Abstract
Background
The supplemental oxygen flow rate is a common bedside measure of gas exchange impairment. We aimed to determine whether a titrated oxygen requirement predicted mortality in idiopathic pulmonary fibrosis.
Methods
We examined 104 adults with idiopathic pulmonary fibrosis enrolled in a prospective cohort study and a validation cohort of 151 adults with a variety of interstitial lung diseases. The titrated oxygen requirement was defined as the lowest oxygen flow rate required to maintain an oxyhemoglobin saturation of 96% while standing. Cox proportional hazards models and time-dependent receiver operating characteristic curves were used to examine survival time.
Results
A higher titrated oxygen requirement was associated with a greater mortality rate independent of forced vital capacity and six-minute walk test results in idiopathic pulmonary fibrosis (adjusted hazard ratio per 1 L/min = 1.10, 95% confidence interval 1.01 to 1.20). The titrated oxygen requirement was at least as accurate as pulmonary function and six-minute walk testing at predicting 1-year mortality. Findings were similar in other interstitial lung diseases.
Conclusion
The titrated oxygen requirement is a simple, inexpensive bedside measurement that aids prognostication in idiopathic pulmonary fibrosis.
Keywords: Idiopathic pulmonary fibrosis, Interstitial lung diseases, Outcome prediction, Pulmonary fibrosis, Pulmonary gas exchange
INTRODUCTION
The interstitial lung diseases (ILDs) are a heterogeneous group of diseases characterized by inflammation and fibrosis of the lung parenchyma [1]. Idiopathic pulmonary fibrosis (IPF), a common ILD that affects older adults, carries a median survival time of approximately 3 years [2, 3]. However, a significant fraction of affected individuals survive for more than 7 to 10 years [4, 5]. Accurate tools to improve prognostication in IPF would aid clinicians and researchers by informing guidelines for the referral and prioritization of patients for lung transplantation, facilitating discussions of end-of-life planning, and identifying appropriate candidates for experimental therapies.
A number of studies have identified associations between reduced exercise test performance and higher mortality rates in IPF [4–11]. For example, both reduced maximal exercise capacity during cycle ergometry [5, 7, 11] and a shorter distance walked during hallway walk testing are associated with higher mortality rates [8–10]. Well-designed studies have indicated that exertional desaturation during six-minute walk testing may explain the association between decreased exercise capacity and mortality in IPF [4, 6].
Exercise capacity and exertional desaturation, however, are strongly influenced by the fraction of inspired oxygen administered during testing [12, 13]. Current guidelines suggest that six-minute walk testing be performed using the patient’s “standard rate” of oxygen flow [14]. Previous studies have excluded those with low resting oxyhemoglobin saturation (SpO2) and have administered supplemental oxygen in a non-standardized fashion during six-minute walk testing [4, 6, 8–10], limiting the widespread application and interpretation of submaximal exercise test results in this population
The amount of supplemental oxygen required to maintain SpO2 at physiologic levels is a common bedside measure of disease severity across a wide spectrum of lung diseases, and the degree of gas exchange impairment at rest is linked to higher mortality rates in IPF [4, 5, 15–17]. However, there are no data examining the reliability and predictive validity of a standardized measure of oxygen requirement in IPF, limiting the ability to examine oxygen requirement in epidemiologic studies and clinical trials. At our center, we implemented an oxygen titration protocol to standardize the use of supplemental oxygen during six-minute walk testing in 2007. In the current study, we hypothesized that the oxygen flow rate required to maintain SpO2 at 96% or greater would predict short-term outcomes in IPF.
