Abstract
Background and Purpose
Ancrod, derived from Malayan pit viper venom, has been tested as ischemic stroke treatment in clinical trials with inconsistent results. We studied the actions of ancrod on fibrinolysis pathways in patient samples and endothelial cell culture systems.
Methods
We analyzed fibrinogen levels during the first six hours of ancrod infusion in patients entered in the Stroke Treatment with Ancrod Trial (STAT). For the in vitro study, human brain microvascular endothelial cells (HBMVEC) or HBMVEC-conditioned medium were incubated with ancrod and/or fibrinogen under normal or oxygen-glucose deprivation conditions over six hours.
Results
Fibrinogen levels decreased both in vivo and in vitro. Ancrod generated fibrinopeptide A, caused visible clot formation, and reduced levels of tissue plasminogen activator (tPA) antigen in HBMVEC system and in a cell-free system with conditioned media.
Conclusions
The in vitro results indicate that ancrod causes local fibrin formation and secondary depletion of tPA by binding to fibrin clot. Ancrod-induced fibrin formation could result in cerebral microvascular occlusion and may explain the suboptimal clinical effects of ancrod in human stroke trials.
Keywords: ancrod, fibrinolysis, ischemic stroke, fibrinogen, defibrinogenation
Introduction
Ancrod has long been viewed as a potential treatment for acute ischemic stroke 1–6. Derived from the venom of the Malayan pit viper Calloselasma rhodostoma, ancrod possesses a serine protease that cleaves fibrinopeptide A (FPA) from fibrinogen 6–9. This fibrinogenolytic effect underlies ancrod’s potential clinical benefit, which would be based upon limited clot propagation, reduced plasma viscosity, improved microcirculatory flow, and activation of endogenous fibrinolysis 6–9.
However, results of ancrod in clinical trials of ischemic stroke have not been uniformly successful. Whereas the Stroke Treatment with Ancrod Trial (STAT) showed a favorable benefit-risk profile for ischemic stroke patients treated within three hours of stroke onset 2, the European Stroke Treatment with Ancrod Trial (ESTAT) and the Ancrod Stroke Program (ASP) showed no benefit in functional outcome for patients given ancrod within six hours of stroke onset 3, 4. To shed light on these disappointing findings, we studied the effects of ancrod on fibrinolysis by analysis of plasma samples from STAT patients and by in vitro studies with human brain microvascular endothelial cells (HBMVEC) including conditions of oxygen-glucose deprivation (OGD) as an in vitro ischemia model.
Materials and Methods
Clinical samples
We analyzed citrated blood obtained at baseline, three, and six hours after starting ancrod administration in ancrod-treated STAT2 patients, in whom local fibrinogen concentrations had been measured with photo-optical instruments based on the Clauss method10 (N=189, excluding 46 ancrod-treated subjects with fibrinogen measured with other instruments and 13 subjects with incomplete measurements). The study was approved by the institutional review board at each participating hospital and written informed consent was obtained from all patients or their representatives.
Cell culture and reagents
HBMVEC were maintained and identified as previously described11. Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen Corporation, Carlsbad, California) without glucose was used for OGD experiments. Human fibrinogen was from Enzyme Research Labs (South Bend, Indiana), human plasminogen was from Sigma (St Louis, Missouri), and ancrod (72 IU/ml) was provided by Neurobiological Technologies, Inc. (NTI, Emeryville, California). The control was the ancrod excipient (NTI).
Experimental design
In vitro experiments were performed with confluent monolayers of HBMVEC, with plasminogen (0.2 mg/mL) and with ancrod (0.004 IU/mL, comparable to that measured in patients after three hours of ancrod infusion) and/or fibrinogen (300 mg/dL) or appropriate control. OGD experiments were performed in a humidified chamber filled with 2% O2 and 5% CO2. After incubation at 37°C for 6 hours, conditioned medium was aliquoted and stored at −80°C. For cell-free tissue plasminogen activator (tPA) depletion studies, HBMVEC were grown to confluence and then incubated with M131 medium containing fibrinogen. After 6 hours, conditioned medium was collected and further incubated with or without ancrod for another 6 hours.
