the median and mean follow-up periods were 47 months and 56±36 months (range: 1–167 months), respectively. The following data were obtained: age, gender, date of surgery, date of death (if applicable), cause of death (if applicable), date of follow-up, location of the primary tumor, tumor size, histologic grade, venous invasion, lymphovascular invasion, depth of invasion, number of retrieved lymph nodes and number of metastatic lymph nodes. Tumors originating from the cecum to the sigmoid colon were defined as colon cancers and tumors located in the rectum or rectosigmoid junction were considered as rectal cancers [14].
Ethics statement
The study was approved by the Research Ethics Committee of China Medical University, China. Written informed consents were obtained from all patients before participating in the study.
Classification methods and Statistical Analysis
According to the 7th edition of the UICC/AJCC TNM staging system, based on the number of tumor-infiltrated lymph nodes, the pN category was stratified into pN0: no positive LNs; pN1a: 1 positive LN; pN1b: 2–3 positive LNs; pN2a: 4–6 positive LNs; and pN2b: ≥7 positive LNs [4]. LNR was defined as the ratio of positive LNs divided by the total number of retrieved LNs, reflecting the probability of positive LNs in the retrieved LNs, which does not significantly depend on the number of LNs harvested [5]. LODDS was estimated by: log 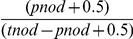 , where the pnod is the number of positive lymph nodes and tnod is the total number of lymph nodes retrieved, and 0.5 is added to both numerator and denomination to avoid singularity [13].
, where the pnod is the number of positive lymph nodes and tnod is the total number of lymph nodes retrieved, and 0.5 is added to both numerator and denomination to avoid singularity [13].
To obtain optimal cut-off values for LNRs and LODDS classifications, running log-rank statistics was applied [15]. Cancer-specific survival was analyzed by Kaplan-Meier survival curves and comparisons were made by the log-rank test. Multivariate analysis was performed using backward stepwise Cox's proportional hazards model [16]. Three-step multivariate analysis was performed to investigate which N staging system had more potential to predict patient outcomes. The p-spline (Fitting Spline Models) function is used to fit a general spline term within the Cox model [17]. The Nagelkerke R2 index (R2 N) was used to score the different Cox models [18]. R2 represents the proportion of variation explained by covariates in regression models [18], [19]. R2 N divides R2 by its maximum attainable value to scale it to within the range 0–1. R2 N is close to 1 for a perfectly predictive model, and close to 0 for a model that does not discriminate between short and long survival times. After each regression, a Harrell's C statistic was run to test the predictive capacity and fit of the model, respectively. A model with perfect predictive capacity (sensitivity and specificity of 100%) would have a Harrell's C statistic of 1.00 and the highest Harrell's C statistic was chosen as the best model [20].
All the statistical analyses and graphics were performed with the SPSS 17.0 statistical package (SPSS, Chicago, IL), Splus 8.0 (Insightful Corporation, Seattle, WA, USA) and STATA MP ver.10 (StataCorp LP, College Station, TX) statistical software. For all analysis, P<0.05 was considered significant.
Results
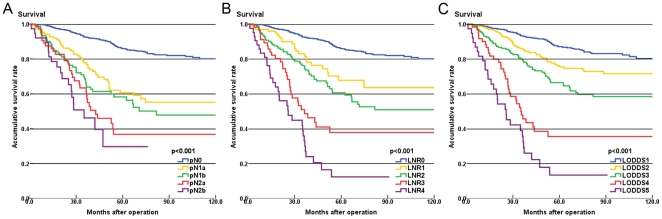
The number of lymph nodes examined in each specimen ranged from 1 to 107 with a mean of 13 and a median of 11. According to the 7th edition of the UICC/AJCC TNM staging system, based on the number of tumor-infiltrated lymph nodes, the patients with different pN categories were divided into pN0: 935(72%); pN1a: 138(11%); pN1b: 121(9%); pN2a: 65(5%); and pN2b: 38(3%). The survival differences were statistically significant (P<0.001; Table 1 and Fig. 1A).