METHODS
Study subjects and study design
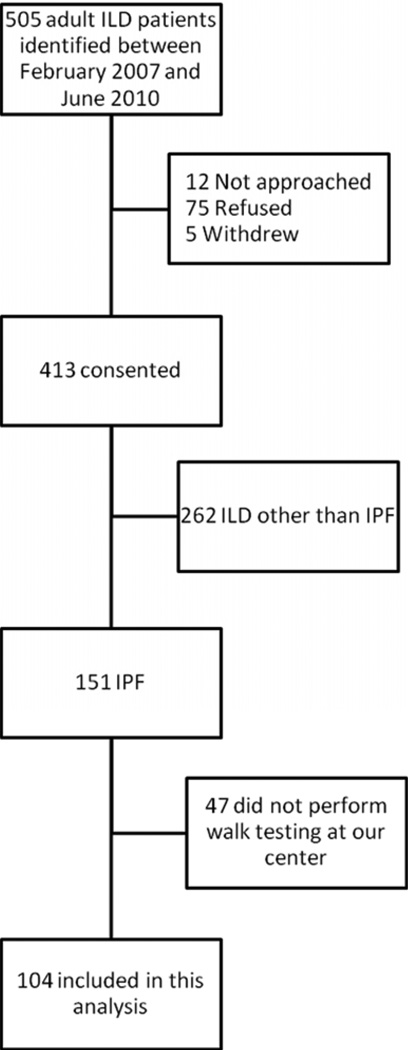
We examined 104 adults with IPF who enrolled in a prospective cohort study of ILD patients at the New York Presbyterian Lung Transplant and Interstitial Lung Disease Programs between February 2007 and June 2010. We screened 505 adults seen by a pulmonologist at our center with suspected or known ILD (Figure 1). Of these, 418 consented and 151 met American Thoracic Society/European Respiratory Society criteria for IPF [18]. We excluded 262 who met criteria for an ILD other than IPF, such as those with evidence of connective tissue disease, a history of occupational or environmental exposures known to cause pneumoconioses, and those with suspected drug-induced lung disease. We excluded 47 who did not undergo oxygen titration testing at our center. The most common reasons for not undergoing testing at our center were: patient preference (n = 17), testing not ordered (n = 12), and testing performed according to a clinical trial protocol (n = 7). The study cohort consisted of 104 participants with IPF who underwent an oxygen titration study at our center (Figure 1). The Columbia University Medical Center Institutional Review Board approved the prospective study. All participants gave written informed consent.
Figure 1.
Study participant flow. ILD = interstitial lung disease; IPF = idiopathic pulmonary fibrosis
To validate our findings, we subsequently assembled a cohort of 152 ILD patients not enrolled in the prospective study by searching the Clinical Data Warehouse at Columbia University for all six-minute walk tests performed for adults with ILD between February 2007 and April 15, 2010. We excluded 1 patient who did not have available spirometry data. The Columbia University Medical Center Institutional Review Board approved this retrospective study and waived informed consent.
Six-minute walk testing and titrated oxygen requirement
Six-minute walk testing was performed in a 100-ft long straight indoor hallway by a single physical therapist in accordance with American Thoracic Society guidelines.[14] Supplemental oxygen was administered according to the results of the oxygen titration study described below using a commercially-available integrated E-cylinder, valve, and regulator device (Linde Integrated Valve; Linde North America, Murray Hill, NJ). Pulse oximetry was measured prior to the six-minute walk test and during patient-initiated rests using a single Nellcor OmiMax N-65 pulse oximeter (Covidien-Nellcor, Boulder, CO). A single pulse oximeter was selected in order to minimize measurement variability. Pulse oximetry was not monitored during ambulation. The distance walked during testing (6MWD), the oxyhemoglobin saturation at the end of the test (end-walk SpO2), and the modified Borg dyspnea scale were measured after each walk. Testing was not terminated for desaturation below 80%.
Immediately prior to six-minute walk testing, an oxygen titration study was performed to determine the lowest oxygen flow rate required to maintain a SpO2 of at least 96% in the standing position (titrated oxygen requirement, or TOR). Titration began with the patient breathing room air. SpO2 was monitored for 1 minute. If the SpO2 was 96% or greater, the study ended. If the SpO2 was less than 96%, the oxygen flow rate was increased each minute to achieve a goal oxyhemoglobin saturation of at least 96% using the following titration “steps”: 1, 2, 3, 4, 5, 6, 8, 12, and 15 L/min. A nasal cannula was used to administer 1 to 6 L/min and a non-rebreather mask was used for 8 to 15 L/min. To examine the inter-observer reliability (precision) of the TOR, two investigators measured the TOR of 10 study participants with IPF in a blinded fashion less than 7 days apart. The results were identical for 9 of 10 participants and disagreed by only 1 L/min in the remaining participant. The weighted kappa (a test of agreement between groups for ordinal variables, such as TOR) was 0.90, suggesting that the TOR is a precise measure. Six-minute walk testing was performed using the TOR; no further changes were made to oxygen flow during six-minute walk testing. We have performed six-minute walk testing using the titrated oxygen flow rate in over 300 patients with ILD with no adverse events other than dyspnea.