Assays
Plasminogen, tPA, urokinase plasminogen activator (uPA), plasminogen activator inhibitor-1 (PAI-1), and fibrinopeptide A (FPA) antigens were determined by enzyme immunoassay (American Diagnostica, Greenwich, Connecticut). Fibrinogen antigen was measured by immunoassay (Diapharma, West Chester, Ohio).
Statistical Analysis
Statistical analysis was performed using paired t-tests (for the clinical analysis) and analysis of variance with Tukey’s test. A p-value of <.05 was considered statistically significant.
Results
Mean fibrinogen concentration in plasma of patients receiving ancrod decreased from 358 mg/dL at baseline to 274 mg/dL (77% of baseline, p<.0001) at three hours and to 121 mg/dL (34% of baseline, p<.0001) at six hours (Figure 1). After incubation of ancrod plus fibrinogen with HBMVEC in vitro, fibrin clot was present at six hours (Figure 2); clot was not formed with fibrinogen alone or ancrod alone. Fibrinogen levels in conditioned medium of ancrod-treated HBMVEC decreased to 91 mg/dL after 6 hours, 31% of that present using fibrinogen without ancrod (Figure 3A) (p<.0001). FPA concentration was 8.7 ng/ml with ancrod (p<.0001) compared with negligible levels in cells treated with fibrinogen without ancrod (Figure 3B).
Figure 1. Plasma fibrinogen concentration in patients treated with ancrod.
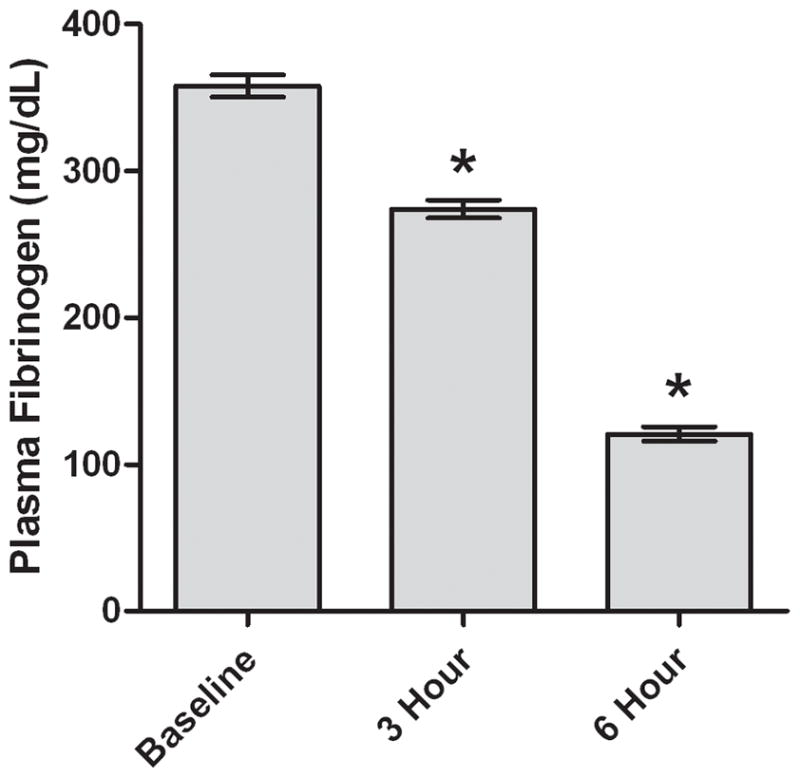
Plasma samples (N=189) were analyzed at baseline and at three and six hours after start of ancrod administration. Values represent mean; error bars represent standard error. *p<.0001 vs baseline.
Figure 2. In vitro clot formation generated by ancrod.
