Abstract
We presented naturalistic combinations of virtual self-movement stimuli while recording neuronal activity in monkey cerebral cortex. Monkeys used a joystick to drive to a straight ahead heading direction guided by either object motion or optic flow. The selected cue dominates neuronal responses, often mimicking responses evoked when that stimulus is presented alone. In some neurons, driving strategy creates selective response additivities. In others, it creates vulnerabilities to the disruptive effects of independently moving objects. Such cue interactions may be related to the disruptive effects of independently moving objects in Alzheimer's disease patients with navigational deficits.
Keywords: cortex, extrastriate, object motion, optic flow, visual motion
Introduction
Driving requires the visual processing of self-movement cues derived from the relative motion of discrete objects and the global patterns of optic flow (Gibson 1950). When the heading of object motion matches that in the surrounding optic flow, it suggests that the object is an earth-fixed landmark that can aid navigation. When object motion does not match the surrounding optic flow, it suggests that the object is an independently moving animate object to be evaded or intercepted. Moving observers must parse the interrelations between these stimuli to navigate through the world and identify potential predators and prey.
Neurons in dorsal medial superior temporal cortex (MSTd) of macaque monkeys respond to self-movement heading directions represented by the patterns of optic flow (Saito et al. 1986; Duffy and Wurtz 1991, 1995; Graziano et al. 1994; Lagae et al. 1994; Lappe et al. 1996). These neurons also show direction selective responses to the movement of discrete objects across the visual field (Tanaka et al. 1986; Komatsu and Wurtz 1988a). These response properties combine with their ability to integrate stimulus attributes across their large receptive fields (Heuer and Britten 2007) to form a potential neuronal substrate for the interactions between optic flow and object motion needed during self-movement through complex environments.
MSTd's neuronal responses are shaped by spatial attention to local motion stimuli (Treue and Martinez Trujillo 1999) and the global patterns of optic flow (Dubin and Duffy 2007). Tasks that alter the behavioral relevance of local motion and global pattern cues can shift neuronal responses between firing that reflects the local versus global features of an optic flow field (Page and Duffy 2007). When optic flow is combined with object motion during a simple fixation task, individual neurons change their responses depending on the momentary details of the stimulus configuration (Sato et al. 2010).
These observations lead us hypothesize that rapid changes in neuronal responsiveness to combined optic flow and object motion might reflect the attentional gating of local versus global processing. In the current studies, we test this hypothesis by engaging our monkeys in a naturalistic driving task with alternating periods of driving by either the optic flow or the object motion in combined stimuli. We find that the monkey's driving strategy powerfully influences MSTd's responses in a manner that may account for the variability seen when attention is allowed to wander.
Materials and Methods
Animal Preparation
Animals were accommodated to the laboratory routine and then underwent surgery in preparation for single neuron recording. Surgery was preceded by sedation with Ketamine (15 mg/kg intramuscluar [im]) and Robinul (0.011 mg/kg im), followed by venous catheterization, endotracheal intubation, and general anesthesia using inhaled Isoflurane. During surgery, we monitored heart rate, core temperature, expired CO2, and the surgical plane of anesthesia.
We excised a 3 × 4 cm section of scalp, debrided the exposed calvarium, and inserted 24 dental pins around the edge of the exposed bone as implant anchors. Bilateral scleral search coils were placed by peri-limbotomy, tunneling the leads to the scalp excision (Judge et al. 1980). A head holder socket was placed at the frontal midline and bilateral recording cylinders were placed over 2 cm trephine holes above area MSTd (anterior-posterior –2 mm, medial-lateral ± 15 mm, angle 0). The dental pins, coil connectors, head holder, and recording chambers were then encased in a dental acrylic cap.
Postoperatively, the animal was returned to its home cage when it showed postural stability and coordinated movements. Monitoring of the monkeys' behavior guided the administration of Banamine (1 mg/kg im) analgesia as judged to be appropriate by veterinary staff. The excised scalp edge and the recording chambers were cleaned daily. All protocols were approved by the University of Rochester Committee on Animal Research and complied with U.S. Public Health Service and Society for Neuroscience policy on the care of laboratory animals.
When the animals fully recovered from surgery, they were trained to maintain visual fixation during visual stimulus presentation as confirmed by eye position monitoring using magnetic search coils. The monkeys were required to maintain fixation (±3°) while viewing object motion and optic flow stimuli simulating translational self-movement. Fixation duration was gradually increased over several months to train the monkeys to repeatedly fixate for 5–8 s. Successful completion of a trial was followed by the automated delivery of an audible tone and a liquid reward.
Visual Stimuli
Visual stimuli were generated by a personal computer driving a television projector at 60 Hz to illuminate a rear projection screen that covered the monkeys' central (90° × 90°) visual field. All stimuli were composed of pixel white dots (0.19° at 2.61 cd/m2) presented on a dark background (0.18 cd/m2). These stimuli simulated forward translational movement in the ground plane with heading deflections limited to ±30° along the horizontal meridian.
Optic Flow Stimuli
The optic flow stimuli simulated self-movement through a 3D cloud of illuminated points filling a 9 m depth field in front of the observer who was moving at 3 m/s. The optic flow stimuli were composed of 1000 illuminated white pixels (0.19° at 2.61 cd/m2) presented on a dark background (0.18 cd/m2). The optic flow dots moved in a radial pattern simulating translational movement of the observer. In the first frame, dots were distributed in a random pattern filtered to smooth dot density across the screen. Dots were removed either by passing out of the 90° × 90° field of view or by the expiration of a randomly distributed, preassigned lifetime ranging from 1 to 60 frames. Dot speed was a sin2 function of dot eccentricity measured as the angle between the monkey's line of sight and each dot's location on the screen and simulated distance. An average dot speed of ∼20°/s was maintained across all stimuli to match the range of speed preferences of MSTd neurons (Orban et al. 1995; Duffy and Wurtz 1997).