Table 1. Univariate analysis of the prognostic factors for patients with colorectal cancer.
| Na | 5-YSRb(%) | P value* | |
| Sex | 0.014 | ||
| Male | 715 | 74 | |
| Female | 582 | 81 | |
| Age | 0.003 | ||
| ≤60 | 594 | 81 | |
| >60 | 703 | 74 | |
| Tumor location | 0.931 | ||
| Rectum | 711 | 76 | |
| Colon | 586 | 78 | |
| Tumor size | 0.947 | ||
| ≤5 | 782 | 77 | |
| >5 | 515 | 77 | |
| Histologic grade | <0.001 | ||
| Well | 646 | 82 | |
| Moderate | 564 | 72 | |
| Poor | 87 | 63 | |
| Venous invasion | 0.701 | ||
| Positive | 7 | 67 | |
| Negative | 1290 | 77 | |
| Lymphovascular invasion | <0.001 | ||
| Positive | 60 | 52 | |
| Negative | 1237 | 78 | |
| pT stage | <0.001 | ||
| T1 | 36 | 92 | |
| T2 | 316 | 88 | |
| T3 | 795 | 76 | |
| T4 | 150 | 58 | |
| pN stage | <0.001 | ||
| N0 | 935 | 86 | |
| N1a | 138 | 61 | |
| N1b | 121 | 56 | |
| N2a | 65 | 37 | |
| N2b | 38 | 30 | |
| LNR | <0.001 | ||
| LNR0 | 935 | 86 | |
| LNR1 | 99 | 68 | |
| LNR2 | 164 | 59 | |
| LNR3 | 57 | 38 | |
| LNR4 | 42 | 12 | |
| LODDS | <0.001 | ||
| LODDS1 | 774 | 87 | |
| LODDS2 | 223 | 75 | |
| LODDS3 | 201 | 66 | |
| LODDS4 | 61 | 36 | |
| LODDS5 | 38 | 13 |
Na: Number of patients.
5-YSRb: 5-year accumulative survival rate.
: P values were made by log-rank test.
Figure 1. Survival curves of colorectal cancer patients according to three classifications (pN, LNR, LODDS) are depicted.
Using running log-rank statistics, we calculated the best cut-off LNR values and proposed a novel LNR category: LNR0: 0%; LNR1: 0%<LNR≤11%; LNR2: 11%<LNR≤36%; LNR3: 36%<LNR≤66% and LNR4>66%. Patients were categorized into five groups according to the LNR category: 935(72%) were as LNR0; 99(8%) were as LNR1; 164(13%) were as LNR2; 57(4%) were as LNR3 and 42(3%) were as LNR4. The 5-year cancer-specific survival rate decreased significantly with increasing LNRs: LNR0 = 86% survival rate; LNR1 = 68% survival rate; LNR2 = 59% survival rate; LNR3 = 38% survival rate; and LNR4 = 12% survival rate (P<0.001; Table 1 and Fig. 1B).
As shown in Table 1 and Fig. 1C, based on the LODDS classification, five groups were identified by running log-rank statistics: LODDS1≤−2.510; −2.510<LODDS2≤−1.680; −1.680<LODDS3≤−0.510; −0.510<LODDS4≤0.730; and LODDS5>0.730. The 5-year cancer-specific survival rates were 87%, 75%, 66%, 36% and 13%, respectively. The survival rate decreased significantly with increasing LODDS (P<0.001). Moreover, in univariate analysis, sex, age, histologic grade, lymphovascular invasion, and pT stage were also significantly correlated with prognosis (Table 1).
Then, we used univariate and three-step multivariate analysis (Cox Proportional Hazard Model) to find the most significant prognostic factors (Table 2). In univariate analysis, sex, age, histologic grade, lymphovascular invasion, pT stage, pN stage, LNR classification and LODDS classification were significant prognostic factors. Next, the step 1 multivariate analysis showed, pN classification, sex, age, histologic grade, lymphovascular invasion and pT classification were confirmed to be independent prognostic factors. After that, LNR classification was added to construct the model in the step 2 multivariate analysis, and LNR classification became significant, while pN classification and histologic grade dropped out of the model. Moreover, when all 3 N classifications were included in the step 3 multivariate analysis, LODDS and pN classifications were substituted by the LNR classification (Table 2).