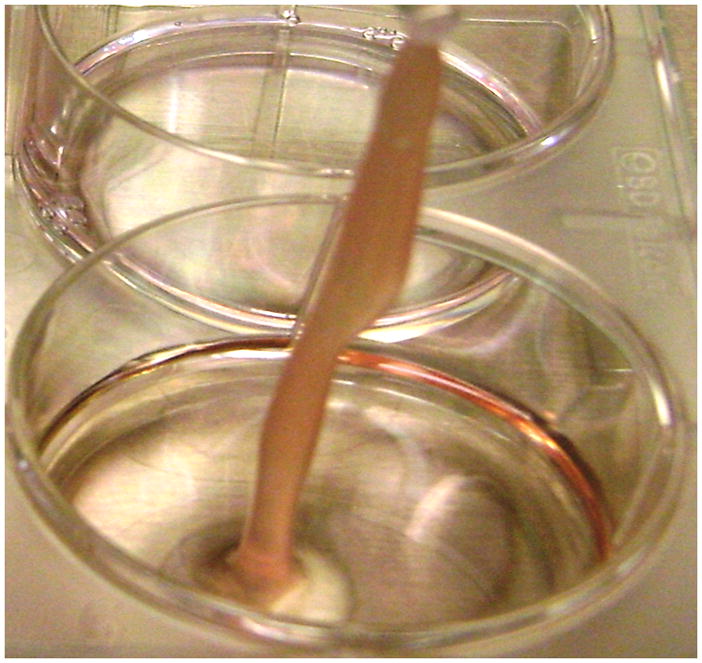
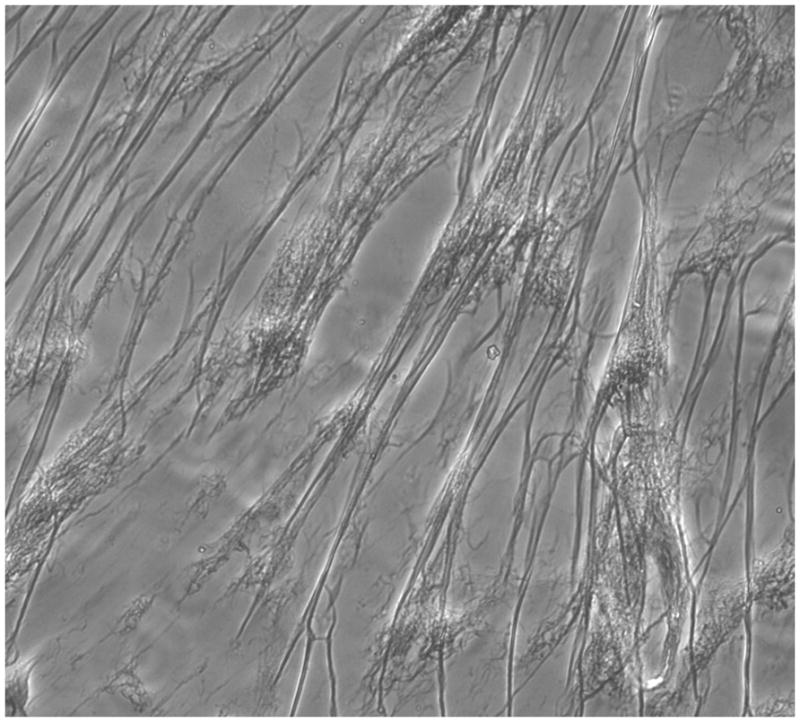
Ancrod plus fibrinogen added to HBMVEC induced clot formation (2A). Microscopic view (200X magnification) showed typical strands of fibrin (2B).
Figure 3. In vitro effects of ancrod.