Object Motion Stimuli
The object motion stimuli consisted of a cube with an X on each of its 3 visible faces. The object was formed by an outline of 35 (far) to 120 (near) dots, varying with object size across stimulus frames to maintain visible structure and comparable dot densities across all stimulus frames. The height and width of the object varied with simulated distance from 4.5° to 9.0° to create repeated cycles of slowly looming forward for ∼1 s and instantaneously reappearing along the same heading at a the smaller size, much like a succession of objects seen as one travels forward along a path. When heading veered to the right, the object drifted to the left side of the screen, and vice versa, again like a series of objects encountered along a path of forward translational self-movement.
Combined Stimuli
The combined stimuli contained superimposed optic flow and object motion identical to those presented alone but in these trials presented together with either matched or nonmatched headings. In matched heading combined stimuli, the heading simulated by the object motion matched that in the surrounding optic flow field to simulate an earth-fixed landmark object. In nonmatched heading combined stimuli, the heading simulated by object motion violated that of the surrounding optic flow field to simulate independently moving (animate) objects. The object did not occlude the flow; that is, pixels within the bounds of an object, which were not illuminated as part of the object, could be illuminated as part of the optic flow.
Behavioral Paradigms
Behavioral Control
We trained monkeys to drive their self-movement heading direction as simulated by either an optic flow field or a moving object during a joystick steering task guiding the heading of their simulated translational movement. Trials of the selected driving condition followed one another such that blocks of trials lasted up to 90 s. The monkeys completed up to 15 trials during that period which was followed by a pause of ∼30 s. Blocks of trials typically continued for up to 30 min, resulting in ∼30 trials per combined stimulus condition.
If visual fixation or joystick grasp failed during a trial, the monkey had 500 ms to self-correct or that block of trials was ended with an audible error tone, the visual stimuli were extinguished, and there was a 3–5 s time-out period. If the monkey was able to self-correct within 500 ms, the visual stimuli continued with the data from that period dropped from the record. The monkeys achieved a ∼90% success rate across all conditions of this task.
Eye position was monitored by search coils, head position was fixed in the apparatus, and body position was constrained in the monkey chair. At no time during the presentation of any analyzed data segment did the monkey moves its eyes, head, or body orientation.
Task Paradigm
The monkey initiated a block of trials by fulfilling 2 sustained conditions: First, centering the joystick, by centering the square joystick cursor over the fixation spot. Second, maintaining gaze fixation on the centered fixation spot for 1 s. Each block of trials began with either optic flow or object motion presented alone. Those first trials cued the monkey as which cue it should use throughout that block of trials (Figs 1A,B and 2A; see also Supplementary Fig. S1A,B). The heading simulated by the target cue was randomly deviated to the left or right by imposing a horizontal drift velocity. The monkey then manipulated the joystick to guide the visual stimulus to return to a centered simulated heading. The monkey maintained that centered heading for 1 s to earn a liquid reward. As soon as a reward was received, a new trial began with the introduction of a new heading drift condition, initiated without an interruption in the visual stimulus. Thus, the task consisted of a series of changes in the imposed drift velocity with the monkey responding to recenter the heading simulated by the selected cue to earn a liquid reward (Fig. 1).
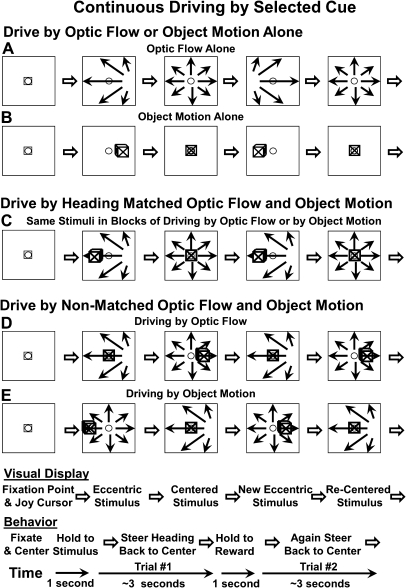
Figure 1.
Diagram of stimuli and tasks showing 2 consecutive trials in each of the task conditions. The monkey initiated a block of trials by centering the joystick cursor (small square in stimulus frames) and establishing fixation on the centered fixation target (small open circle in stimulus frames). The monkeys then maintain centered gaze and moved the joystick to recenter their simulated heading in a continuous driving task using optic flow and/or object motion. Each block of trials (total ∼ 1.5 min) began with either optic flow (A) or object motion alone (B), cueing the monkey to use that cue throughout the trials (3–4 s) of that block. Here, the simulated heading is randomly deviated to the left by imposing a drift velocity in that direction. The monkey drives to center the heading to earn liquid reward. When centering leftward deviated optic flow, the monkey makes a rightward joystick deflection. When centering leftward deviated object motion, the monkey makes a left joystick deflection. (C) The optic flow and moving object are then superimposed such that the movements are along matched headings shown here by the heading displacement to the right resulting in the earth-fixed object moving to the left. In other blocks of trials, the optic flow and object motion are nonmatched heading and the irrelevant stimulus is a distracter. (D) An illustration of a nonheading trial in which the monkey uses the optic flow to center its heading. (E) An illustration of a nonmatched heading trial in which the monkey uses the object motion to center the object in the middle of the screen.