Analysis
The cohort was divided into rough quartiles of TOR. We examined associations between TOR and the rate of death using Cox proportional hazards models censored at the time of transplantation. Stratified Cox models with strata for diagnostic category were used in the validation cohort in order to account for variation by diagnosis. We included purposefully selected covariates known to be important prognostic factors in IPF: age, 6MWD, end-walk SpO2, and forced vital capacity (FVC). We examined the ability of TOR and other established prognostic factors to predict the risk of death and transplantation using time-dependent receiver operative characteristic (ROC) curves (survivalROC package in R) [19]. Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, North Carolina), and R version 2.8.1 (R Foundation, Vienna, Austria). P values less than 0.05 were considered statistically significant.
RESULTS
IPF Participant Characteristics
The mean (SD) age was 62 (7) years, and 79% were male. Forty-five percent had diagnoses confirmed by surgical lung biopsy. The mean (SD) FVC was 54 (21) %, and the mean (SD) diffusing capacity of carbon monoxide (DLCO) was 37 (13) %. The mean 6MWD (SD) was 394 (133) m, and 56% had an end-walk SpO2 of 88% or less (interquartile range 82 to 92%). Compared to those who performed an oxygen titration study, excluded participants were older (mean age 68 vs. 62 years, p < 0.001), more frequently female (38 vs. 21%, p = 0.03), and had lower FVC (mean 46 vs. 54% predicted, p = 0.046).
The median TOR was 2 L/min (interquartile range 0 to 4 L/min) and the mean (SD) TOR was 3.3 (4.6) L/min. Other than shortness of breath, no adverse events occurred during six-minute walk testing or oxygen titration. Those with higher TOR values were more likely to be male and tended to have lower FVC, lower total lung capacity, lower DLCO, higher pulmonary artery pressure, and lower 6MWD (Table 1).
Table 1.
Participant characteristics in the derivation cohort
| Titrated oxygen requirement (L/min) | |||||
|---|---|---|---|---|---|
| No. | 0 | 1 to 2 | 3 to 6 | 8 to 15 | |
| No. | 104 | 42 | 20 | 28 | 14 |
| Age, yrs | 104 | 62 (12) | 62 (6) | 62 (7) | 64 (4) |
| Male, % | 104 | 79 | 70 | 79 | 93 |
| Body mass index, kg/m2 | 104 | 28 (5) | 30 (6) | 28 (4) | 30 (5) |
| Ever-smoker, % | 104 | 71 | 70 | 61 | 64 |
| Forced vital capacity, % predicted | 104 | 65 (21) | 45 (17) | 44 (16) | 50 (16) |
| Total lung capacity, % predicted | 103 | 67 (18) | 48 (11) | 49 (9) | 53 (10) |
| DLCO, % predicted | 101 | 45 (13) | 37 (8) | 33 (11) | 27 (10) |
| Resting supplemental oxygen prescription | |||||
| L/min | 104 | 0 (0 – 0) | 2 (0 – 2.5) | 3 (2 – 4) | 3.5 (0 – 4) |
| “Room air” (0 L/min) | 104 | 79 | 40 | 18 | 43 |
| Mean pulmonary artery pressure, mm Hg* | 83 | 19 (4) | 22 (4) | 23 (7) | 27 (11) |
| Pulmonary vascular resistance, Wood units | 76 | 2.3 (0.7) | 2.5 (0.7) | 3.2 (1.3) | 3.0 (1.4) |