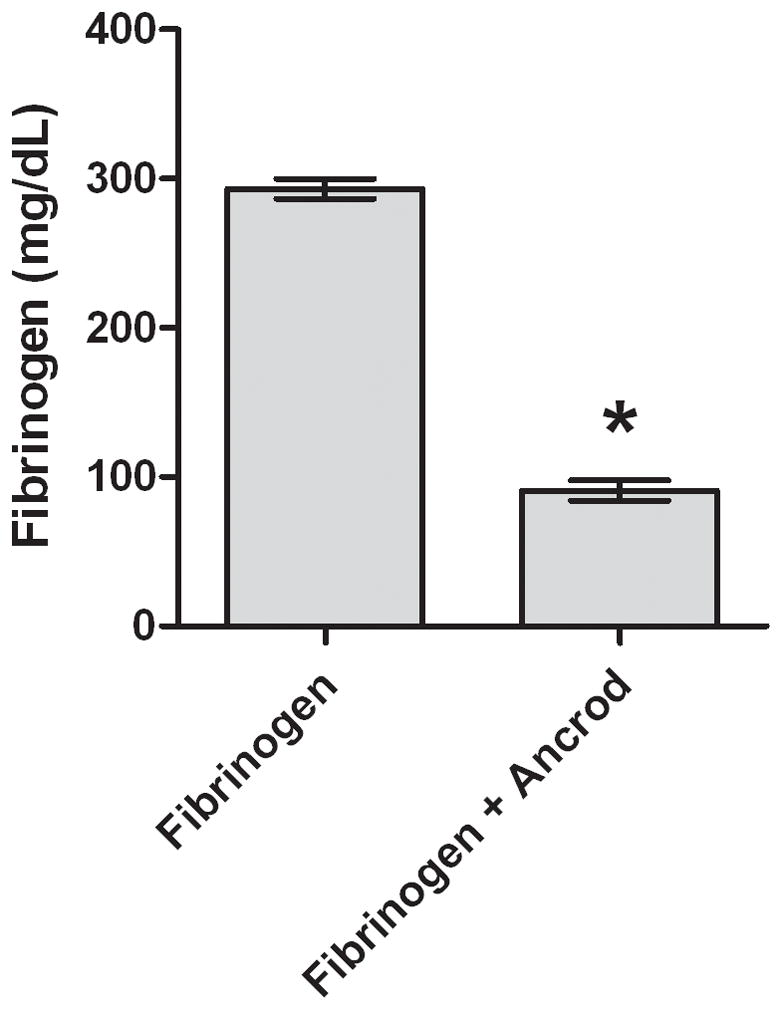
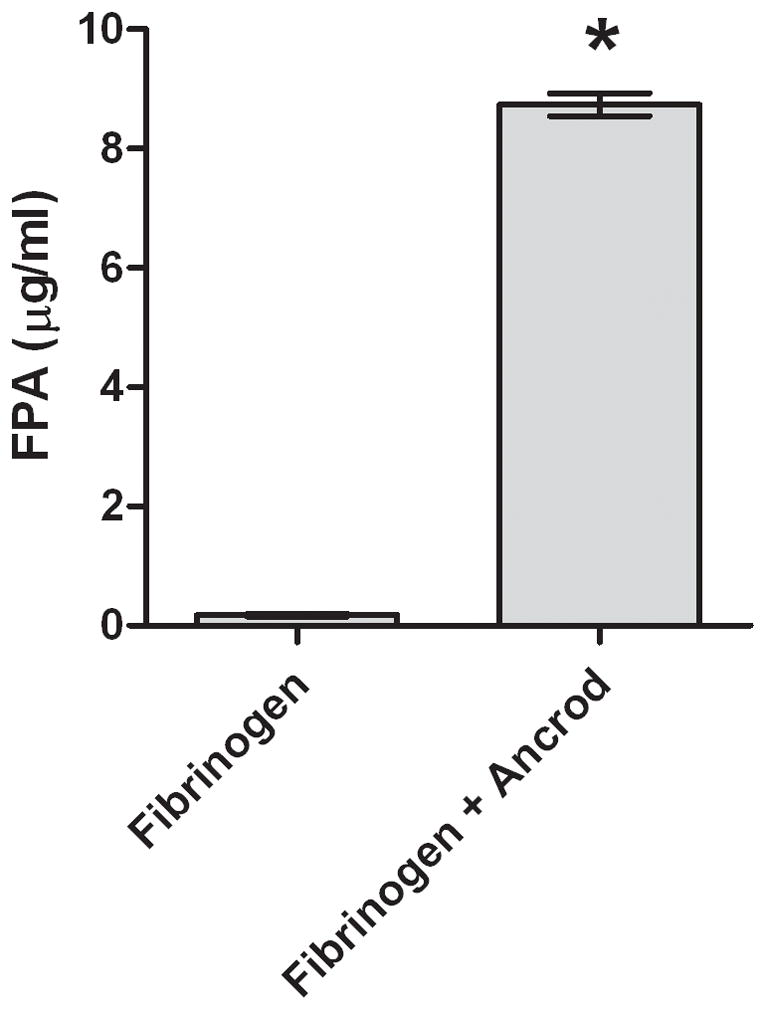
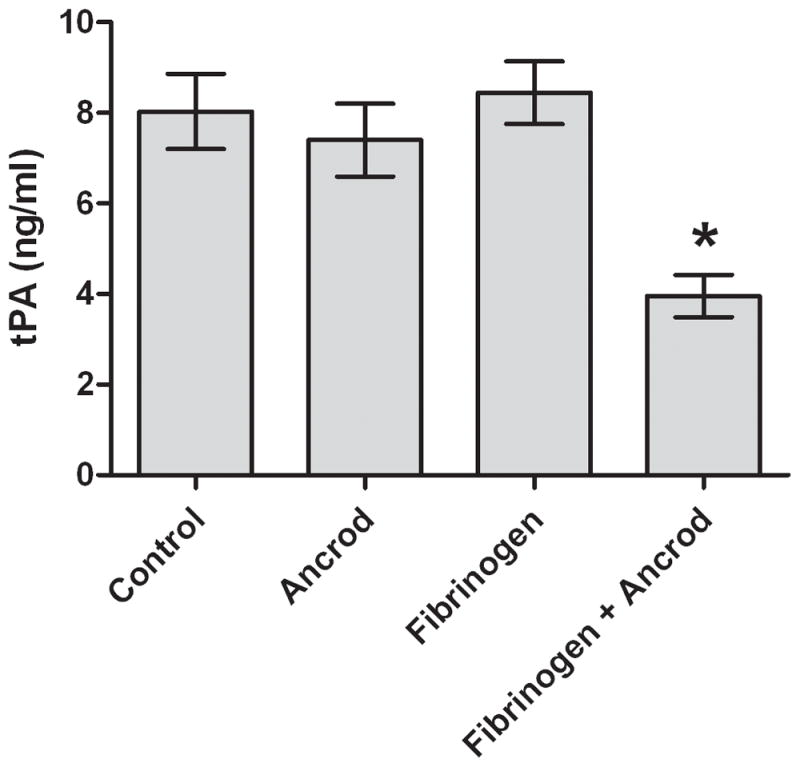
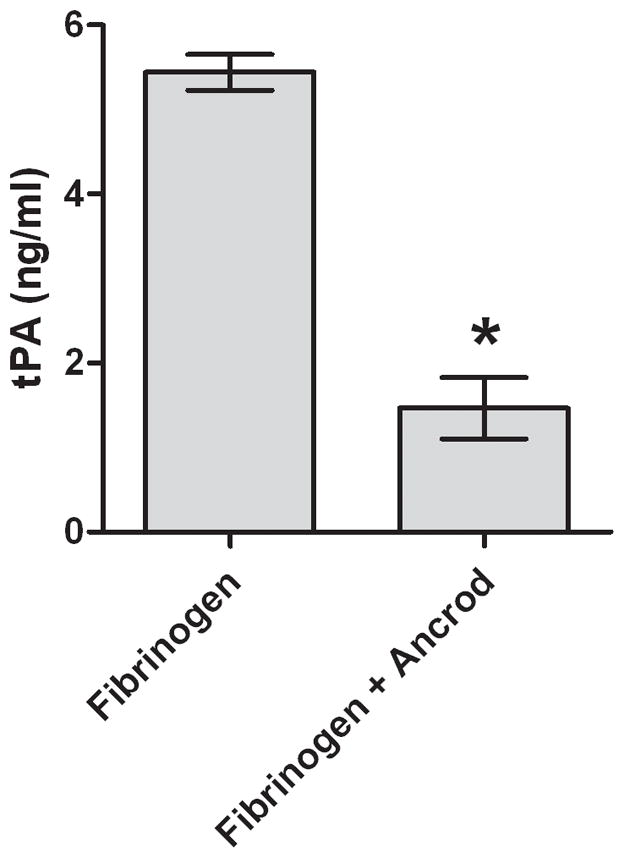
Fibrinogen (3A) and FPA (3B) antigen concentrations were measured in media conditioned by HBMVEC after incubation with ancrod for six hours. HBMVEC incubated with control, ancrod, fibrinogen, and fibrinogen+ancrod for six hours (3C). Six-hour endothelial conditioned media containing fibrinogen was isolated from HBMVEC and further incubated with ancrod for another six hours (3D). Pooled results from three independent experiments: values represent mean; error bars represent standard error. * p<.0001 vs fibrinogen (3A–B); vs control, ancrod, and fibrinogen (3C); or vs fibrinogen (3D).