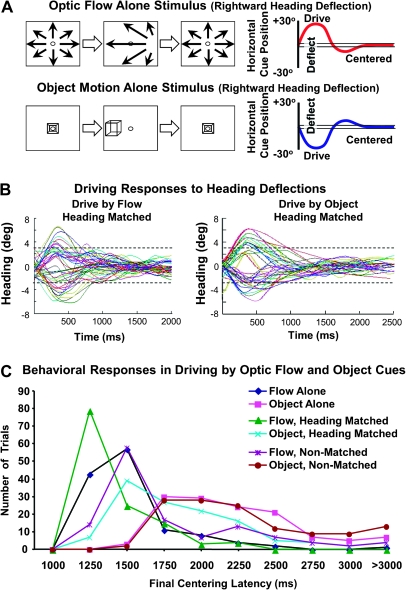
Figure 2.
Behavioral paradigm and performance in cue selective driving. (A) Schematic diagram of heading recentering stimuli (left) and task performance (right) presented with optic flow (top) and object motion (bottom). A centered heading is displaced randomly to the left or right and the monkey uses a joystick to drive it back to the center. The graph shows a schematic representation of an initially centered heading that is displaced toward a rightward heading and causes the monkey to drive it back to the center. (B) Numerous trials (colored lines) are shown for the matched heading stimulus conditions in which the monkey drives with the optic flow (left) and object motion (right). In both cases, the trials began with minimal heading error (ordinate) and the heading is either deflected to the right of center (upward deflections) or left of center (downward deflections). In the majority of trials, the monkey realigns the heading faster when driving with the optic flow. (C) Recentering response latencies (abscissa) are averaged across all trials (ordinate) for each of the 6 driving conditions (colored lines). The latency to the monkeys' maintaining a centered heading for 1 s, and earning a reward, was shortest for the trials in which the monkey was directed to drive by the optic flow pattern with heading matched object motion, simulating an earth-fixed object. The trials in which the monkey was directed to drive by the flow, regardless of the relationship to the object stimulus, were shorter than trials where the monkey was directed to drive by the object stimulus.
In centering the optic flow and the object motion cues, the monkeys performed 2 distinct, although naturalistically linked, tasks. When the optic flow's focus of expansion shifted to the right, it simulated a rightward deflection of the monkeys' heading. In response, the monkeys had to deflect the joystick to the left, in order to recenter the focus of expansion. When the moving object shifted to the right, it simulated a leftward deflection of the monkeys' heading, as when you steer to the left in driving, and objects that had been directly in front of you gradually move in to your right visual field. In response, the monkey had to deflect the joystick to the right, in order to recenter the object (Supplementary Fig. S1).
After 6 successful trials with optic flow or object motion alone, the 2 types of stimuli were combined and the monkey continued to use the selected stimulus to guide its steering to center throughout that block of trials. In each of the combined stimulus trials, the computer randomly chose whether the irrelevant cue matched the heading of the target cue or moved along a nonmatched heading. In the matched heading condition, the simulated self-movement heading depicted by the optic flow and the object motion was the same (Fig. 1C; see also Supplementary Fig. S1C,D). In the nonmatched heading condition, the task-irrelevant stimulus moved independently of the target stimulus and of joystick manipulations, varying its right or left velocity in a sinusoidal pattern across the screen (Fig. 1D,E; see also Supplementary Fig. S1E,F). Whether the task-irrelevant cue was heading matched or nonmatched, the task remained the same, steering to center the selected target cue.
Justification of This Paradigm
In the first set of 6 trials in each block, the monkey learned to use the selected cue (optic flow or object motion) as the centering target. These trials yielded neuronal responses to single cue stimuli that contributed to our analysis of responses to combined stimuli. In matched heading combined trials, the monkey could use either the optic flow or the object motion cue. These trials served the primary goal of this study: to compare neuronal responses to matched, centered heading, stimuli during driving by optic flow versus driving by object motion. The nonmatched heading combined trials were included primarily to verify the monkey's strategy in the task. In these trials, centering of the nontarget cue would never lead to sustained centering of the target and would not yield a reward. The random interleaving of matched and nonmatched heading trials imposed the strategy of continuing to use the selected target cue throughout each block of trials.
Single Neuron Recording
We recorded single neuron action potentials using epoxy-coated tungsten microelectrodes (FHC, Inc. and Microprobe, Inc.) passed through a transdural guide tube positioned within a recording chamber (Crist et al. 1988). As the electrode was advanced, we monitored neural activity to identify gray and white matter layers and the relative depth of physiological landmarks. After isolating a neuron's responses, its receptive field was mapped using hand-held projectors. MSTd neurons were identified by their: large receptive fields (>20°) that included the fixation point and showed a preference for large moving patterns, with direction selective responses evident in the audible representation of their activity (Komatsu and Wurtz 1988a; Duffy and Wurtz 1991, 1995). A template matching algorithm (Alpha Omega, Inc. Atlanta, GA) was used to digitize neuronal discharge times that were stored with stimulus and behavioral event markers for offline analysis using the REX system (Hays et al. 1982).
Recording Sites
Single neuron recordings were directed to MSTd using stereotaxic positioning. When experiments were completed, electrolytic marks (25 μA × 25 s) were made at selected depths in several guide tubes in 1 of the 2 monkeys. After pentobarbital euthanasia, the animal was perfused with heparinized saline followed by formalin, and the brain was removed. When formalin fixation was complete, cortical regional blocks were cut in 50 μm sections with every fourth and fifth section stained by the Nissl and Luxol Fast Blue methods, respectively. We identified the electrolytic lesions relative to anatomic landmarks to extrapolate the position of the recording sites. This analysis indicates that the neurons studied were located in the anterior bank of the superior temporal sulcus that is included in cortical area MSTd (Komatsu and Wurtz 1988b). Although the boundaries between areas within the superior temporal sulcus(STS) are nebulous, we did not record neurons further up the STS in to area 7a, further anteriorly along the STS in to fundal superior temporal cortex nor across the STS in to the middle temporal area (Nelissen et al. 2006). We have applied the rule that gray matter layers that include neurons with the large, direction selective, pattern motion preferring receptive fields of MSTd are considered to be part of MSTd (Duffy and Wurtz 1991, 1995). In these areas, we occasionally encounter neurons with smaller, more central receptive fields that do not respond to our hand controlled spot or bar stimuli but only to large projected patterns that cross the central field as they are moved over the screen.