| 6MWD, m | 104 | 456 (134) | 372 (133) | 335 (101) | 357 (119) |
| End-walk SpO2, % | 104 | 89 (7) | 84 (7) | 83 (7) | 92 (6) |
Data are mean (SD), median (IQR), and percentage
Abbreviations: DLCO = diffusing capacity of carbon monoxide, 6MWD = six-minute walk distance, end-walk SpO2 = oxyhemoglobin saturation determined by pulse oximetry at the end of six-minute walk testing;
Missing for n = 14 in the 0 L/min group, n=3 in the 1 to 2L/min group, n = 2 in the 3 to 6 L/min group, n = 2 in the 8 to 15 L/min group
Associations of TOR with survival time in IPF
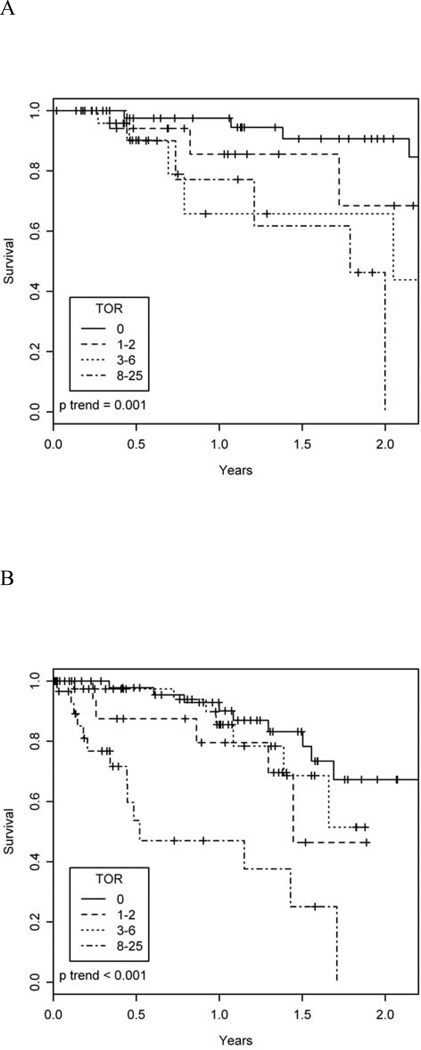
During a median follow-up time of 11 months (interquartile range 5 to 21 months), 17 study participants died without undergoing lung transplantation, and 35 underwent lung transplantation. Figure 2A shows unadjusted survival by TOR group. After adjustment for age, 6MWD, end-walk SpO2, and FVC, greater TOR was associated with a higher mortality rate (Table 2). For example, after adjustment for potential confounders, a TOR of 8 L/min or greater was associated with a 6.7-fold increased mortality rate (95% CI 1.7 to 25, p = 0.005) compared to a TOR of 0 L/min. Examination of TOR as a quasi-continuous variable showed a similar finding with an adjusted hazard ratio for death of 1.16 (95% CI 1.06 to 1.27, p = 0.001) per 1 L/min increase in TOR. Survival time was shorter for those with a TOR of at least 1 Lmin compared to those with a TOR of 0 L/min (multivariable-adjusted hazard ratio 3.2, 95% CI 1.11 to 9.3, p = 0.003, Figure E1).
Figure 2.
Unadjusted survival in the (A) derivation (p for trend = 0.001) and (B) validation (p for trend < 0.001) cohorts.
Table 2.
Associations of titrated oxygen requirement with survival time
| Titrated oxygen requirement (L/min) | Hazard ratio per 1 L/min increase in titrated oxygen requirement |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 to 2 | 3 to 6 | 8 to 15* | p for trend | Hazard ratio (95% CI) |
P value | |
| Derivation cohort (n = 104) | |||||||
| Unadjusted hazard ratio (95% CI) | Ref | 3.7 (1.1 – 13) | 5.3 (1.6 – 18) | 7.5 (2.1 – 27) | 0.003 | 1.14 (1.06 – 1.24) | <0.001 |
| Adjusted hazard ratio (95% CI) | |||||||
| Model 1 | Ref | 1.8 (0.5 – 6.9) | 3.1 (0.8 – 11) | 6.7 (1.7 – 26) | 0.004 | 1.16 (1.06 – 1.27) | 0.001 |
| Model 2 | Ref | 1.9 (0.5 – 7.3) | 3.0 (0.8 – 11) | 6.7 (1.7 – 25) | 0.005 | 1.16 (1.06 – 1.27) | 0.001 |
| Validation cohort (n = 151) | |||||||
| Unadjusted hazard ratio (95% CI) | Ref | 2.2 (0.7 – 7.2) | 1.2 (0.4 – 3.3) | 7.7 (2.7 – 22) | <0.001 | 1.13 (1.06 – 1.20) | <0.001 |
| Adjusted hazard ratio (95% CI) | |||||||
| Model 1 | Ref | 1.4 (0.4 – 5.4) | 0.7 (0.2 – 2.4) | 3.8 (1.01 – 15) | 0.01 | 1.10 (1.01 – 1.19) | 0.02 |
| Model 2 | Ref | 1.4 (0.4 – 5.3) | 0.7 (0.2 – 2.3) | 3.9 (1.05 – 15) | 0.01 | 1.10 (1.01 – 1.18) | 0.02 |
Abbreviations: CI = confidence interval
Two participants in the validation cohort who were titrated to 25 L/min via non-rebreather mask are included in this group.