Ancrod reduced the level of tPA antigen in fibrinogen-enriched HBMVEC-conditioned medium to 4.0 ng/ml, compared with 7.4–8.4 ng/ml under three control conditions (p<.0001 vs ancrod alone, fibrinogen alone, and neither, Figure 3C). To analyze the possibility that ancrod may have reduced tPA release by HBMVEC, we collected media (containing fibrinogen) conditioned six hours by HBMVEC; we then incubated the conditioned media with ancrod for an additional six hours without HBMVEC. Under these conditions, clot was formed and tPA levels decreased to 1.5 ng/ml compared with 5.4 ng/ml (p<.0001) in control (Figure 3D). These findings indicated that decline in tPA levels was not dependent on presence of endothelial cells.
Fibrinogen and ancrod treatment did not induce significant decrease in plasminogen antigen levels when compared with fibrinogen alone. Ancrod induced no significant change in levels of PAI-1 or uPA antigen. Under OGD conditions, ancrod produced similar but generally milder effects with regard to FPA generation and tPA depletion (data not shown).
Discussion
We demonstrated ancrod-induced decline in fibrinogen levels in vivo and in vitro, along with in vitro generation of FPA (released from fibrinogen), decline in tPA antigen levels, and production of fibrin clot. While ancrod-induced fibrin generation is well known, the fibrin produced has usually been understood to be non-cross-linked, soluble, readily degraded, and rapidly removed from the circulation 9, 12. Ancrod-induced insoluble fibrin has been previously described in a plasma-based in vitro system using ancrod at a concentration at least two to three orders of magnitude higher than was used in the current study 13. For the present work, we used a concentration of ancrod comparable to that utilized in stroke clinical trials (DE Levy, unpublished observations). The rapid decline of fibrinogen levels, release of FPA, and generation of insoluble fibrin clot are consistent with what is encountered in disseminated intravascular coagulation (DIC). Fibrin generation consequent to ancrod use may not be inconsequential and could contribute to cerebral microvascular occlusion 14.
Levels of tPA were substantially reduced when HBMVEC were incubated with ancrod and fibrinogen. In the cell-free system, tPA antigen showed a 73% decline in conditioned media, comparable to that in the presence of cells (approximately 50% depletion). This indicates that soluble tPA was depleted in the presence of clot formation, and that tPA was likely bound to clot resulting in low levels of soluble tPA in conditioned media. Prior in vivo work showed no change in levels of circulating tPA with ancrod treatment 6, 12, but levels of tPA in the systemic circulation may not reflect actions in the brain vasculature and microcirculation.
It is tempting to assume that fibrin clot generation observed in our cell culture system is analogous to what occurs in vivo. However, the relationship between endothelial cell surface area and fibrin clot will likely differ substantially between in vitro and in vivo systems, the fibrinolytic capacity of our cell culture system will also differ from what is encountered in vivo, and we did not measure direct indicators of fibrinolysis (e.g., fibrin D-dimer). Furthermore, variable results among the different ancrod clinical trials may reflect differences in study design and patient population, rather than generation of fibrin clot formation in the microvasculature, and there is no current evidence relating in vivo clot formation to poor outcome in ancrod clinical trials.
Despite the positive results in STAT, ancrod did not improve outcome for stroke patents in ESTAT or ASP. The variable clinical findings do not rule-out the possibility that ancrod may have a role in a subset of stroke patients with elevated levels of fibrinogen. Further clinical studies should analyze effects of ancrod on indices of fibrinolysis in patient subsets with differing stroke outcome. Nevertheless, ancrod-induced microvascular thrombotic occlusion is a potential explanation for adverse outcomes and could explain the lack of consistent effects of ancrod in stroke treatment.
Acknowledgments
We thank UCI undergraduate students Salma Rashan, Sabrina Rebollar, Christina Nguyen, and Que-Huong Thi Duong for their assistance.
Sources of Funding
This research was supported by Neurobiological Technologies, Inc. and by NIH NS 20989.