Data Analysis
Neuronal spike times were convolved with a 20 ms Gaussian to produce trialwise spike density response functions averaging the 6–8 presentations of each stimulus. Average spike densities were plotted on a time line to relate changes in firing rates to the parameters of object motion and optic flow. We did not change timing to account for response latency differences within or between neurons.
We used 2-way analyses of variance (ANOVAs) with main effects of stimulus type (alone vs. matched combined vs. nonmatch combined) and driving condition (drive by optic flow vs. drive by object motion) to identify neurons with significant effects. Follow-up 1-way ANOVAs of driving condition effects were conducted for each stimulus type as reported in the text. We used regression analysis to examine relations between neuronal firing rates in the combined stimulus conditions as a function of firing rates in the object motion and optic flow alone conditions. To do so, we compared activity in each 1-s interval of each combined condition with activity in the 1-s intervals from the alone conditions that presented the corresponding component stimuli. Our approach was to separately examine firing rate relations in each combined stimulus condition, although in some cases (specified below), we joined data across conditions (e.g., both single cue conditions were entered in to multiple linear regressions to model heading matched and nonmatched responses). All statistical analyses were carried-out using SPSS (SPSS Inc. 2007).
Results
We conducted studies of 141 neurons from 3 hemispheres of 2 adult Rhesus monkeys; 127 of these neurons yielded a complete data set under all stimulus conditions. Single neuron recordings were made at sites that included neurons with large receptive fields, often more than a full quadrant of the visual field, including the fovea. All neurons showed direction selective motion sensitivity preferring large pattern motion to moving bars as is typical in MSTd (Komatsu and Wurtz 1988b).
Monkeys were engaged in up to 30 min of nearly continual driving during the recording of each neuron. The monkeys fixated the center of the screen and viewed either optic flow or object motion alone (Fig. 2A). Every 3–4 s, the heading simulated by the stimulus was randomly deflected to the left or right and the monkey used a joystick to drive it back to the center. After a series of such trials, the optic flow and object motion stimuli were superimposed to create a series of matched or nonmatched heading combined cue stimuli (see Materials and Methods, Behavioral Paradigms) while the monkey continued to drive using the cue that was presented at the beginning of that block of trials (Fig. 1; see also Supplementary Fig. S1).
The final 1 s of every trial that led to a reward, and accepted response data, presented the continually centered target stimulus (optic flow and/or object motion). The final 1 s in matched heading combination trials centered both cues at the fixation point, allowing us to compare responses to matched stimuli during different driving conditions. That period in nonmatched heading combination trials centered the selected cue, leaving the other cue off center, forcing the monkeys' cue selection. The impact of cue selection was evident in the timing of the monkey's responses. (Fig. 2B,C).
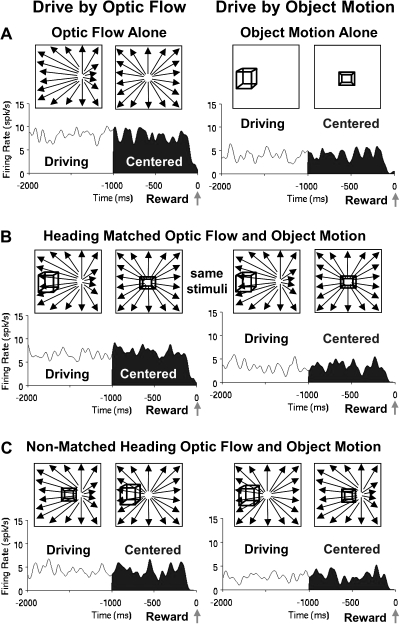
Neuronal responses to the stimulus and driving conditions are illustrated by several examples (Figs 3–5) with descriptive statistics of the samples from which they are drawn. Many neurons responded to the combined stimuli as if the selected cue was the only one present. The neuronal responses in Figure 3 show strong activation by optic flow alone and little activation by object motion alone. When the monkey was driving by optic flow, the neuron responded to the combined stimuli as if responding to optic flow alone. When the monkey was driving by object motion, the neuron responded to the combined stimuli as if responding to object motion alone. Thus, the neurons' visual responsiveness depended on the monkeys' driving strategy. This pattern of responses should be contrasted with that which can be expected from purely visual sensory effects (Supplementary Figs S2 and S3).
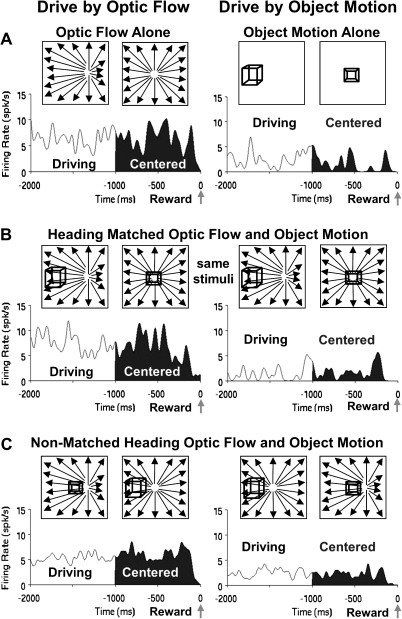
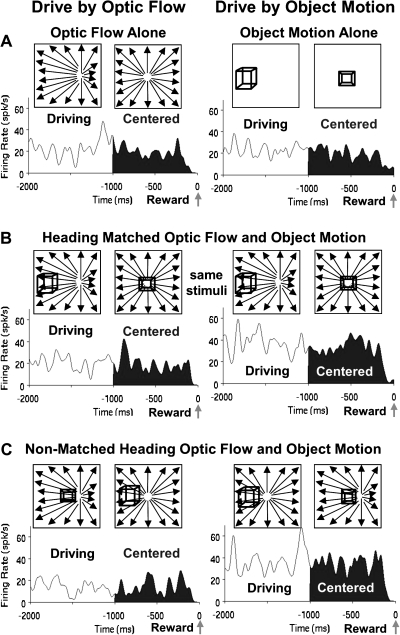
Figure 3.