Table 2. Univariate and Three-step Multivariate Analysis (Cox Proportional Hazard Model) of Prognostic Factors.
| Univariate Analysis | Multivariate Analysis 1 | Multivariate Analysis 2 | Multivariate Analysis 3 | |||||||||
| RRa | 95% CIb | P | RR | 95% CI | P | RR | 95% CI | P | RR | 95% CI | P | |
| Sex, female vs male | 0.730 | 0.567–0.940 | 0.015 | 0.714 | 0.555–0.920 | 0.009 | 0.762 | 0.592–0.982 | 0.035 | 0.762 | 0.592–0.982 | 0.035 |
| Age* | 1.016 | 1.005–1.027 | 0.004 | 1.023 | 1.012–1.034 | <0.001 | 1.019 | 1.008–1.030 | 0.001 | 1.019 | 1.008–1.030 | 0.001 |
| Tumor location, colon vs rectum | 0.989 | 0.773–1.266 | 0.931 | |||||||||
| Tumor size* | 1.009 | 0.955–1.067 | 0.739 | |||||||||
| Histologic grade, well vs moderate vs poor | 1.521 | 1.264–1.829 | <0.001 | 1.229 | 1.012–1.493 | 0.037 | ||||||
| Venous invasion, positive vs negative | 1.312 | 0.326–5.277 | 0.702 | |||||||||
| Lymphovascular invasion, positive vs negative | 2.733 | 1.795–4.162 | <0.001 | 2.193 | 1.409–3.414 | 0.001 | 2.603 | 1.691–4.008 | <0.001 | 2.603 | 1.691–4.008 | <0.001 |
| pT stage, T1 vs T2 vs T3 vs T4 | 2.047 | 1.695–2.473 | <0.001 | 1.764 | 1.446–2.152 | <0.001 | 1.735 | 1.428–2.107 | <0.001 | 1.735 | 1.428–2.107 | <0.001 |
| pN stage, N0 vs N1a vs N1b vs N2a vs N2b | 1.796 | 1.646–1.961 | <0.001 | 1.676 | 1.531–1.835 | <0.001 | ||||||
| LNR, LNR0 vs LNR1 vs LNR2 vs LNR3 vs LNR4 | 1.915 | 1.759–2.086 | <0.001 | 1.793 | 1.644–1.955 | <0.001 | 1.793 | 1.644–1.955 | <0.001 | |||
| LODDS, LODDS1 vs LODDS2 vs LODDS3 vs LODDS4 vs LODDS5 | 1.939 | 1.765–2.130 | <0.001 | |||||||||
RRa: relative risk.
CIb: confidence interval.
: continuous variable.
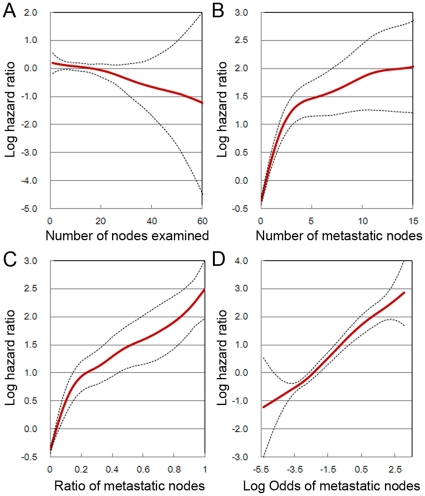
Furthermore, in fitting spline models, the number of nodes examined and pN exhibited marked nonlinearity and widely diverging confidence intervals (Fig. 2A and 2B). The linearity improved for LNR and LODDS classifications, which also showed more homogeneously distributed confidence intervals (Fig. 2C and 2D).
Figure 2. In fitting spline models, colorectal cancer mortality as a function of different classifications.
Dotted lines indicate the 95% confidence interval.