Footnotes
Disclosure(s)
None
References
- 1.Hossmann V, Heiss WD, Bewermeyer H, Wiedemann G. Controlled trial of ancrod in ischemic stroke. Arch Neurol. 1983;40:803–808. doi: 10.1001/archneur.1983.04050120053007. [DOI] [PubMed] [Google Scholar]
- 2.Sherman DG, Atkinson RP, Chippendale T, Levin KA, Ng K, Futrell N, et al. Intravenous ancrod for treatment of acute ischemic stroke: The STAT study: A randomized controlled trial. StrokeTreatment with Ancrod Trial. JAMA. 2000;283:2395–2403. doi: 10.1001/jama.283.18.2395. [DOI] [PubMed] [Google Scholar]
- 3.Hennerici MG, Kay R, Bogousslavsky J, Lenzi GL, Verstraete M, Orgogozo JM. Intravenous ancrod for acute ischaemic stroke in the european stroke treatment with ancrod trial: A randomised controlled trial. Lancet. 2006;368:1871–1878. doi: 10.1016/S0140-6736(06)69776-6. [DOI] [PubMed] [Google Scholar]
- 4.Levy DE, del Zoppo GJ, Demaerschalk BM, Demchuk AM, Diener HC, Howard G, et al. Ancrod in acute ischemic stroke: Results of 500 subjects beginning treatment within 6 hours of stroke onset in the ancrod stroke program. Stroke. 2009;40:3796–3803. doi: 10.1161/STROKEAHA.109.565119. [DOI] [PubMed] [Google Scholar]
- 5.Dempfle CE, Argiriou S, Alesci S, Kucher K, Muller-Peltzer H, Rubsamen K, et al. Fibrin formation and proteolysis during ancrod treatment. Evidence for des-a-profibrin formation and thrombin independent factor xiii activity. Ann N Y Acad Sci. 2001;936:210–214. [PubMed] [Google Scholar]
- 6.Dempfle CE, Argiriou S, Kucher K, Muller-Peltzer H, Rubsamen K, Heene DL. Analysis of fibrin formation and proteolysis during intravenous administration of ancrod. Blood. 2000;96:2793–2802. [PubMed] [Google Scholar]
- 7.Lowe GD, Morrice JJ, Forbes CD, Prentice CR, Fulton AJ, Barbenel JC. Subcutaneous ancrod therapy in peripheral arterial disease: Improvement in blood viscosity and nutritional blood flow. Angiology. 1979;30:594–599. doi: 10.1177/000331977903000903. [DOI] [PubMed] [Google Scholar]
- 8.Soszka T, Kirschbaum NE, Stewart GJ, Budzynski AZ. Effect of fibrinogen-clotting enzymes on secretion of plasminogen activators from cultured human endothelial cells. Fibrinolysis. 1988;2:49–57. [Google Scholar]
- 9.Levy DE, Del Zoppo GJ. Ancrod: A potential treatment for acute, ischemic stroke from snake venom. Toxin reviews. 2006;25:323–333. [Google Scholar]
- 10.Clauss A. Rapid physiological coagulation method in determination of fibrinogen. Acta Haematologica. 1957;17:237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- 11.Kim JA, Tran ND, Li Z, Yang F, Zhou W, Fisher MJ. Brain endothelial hemostasis regulation by pericytes. J Cereb Blood Flow Metab. 2006;26:209–217. doi: 10.1038/sj.jcbfm.9600181. [DOI] [PubMed] [Google Scholar]
- 12.Prentice CR, Hampton KK, Grant PJ, Nelson SR, Nieuwenhuizen W, Gaffney PJ. The fibrinolytic response to ancrod therapy: Characterization of fibrinogen and fibrin degradation products. Br J Haematol. 1993;83:276–281. doi: 10.1111/j.1365-2141.1993.tb08283.x. [DOI] [PubMed] [Google Scholar]
- 13.Pizzo SV, Schwartz ML, Hill RL, McKee PA. Mechanism of ancrod anticoagulation. A direct proteolytic effect on fibrin. J Clin Invest. 1972;51:2841–2850. doi: 10.1172/JCI107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okada Y, Copeland B, Fitridge R, Koziol J, del Zoppo G. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994;25:1847–1853. doi: 10.1161/01.str.25.9.1847. [DOI] [PubMed] [Google Scholar]