Average spike density histograms presenting the final 2 s of the trials in each condition, the final 1 s of which (filled) presented continually centered targets. These responses illustrate the influence of the monkey's driving strategy on neuronal activation. (A) The responses of a neuron showing much stronger activation to driving optic flow alone (left) compared with driving object motion alone (right). (B) These differences are also evident when the optic flow and object motion are combined in the matched heading stimuli, despite the presentation of identical stimuli during driving by optic flow (left) and driving by object motion (right). (C) The same effects of the selected driving cue are evident in responses to the nonmatched heading stimuli even though they contain somewhat different stimulus configurations in the 2 driving conditions.
Figure 4.
Comparison of the stimulus alone and heading matched combined stimulus conditions indicate the irrelevant stimulus can interfere with the response. Average spike density histograms for this neuron shows modest responses to the object alone condition (A, right) that increases when the optic flow is added for both the matched (B, right) and the nonmatched (C, right) heading conditions during driving by object motion. This suggests a selective additivity of the 2 alone responses for the object driving combined stimulus conditions. There is no comparable response additivity when driving by optic flow (A–C, left).
Figure 5.
The irrelevant cue in the nonmatched heading combined stimulus condition could also interfere with the differential responses in the 2 driving conditions. Average spike density histograms for this neuron show stronger response in the optic flow driving conditions in both the alone (A) and the heading matched (B) conditions, but these differences are reduced during the nonmatched heading condition (C).
In this sample of neurons, 43% (55/127) showed significant effects of driving condition (P < 0.05) in the last 1 s of responses to heading matched stimuli when the monkey was driving by optic flow versus driving by object motion. A substantial number of these neurons (60%, 33/55) were among the 56% (66/127) that showed significant effects in the comparable periods when each cue was presented alone. Nevertheless, comparing relative firing rates showed that 81% (101/125) of the neurons have the same object motion versus optic flow driving preferences in the alone and matched heading combined stimulus conditions. That is, the alone stimulus that evoked the higher firing rate (optic flow or object motion) was also the combined stimulus driving condition (drive by optic flow or drive by object motion) that showed higher firing rate.
The similar numbers of neurons with significant responses evoked by the alone and combined stimuli does not reflect an obligate mirroring of the alone responses by responses evoked by the combined stimuli. To the contrary, the sample includes examples of driving condition dependent additive response interactions and driving condition dependent subtractive response interactions.
Driving condition dependent additive response interactions are seen in neurons that show responses to combined stimuli that approximate the sum of the responses to optic flow and object motion presented alone, rather than matching responses to the selected cue only. A unique aspect of these responses is that the response additivity occurs only when one or the other cue is selected to guide driving (Fig. 4). Thus, we find evidence of visual response interactions that depend on the monkeys' behavior; that is, driving strategy dependent response additivity.
Interactions between visual self-movement cues were also seen in responses to nonmatched heading combined stimuli which were dominated by the nonselected cue. This occurred despite the selected cues' dominating those neurons' responses to matched heading combined stimuli. Figure 5 exemplifies this effect with responses to the matched heading combined stimuli that are like those evoked by each cue presented alone. However, this neuron's responses to nonmatched heading combined stimuli during driving by optic flow are smaller than the responses to optic flow alone. In this case, responses to object motion appear to have suppressed the larger responses to optic flow; the nonselected stimulus suppressed the response to the selected stimulus but only in the nonmatched heading condition.
We characterized relationships between the single stimulus responses and the combined stimulus responses across the sample of neurons, both in terms of the relative influence of optic flow and object motion stimuli on responses to the heading matched and nonmatched combined stimuli (Fig. 6), and in terms of the additivity of response interactions in the heading matched driving conditions (Fig. 7); that is, how responses in each of the combined stimulus conditions depends on the responses in each of the alone stimulus conditions.
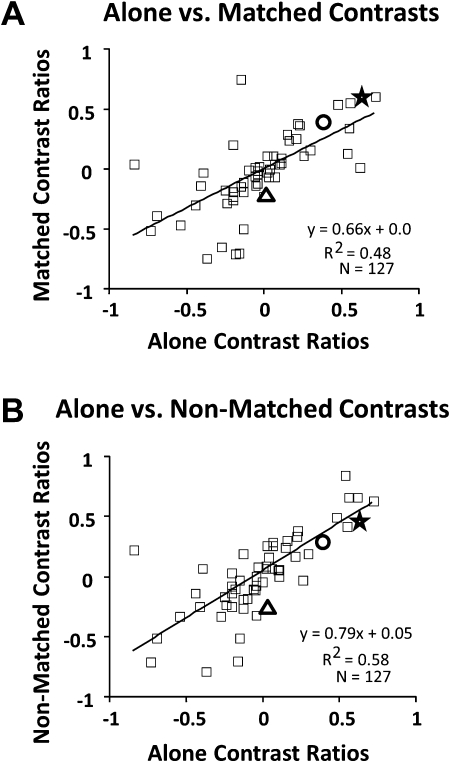
Figure 6.