Based on R2 N, the results showed a comparison between proportional hazards models that included pN(R2 N = 0.100), LNR(R2 N = 0.130) and LODDS(R2 N = 0.119). The best predictive covariate model was LNR, obviously. Then, we used Harrell's C statistic to test the predictive capacity and fit of the model. The Harrell's C value and 95% CI of LNR (0.707, 0.675–0.739) and LODDS (0.708, 0.674–0.741) were similar and better than that of pN classification (0.698, 0.666–0.730). Comparing the predictive power of survival models with pN, LNR was significant (P = 0.002), but LODDS was not (P = 0.348). When we compared the predictive power between LNR and LODDS, there was no significant difference (P = 0.962).
Table 3 listed cancer-specific survival rates on the basis of pN and LODDS classification according to the LNR staging system. As shown, for patients in each LNR classification, prognosis was highly homologous between those in different pN or LODDS classifications. However, for patients in pN1a, pN1b, LODDS2 and LODDS3 classifications, significant differences in survival could always be observed among patients in different LNR classifications.
Table 3. Cancer-specific survival rates on the basis of pN and LODDS classification according to the LNR staging system.
| LNR0 | LNR1 | LNR2 | LNR3 | LNR4 | Pa | ||||||
| Nd | 5-YSRe (%) | Nd | 5-YSRe (%) | Nd | 5-YSRe (%) | Nd | 5-YSRe (%) | Nd | 5-YSRe (%) | ||
| pN classification | |||||||||||
| pN0 | 935 | 86 | 0 | 0 | 0 | 0 | - | ||||
| pN1a | 0 | 84 | 66 | 46 | 61 | 5 | 20 | 3 | 0 | <0.001 | |
| pN1b | 0 | 15 | 82 | 83 | 61 | 13 | 57 | 10 | 0 | <0.001 | |
| pN2a | 0 | 0 | 28 | 53 | 26 | 29 | 11 | 25 | 0.150 | ||
| pN2b | 0 | 0 | 7 | 67 | 13 | 56 | 18 | 13 | 0.093 | ||
| Pb | - | 0.409 | 0.972 | 0.719 | 0.115 | ||||||
| LODDS classification | |||||||||||
| LODDS1 | 745 | 87 | 29 | 79 | 0.412 | ||||||
| LODDS2 | 137 | 81 | 70 | 63 | 16 | 68 | 0.017 | ||||
| LODDS3 | 53 | 80 | 148 | 58 | 0.002 | ||||||
| LODDS4 | 57 | 38 | 4 | 0 | 0.362 | ||||||
| LODDS5 | 38 | 13 | - | ||||||||
| Pc | 0.346 | 0.153 | 0.471 | - | 0.884 | ||||||
Pa: Comparison of survival rates between different LNR groups.
Pb: Comparison of survival rates between different pN groups.
Pc: Comparison of survival rates between different LODDS groups.
Nd: Number of patients.
5-YSRe: 5-year accumulative survival rate.
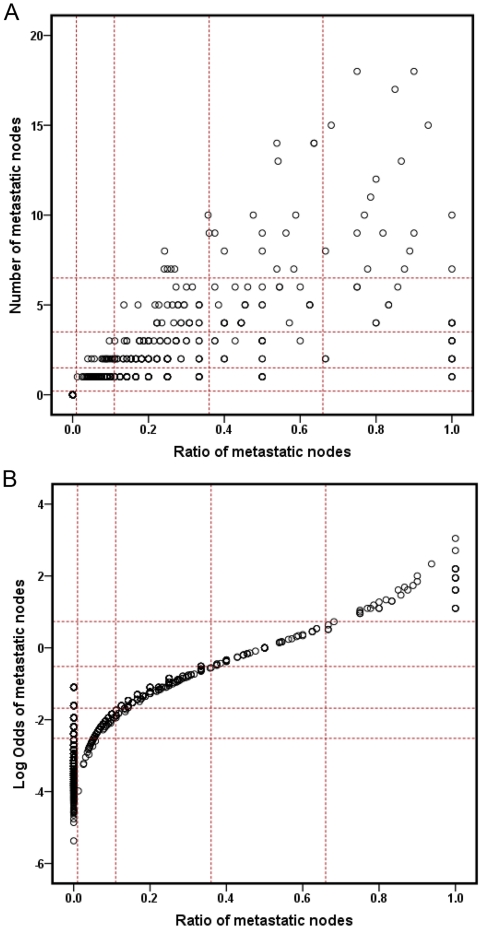
To explain why the LODDS classification was similar to LNR, we plotted scatter plots of the relationship among the three classifications. As shown in Fig. 3A, every pN classification can be divided into different LNR classifications. However, Fig. 3B showed that the patient distribution of LODDS classification was similar to the LNR classification and the value of LODDS increased with LNR increasing, indicating there was a close correlation between LODDS and LNR (except LNR = 0). When the LNR was 0, the value of LODDS was heterogeneous. However, Table 3 showed, for patients in the LNR0, prognosis was highly homologous between those in LODDS1, LODDS2 and LODDS3 classifications.