Comparisons between responses evoked during driving by optic flow and object motion across stimulus conditions. Each scatter plot shows a data point for each neuron, the points representing the contrast ratios for driving by optic flow minus driving by object motion, divided by the sum of those values. The neurons illustrated in the preceding figures are indicated as: Figure 3, star; Figure 4, triangle; Figure 5, circle. (A) Plot of the contrast ratios to each stimulus presented alone (abscissa) versus the matched heading combined stimuli (ordinate) showing that most neurons maintain similar selectivities under both conditions. (B). Plot of the contrast ratios to each stimulus presented alone (abscissa) versus the nonmatched heading combined stimuli (ordinate) again showing that most neurons maintain similar selectivities under both conditions. In both cases, but more clearly with nonmatched combinations, there is less variability among optic flow preferring neurons (positive ratios) than among object motion preferring neurons (negative ratios).
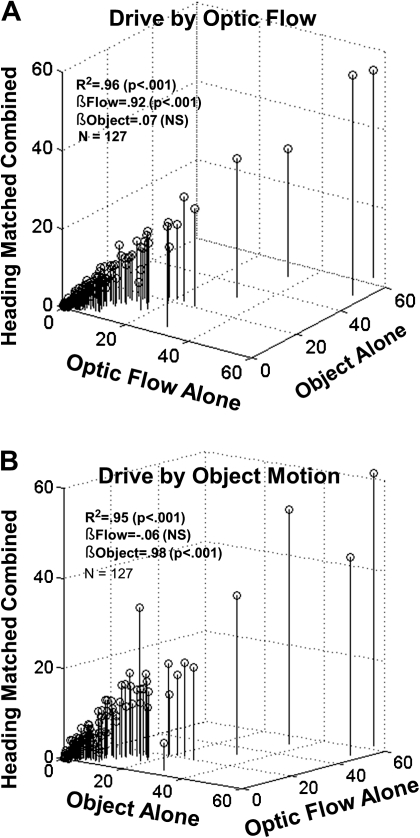
Figure 7.
The relative contribution/weighting of individual stimuli for the neuronal responses depends on the driving strategy. (A) Three-dimensional scatter plot of the responses to optic flow alone, object motion alone, and the drive by optic flow, matched heading condition. A multiple linear regression indicated a strong correlation (r2 = 0.96) with a large significant beta weight for optic flow (0.92) and small nonsignificant beta weight for object motion (0.07). (B) Three-dimensional scatter plot of the responses to optic flow alone, object motion alone, and the drive by matched heading object motion condition. A multiple linear regression indicated a strong correlation (r2 = 0.95) with a small nonsignificant beta weight for optic flow (−0.06) and a large significant beta weight for object motion (0.98). Thus, in identical matched heading stimulus conditions, the weighting for the cued driving stimulus increases while the irrelevant stimulus decreases.
To compare the relative influence of optic flow and object motion on the combined responses, we derived contrast ratios of the responses recorded during driving by optic flow minus driving by object motion, divided by the sum of those values. These contrast ratios were derived for the alone stimulus condition and the matched and nonmatched heading combined stimulus conditions. The contrast ratios for the alone and matched heading combined conditions showed a significant linear relationship (r2 = 0.48, P < 0.001) reflecting the matched heading response's mirroring the alone responses (Fig. 6A). The contrast ratios for the alone and nonmatched heading combined conditions also showed a strong linear relationship (r2 = 0.58, P < 0.001) (Fig. 6B). The scatter in both relationships is concentrated at the negative contrast ends of the spectrum, among neurons with stronger responses to object motion than optic flow. In these neurons, object motion appeared to have a greater influence on combined responses, especially with nonmatched heading combined stimuli.
We further explored the relative impact of optic flow and object motion on neuronal activity by comparing the relationships between the alone and matched heading combined responses. The overall effect of driving strategy on responsiveness to visual self-movement cues is seen in the relationship between responses to the alone and matched heading combined conditions. This relationship is described by multiple linear regression analyses in which the response amplitude to combined stimuli is modeled as a function of responses to the alone stimuli. The results of those regression analyses are presented graphically in Figure 7.
Responses to matched heading combined cues showed strong relationships to responses to the optic flow and object motion cues presented alone: During driving by optic flow (R2 = 0.96), the influence of optic flow responses was determinative (βflow = 0.92, P < 0.001; βobject = 0.07, not significant) (Fig. 7A). In contrast, during driving by object motion (R2 = 0.95), the influence of object motion was determinative (βflow = −0.06, not significant; βobject = 0.98, P < 0.001) (Fig. 7B). Thus, the cue selected to guide driving dominated visual responsiveness.
A different result was obtained in assessing the impact of self-movement cues on responses to nonmatched heading combined stimuli. Like the matched heading responses, those evoked by nonmatched heading cues showed strong relationships to the optic flow and object motion responses: Unlike the matched heading responses, those evoked by nonmatched heading cues during driving by optic flow (R2 = 0.94) showed significant effects of the nonselected (βobject = 0.18, P = 0.001) as well as the selected (βflow = 0.80, P < 0.001) cue. This was not the case during driving by object motion (R2 = 0.97) that was significantly related to the selected cue only (βflow = −0.005, not significant; βobject = 0.99, P < 0.001). Thus, driving by optic flow showed a unique effect of significant interference by nonmatched heading object motion.
Discussion
Interactions between object motion and optic flow responses are evident in object motion's confounding human heading estimation (Warren and Saunders 1995; Habak et al. 2002). Larger confounding effects have been seen when the objects cross the self-movement heading simulated by the optic flow (Royden 2002; Royden and Conti 2003). Conversely, object motion perception may be enhanced by superimposed optic flow, particularly where there are larger differences between the direction and speed of object motion and that in the immediately surrounding optic flow (Royden and Connors 2010).