Figure 3. The distribution of the pN & LNR(A) and LODDS & LNR(B).
Discussion
Although UICC/AJCC TNM classification was revised significantly from the 5th edition to the 7th edition, especially in regard to the pN categories [4], [21], [22], the pN categories still have some deficiencies. The primary flaw of the number-based UICC/AJCC pN classification is that the accuracy of the predicting prognosis was significantly influenced by the total number of nodes retrieved. According to the guidelines for colorectal cancer from the AJCC/UICC, only when the number of LNs that were retrieved and examined was 12 or more, it could be regarded as an adequate lymphadenectomy for accurate staging [4]. However, cases with insufficiently retrieved and examined LNs are not unusual in clinical practice. This led to the development and adoption of new prognostic indices that incorporate all the lymph node information in a single identifiable parameter. Among the indices, the important and promising classifications are the LNR and LODDS classifications [8], [13].
LNR has been identified as a significant prognostic value in breast cancer [23], pancreatic cancer [24], gastric cancer [25]. Furthermore, an increasing number of studies have demonstrated that the LNR classification is superior to the pN classification in colorectal cancer [5]–[10], [26]. LODDS, a novel indicator of predicting the status of lymph nodes, provides a new chance to improve the accuracy of N classification for prognostic assessment. But research on LODDS has mainly focused on breast and gastric cancer [11], [12], [27]. Only the study of Wang et al. revealed that LODDS was a better prognostic factor than LNR classification [13].
In our study, pN, LNR and LODDS classifications were all identified as significant prognostic factors in univariate analysis. To investigate whether one N classification was superior to the others, multistep multivariate analysis has often been used [27], [28]. For example, to prove the LNR classification was superior to the pN classification, we performed a three-step multivariate analysis. In the step 1 multivariate analysis, pN classification was one of the independent prognostic factors, whereas in the step 2 multivariate analysis, pN classification was substituted by the LNR classification. In addition, we performed a step 3 multivariate analysis, including all the 3 N classifications (pN, LNR and LODDS). The results indicated that the LNR classification was superior to both the pN classification and the LODDS classification. On the other hand, the results of the Nagelkerke R2 index and a Harrell's C statistic showed that LNR and LODDS classification were similar and LNR was a little better than the other two classifications.
LODDS classification divided the patients with negative lymph node into three groups: LODDS1, LODDS2 and LODDS3. In contrast,patients with negative lymph node were staged only as pN0 or LNR0 in pN or LNR classifications. Unfortunately, no significant survival difference was found among the patients in three LODDS classifications in the present study. Therefore, the prognostic effect of LODDS classification for negative lymph nodes colorectal cancer patients need further investigation in larger samples. Furthermore, our results further confirmed the superiority of the LNR classification: for patients in each LNR classification, prognosis was highly homologous among those in different pN or LODDS classifications. However, for patients in pN1a, pN1b, LODDS2 and LODDS3 classifications, significant differences in survival could always be observed among patients in different LNR classifications. Thus, we think the LNR classification is superior to the pN and LODDS classifications and it can contribute to accuracy in prognostic assessment.