MSTd neurons exhibit similar 3D heading direction selectivities for both optic flow and object motion stimuli (Logan and Duffy 2006). Combined optic flow and object motion evoke veridical neuronal population heading selectivity when both stimuli simulate the same heading. When a wide variety of relative heading combinations are presented using superimposed optic flow and object motion single MSTd neurons show a wide range of additive and nonadditive response interactions (Sato et al. 2010). In single neurons, these responses included idiosyncratic preferences for particular stimulus combinations. However, across the neuronal population, there is no particular combination of matched or unmatched headings that is preferred; all stimulus combinations evoked similar levels of population activity.
The visual motion responses of MSTd neurons are modulated by ongoing behavioral tasks. Responses to local motion patches are augmented by the monkeys' directing spatial attention to the location of the motion patch (Treue and Maunsell 1996). Although motion patches are critically different from moving objects, the former lacking the movement of its boundaries, there is clearly a potential parallel with instantaneous local motion. MSTd neuronal responses to optic flow are also augmented by the monkey's directing spatial attention, in this case to the site of the simulated heading direction (Dubin and Duffy 2007). These effects can be seen as discrete waves of response enhancement associated with the timing of covert reorientation to the heading direction in sustained presentations of optic flow stimuli (Dubin and Duffy 2009).
Considering the rapid time course of covert reorientation indicated by our earlier studies, we considered that attentional shifts between object motion and optic flow could account for the varying response additivities seen with combined stimuli.
We tested this hypothesis in the current work, finding that over 40% of the neurons showed changes in their responses to heading matched combinations of optic flow and object motion depending on the monkeys' use of one or the other cue in the driving task (Figs 3–5). In most cases, this driving dependent selectivity caused the responses to the combined stimuli to approximate those recorded when the selected stimulus was presented by itself. In other cases, the effect was seen as a selective additivity of responses, typically showing additive effects during driving by 1 cue with suppressive interactions during driving by the other cue.
In many neurons, driving dependent responses to combined nonmatched heading directions were like those seen with heading matched stimuli. This was surprising because the nonmatched conditions present different stimuli during driving by optic flow and driving by object motion. One interpretation is that that driving dependent responses filter out nonselected stimuli, with little effect of whether the filtered stimulus is centered or displaced. Neurons that show less completely suppressive response interactions, mainly during driving by object motion (Figs 5 and 6), can be seen as forming a continuum with the more selective cells.
Driving dependent response suppression could engage the same mechanisms that inhibit receptive field segments along the vertical meridian to shape MSTd's optic flow selectivity (Yu et al. 2010). Receptive field based inhibitory effects may also contribute to the surprising suppression of MSTd's optic flow responses during centrally focused local motion processing (Page and Duffy 2007). Together, these findings suggest that attentional shifts access inhibitory mechanisms that shape receptive field response properties. Spontaneous fluctuations in such effects could account for the diverse interactions between optic flow and object motion during the free viewing of naturalistically diverse cue combinations (Sato et al. 2010).
The selection of either optic flow or object motion to guide driving was found to have a tremendous impact on the relative influence of these stimuli on the neuronal responses (Fig. 7). When the monkeys drive using optic flow, the responses to the heading matched combined stimuli were closely related to the responses to optic flow alone without significant links to the object motion responses. Likewise, when the monkeys drive using object motion, the responses to the heading matched combined stimuli were closely related to the responses to object motion alone without significant links to the optic flow responses. The cue selection effects seen in these studies are like those modeled as competitive interactions between neurons shaped by directing attention to 1 of 2 objects in the visual fields of temporal cortical neurons (Reynolds et al. 1999). This effect might also be related to the previously seen changes in MSTd's population responses when optic flow and object motion simulate opposite heading directions and the population represents the heading of the object rather than the heading in the optic flow (Logan and Duffy 2006). Together, these findings suggest that momentary changes in the monkey's attention to particular cues in a naturalistic array has an enormous impact on neuronal responses, shaping them to better meet the immediate demands of ongoing behavior.
These findings may have implications for the neuropathophysiology of disease. We have found accurate heading perception based on either optic flow or object motion, without much benefit of matching superimposed stimuli, in young subjects; they do as well with each stimulus alone as they do with them combined. In contrast, Alzheimer's disease (AD) patients show significant benefits from heading matched optic flow and object motion (Mapstone et al. 2006) but are uniquely confounded by nonmatched heading cues, always seeing them as straight ahead self-movement regardless of the heading direction in the stimuli (Mapstone and Duffy 2010).
In light of our current findings, we are drawn to consider that heading errors with nonmatching optic flow and object motion might reflect greater dependence on combined cues, AD patients benefiting when they are heading matched and being confused by them when they are not matched. These effects may reflect a decline in the inhibitory mechanisms that create the capacity for robust cue selection, as seen in our most selective neurons.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
National Eye Institute (grants R01-EY10287 and P30EY01319); National Institute of Mental Health (grant T32EY07125).
Supplementary Material
Acknowledgments
All authors contributed equally to this work. We gratefully acknowledge the scientific and computer programming contributions of William Vaughn and the animal care and training assistance of Sherry Estes. Conflict of Interest: None declared.
References
- Crist CF, Yamasaki DS, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recordings. J Neurosci Methods. 1988;26:117–122. doi: 10.1016/0165-0270(88)90160-4. [DOI] [PubMed] [Google Scholar]
- Dubin MJ, Duffy CJ. Behavioral influences on cortical neuronal responses to optic flow. Cereb Cortex. 2007;17:1722–1732. doi: 10.1093/cercor/bhl083. [DOI] [PubMed] [Google Scholar]
- Dubin MJ, Duffy CJ. Neuronal encoding of the distance traversed by covert shifts of spatial attention. Neuroreport. 2009;20:49–55. doi: 10.1097/WNR.0b013e32831b44b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large-field stimuli. J Neurophysiol. 1991;65:1329–1345. doi: 10.1152/jn.1991.65.6.1329. [DOI] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. Response of monkey MST neurons to optic flow stimuli with shifted centers of motion. J Neurosci. 1995;15:5192–5208. doi: 10.1523/JNEUROSCI.15-07-05192.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. Medial superior temporal area neurons respond to speed patterns in optic flow. J Neurosci. 1997;17:2839–2851. doi: 10.1523/JNEUROSCI.17-08-02839.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JJ. The perception of the visual world. Boston (MA): Houghton Mifflin; 1950. [Google Scholar]
- Graziano MSA, Andersen RA, Snowden RJ. Tuning of MST neurons to spiral motion. J Neurosci. 1994;14:54–67. doi: 10.1523/JNEUROSCI.14-01-00054.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habak C, Casanova C, Faubert J. Central and peripheral interactions in the perception of optic flow. Vision Res. 2002;42:2843–2852. doi: 10.1016/s0042-6989(02)00355-3. [DOI] [PubMed] [Google Scholar]
- Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conference Proceedings. 1982;Vol. 2:1–10. [Google Scholar]
- Heuer HW, Britten KH. Linear responses to stochastic motion signals in area MST. J Neurophysiol. 2007;98:1115–1124. doi: 10.1152/jn.00083.2007. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol. 1988a;60:580–603. doi: 10.1152/jn.1988.60.2.580. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. III. Interaction with full-field visual stimulation. J Neurophysiol. 1988b;60:621–644. doi: 10.1152/jn.1988.60.2.621. [DOI] [PubMed] [Google Scholar]
- Lagae L, Maes H, Raiguel S, Xiao DK, Orban GA. Responses of macaque STS neurons to optic flow components: a comparison of areas MT and MST. J Neurophysiol. 1994;71:1597–1626. doi: 10.1152/jn.1994.71.5.1597. [DOI] [PubMed] [Google Scholar]
- Lappe M, Bremmer F, Pekel M, Thiele A, Hoffmann KP. Optic flow processing in monkey STS: a theoretical and experimental approach. J Neurosci. 1996;16:6265–6285. doi: 10.1523/JNEUROSCI.16-19-06265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan DJ, Duffy CJ. Cortical. area MSTd combines visual cues to represent 3-D self-movement. Cereb Cortex. 2006;16:1494–1507. doi: 10.1093/cercor/bhj082. [DOI] [PubMed] [Google Scholar]
- Mapstone M, Duffy CJ. Approaching objects cause confusion in patients with Alzheimer's disease regarding their direction of self-movement. Brain. 2010;133:2690–2701. doi: 10.1093/brain/awq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapstone M, Logan D, Duffy CJ. Cue integration for the perception and control of self-movement in ageing and Alzheimer's disease. Brain. 2006;129:2931–2944. doi: 10.1093/brain/awl201. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Vanduffel W, Orban GA. Charting the lower superior temporal region, a new motion-sensitive region in monkey superior temporal sulcus. J Neurosci. 2006;26:5929–5947. doi: 10.1523/JNEUROSCI.0824-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA, Lagae L, Raiguel S, Xiao D, Maes H. The speed tuning of medial superior temporal (MST) cell responses to optic-flow components. Perception. 1995;24:269–285. doi: 10.1068/p240269. [DOI] [PubMed] [Google Scholar]
- Page WK, Duffy CJ. Cortical neuronal responses to optic flow are shaped by visual strategies for steering. Cereb Cortex. 2007;18(4):727–739. doi: 10.1093/cercor/bhm109. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royden CS. Computing heading in the presence of moving objects: a model that uses motion-opponent operators. Vision Res. 2002; 42(28):3043–3058. doi: 10.1016/s0042-6989(02)00394-2. [DOI] [PubMed] [Google Scholar]
- Royden CS, Connors EM. The detection of moving objects by moving observers. Vision Res. 2010;50:1014–1024. doi: 10.1016/j.visres.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Royden CS, Conti DM. A model using MT-like motion-opponent operators explains an illusory transformation in the optic flow field. Vision Res. 2003;43:2811–2826. doi: 10.1016/s0042-6989(03)00481-4. [DOI] [PubMed] [Google Scholar]
- Saito H, Yukie M, Tanaka K, Hikosaka K, Fukada Y, Iwai E. Integration of direction signals of image motion in the superior temporal sulcus of the macaque monkey. J Neurosci. 1986;6:145–157. doi: 10.1523/JNEUROSCI.06-01-00145.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Kishore S, Page WK, Duffy CJ. Cortical. neurons combine visual cues about self-movement. Exp Brain Res. Forthcoming 2010;206(3):283–297. doi: 10.1007/s00221-010-2406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS Inc. SPSS 15.0. Upper Saddle River (NJ): Prentiss Hall, Inc; 2007. [Google Scholar]
- Tanaka K, Hikosaka K, Saito H, Yukie M, Fukada Y, Iwai E. Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. J Neurosci. 1986;6:134–144. doi: 10.1523/JNEUROSCI.06-01-00134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Martinez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- Warren WH, Jr, Saunders JA. Perceiving heading in the presence of moving objects. Perception. 1995;24:315–331. doi: 10.1068/p240315. [DOI] [PubMed] [Google Scholar]
- Yu CP, Page WK, Gaborski R, Duffy CJ. Receptive field dynamics underlying MST neuronal optic flow selectivity. J Neurophysiol. 2010;103:2794–2807. doi: 10.1152/jn.01085.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.