To date, although a number of studies have shown that LNR classification was superior to the pN classification, no study comparing the prognostic value among pN, LNR and LODDS classifications for colorectal cancer in Chinese patients has been reported. In our study, we first demonstrated that the LNR classification was superior to the pN and LODDS classifications in 1297 Chinese patients with colorectal cancer. However, Wang et al. studied 24,477 patients with stage III colon cancer that were registered in the SEER database and revealed that LODDS was a better prognostic factor than LNR. It is possible that different cut off points acquired from different statistical methods for subclassification, different populations, different environments and different diet habits contribute to these different results.
In clinical practice, when the LNs that were retrieved and examined were insufficient, a so-called “stage migration” phenomenon [25] appeared due to inappropriate staging in the pN classification and the prognosis of the patient was underestimated. On the other hand, as the LNR classification is easier to calculate than the LODDS classification, LNR is recommended to be used in clinical practice.
Our study has some limitations. Our conclusion results from a Chinese single institutional study in 1297 patients with colorectal cancer. We used running log-rank statistics to calculate our cut-off values which were different from previous studies. Whether our results and cut-off values for LNR and LODDS can be applied to other institutions remains to be demonstrated. We look forward to performing larger sample studies and international multicentric research on LNR and LODDS classifications in colorectal cancer in the near future.
In conclusion, for patients with colorectal cancer, the LNR classification is more suitable than pN and LODDS classifications for prognostic assessment. Although the best and most clinically meaningful cut-off value for LNR classification has yet to be determined, we still believe that the LNR classification is the most reliable N classification to date and should be recognized in China in the future.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Science Foundation of China (No. 30972879 and No. 81172370), Specialized Research Fund for the Doctoral Program of Higher Education (No. 200801590006) and Natural Science Foundation of Liaoning Province (No. 20092129). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ostadi MA, Harnish JL, Stegienko S, Urbach DR. Factors affecting the number of lymph nodes retrieved in colorectal cancer specimens. Surg Endosc. 2007;21:2142–2146. doi: 10.1007/s00464-007-9414-6. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Xu AG, Jiang B, Zhong XH, Liu JH. Clinical epidemiological characteristics of 3870 cases of colorectal cancers in Guangdong region. Zhonghua Nei Ke Za Zhi. 2006;45:9–12. [PubMed] [Google Scholar]
- 4.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, et al. AJCC cancer staging manual, 7th ed. New York: Springer; 2010. [Google Scholar]
- 5.Huh JW, Kim YJ, Kim HR. Ratio of metastatic to resected lymph nodes as a prognostic factor in node-positive colorectal cancer. Ann Surg Oncol. 2010;17:2640–2646. doi: 10.1245/s10434-010-1015-2. [DOI] [PubMed] [Google Scholar]
- 6.Qiu HB, Zhang LY, Li YF, Zhou ZW, Keshari RP, et al. Ratio of metastatic to resected lymph nodes enhances to predict survival in patients with stage III colorectal cancer. Ann Surg Oncol. 2011;18:1568–1574. doi: 10.1245/s10434-010-1528-8. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg R, Engel J, Bruns C, Heitland W, Hermes N, et al. The prognostic value of lymph node ratio in a population-based collective of colorectal cancer patients. Ann Surg. 2010;251:1070–1078. doi: 10.1097/SLA.0b013e3181d7789d. [DOI] [PubMed] [Google Scholar]
- 8.Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol. 2010;17:2847–2855. doi: 10.1245/s10434-010-1158-1. [DOI] [PubMed] [Google Scholar]
- 9.Ng M, Roy-Chowdhury S, Lum SS, Morgan JW, Wong JH. The impact of the ratio of positive to total lymph nodes examined and outcome in colorectal cancer. Am Surg. 2009;75:873–876. [PubMed] [Google Scholar]
- 10.Moug SJ, Saldanha JD, McGregor JR, Balsitis M, Diament RH. Positive lymph node retrieval ratio optimises patient staging in colorectal cancer. Br J Cancer. 2009;100:1530–1533. doi: 10.1038/sj.bjc.6605049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinh-Hung V, Verschraegen C, Promish DI, Cserni G, Van De Steene J, et al. Ratios of involved nodes in early breast cancer. Breast Cancer Res. 2004;6:680–688. doi: 10.1186/bcr934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voordeckers M, Vinh-Hung V, Van de Steene J, Lamote J, Storme G. The lymph node ratio as prognostic factor in node-positive breast cancer. Radiother Oncol. 2004;70:225–230. doi: 10.1016/j.radonc.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Hassett JM, Dayton MT, Kulaylat MN. The prognostic superiority of log odds of positive lymph nodes in stage III colon cancer. J Gastrointest Surg. 2008;12:1790–1796. doi: 10.1007/s11605-008-0651-3. [DOI] [PubMed] [Google Scholar]
- 14.Chok KS, Law WL. Prognostic factors affecting survival and recurrence of patients with pT1 and pT2 colorectal cancer. World J Surg. 2007;31:1485–1490. doi: 10.1007/s00268-007-9089-0. [DOI] [PubMed] [Google Scholar]
- 15.Crowley J, LeBlanc M, Jacobson J, Salmon S. Lecture Notes on Statistics. Proceedings of the First Seattle Symposium in Biostatistics: Survival Analysis. New York: Springer; 1997. Some exploratory tools for survival analysis. pp. 199–229. [Google Scholar]
- 16.Landau S, Everitt BS. A Handbook of Statistical Analyses using SPSS. Boca Raton FL: Chapman & Hall-CRC; 2004. [Google Scholar]
- 17.Eilers P, Marx B. Flexible smoothing with B-splines and penalties. Statistical Science. 1996;11:89–121. [Google Scholar]
- 18.Harrell FE., Jr Cox proportional hazards. 2000. pp. 453–499. In Regression Modeling Strategies with Applications to Survival Analysis and Logistic Regression Charlottesville, VA: University of Virginia.
- 19.Royston P, Sauerbrei W. A new measure of prognostic separation in survival data. Stat Med. 2004;23:723–748. doi: 10.1002/sim.1621. [DOI] [PubMed] [Google Scholar]
- 20.Downing SR, Cadogan KA, Ortega G, Jaji Z, Boloruduro OB, et al. The number of lymph nodes examined debate in colon cancer: how much is enough? J Surg Res. 2010;163:264–269. doi: 10.1016/j.jss.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Fleming ID, Cooper JS, Henderson DE. AJCC Cancer Staging Manual, 5th ed. New York: Springer; 1997. [Google Scholar]
- 22.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, et al. AJCC Cancer Staging Manual, 6th ed. New York: Springer; 2002. [Google Scholar]
- 23.Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, et al. Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol. 2009;27:1062–1068. doi: 10.1200/JCO.2008.18.6965. [DOI] [PubMed] [Google Scholar]
- 24.Bhatti I, Peacock O, Awan AK, Semeraro D, Larvin M, et al. Lymph node ratio versus number of affected lymph nodes as predictors of survival for resected pancreatic adenocarcinoma. World J Surg. 2010;34:768–775. doi: 10.1007/s00268-009-0336-4. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Zhu GL, Lu C, Guo PT, Huang BJ, et al. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient number or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients. Ann Oncol. 2009;20:897–905. doi: 10.1093/annonc/mdn707. [DOI] [PubMed] [Google Scholar]
- 26.Tong LL, Gao P, Wang ZN, Song YX, Xu YY, et al. Can lymph node ratio take the place of pN categories in the UICC/AJCC TNM classification system for colorectal cancer? Ann Surg Oncol. 2011;18:2453–2460. doi: 10.1245/s10434-011-1687-2. [DOI] [PubMed] [Google Scholar]
- 27.Sun Z, Xu Y, Li DM, Wang ZN, Guo PT, et al. Log odds of positive lymph nodes: a novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer. 2010;116:2571–2580. doi: 10.1002/cncr.24989. [DOI] [PubMed] [Google Scholar]
- 28.Persiani R, Rausei S, Biondi A, Boccia S, Cananzi F, et al. Ratio of metastatic lymph nodes: impact on staging and survival of gastric cancer. Eur J Surg Oncol. 2008;34:519–524. doi: 10.1016/j.ejso.2007.05.009. [DOI] [PubMed] [Google Scholar]