Model 1: Adjusted for age, six-minute walk distance, and oxygen saturation at end six-minute walk test.
Model 2: Model 1 + adjustment for forced vital capacity % predicted
After adjusting for age, TOR, and FVC (Model 2 in Table 2), each 100-meter decrement in 6MWD was associated with a 2-fold higher mortality rate (hazard ratio 2.1, 95% CI 1.4 to 3.0, p < 0.001), but end-walk SpO2 was not associated with the risk of death (p = 0.32). Similarly, an end-walk SpO2 < 88% was associated with a non-significant 80% increased mortality rate (adjusted hazard ratio 1.8, 95% CI 0.6 to 5.4, p = 0.31).
Prediction of death at 1 year in IPF
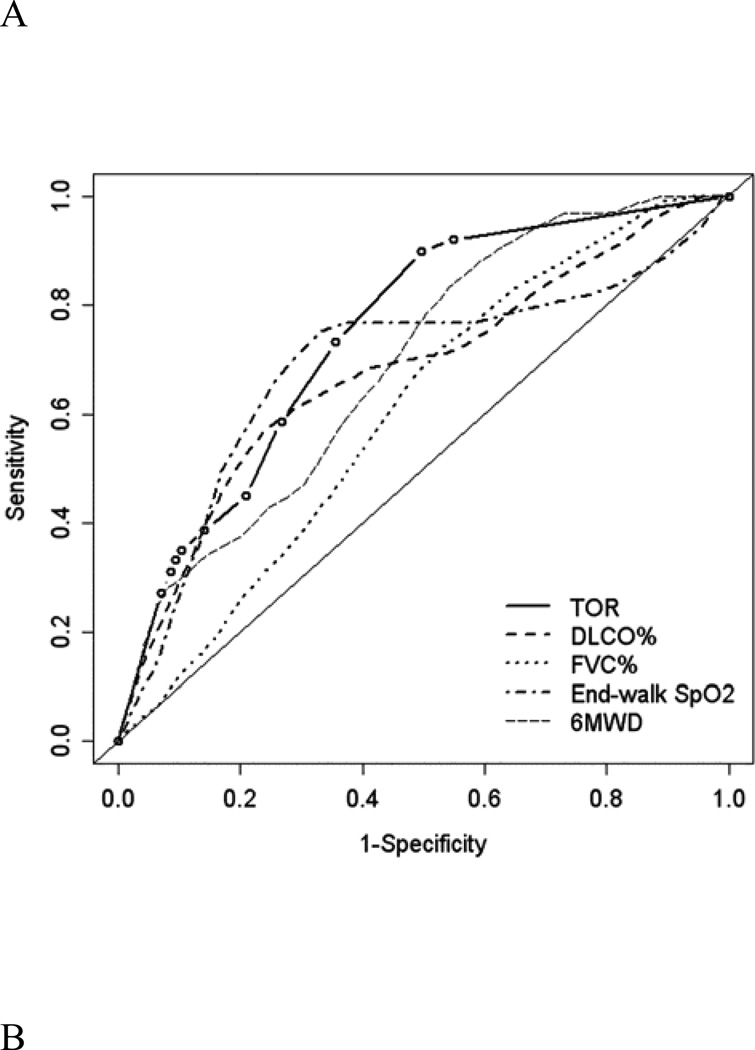
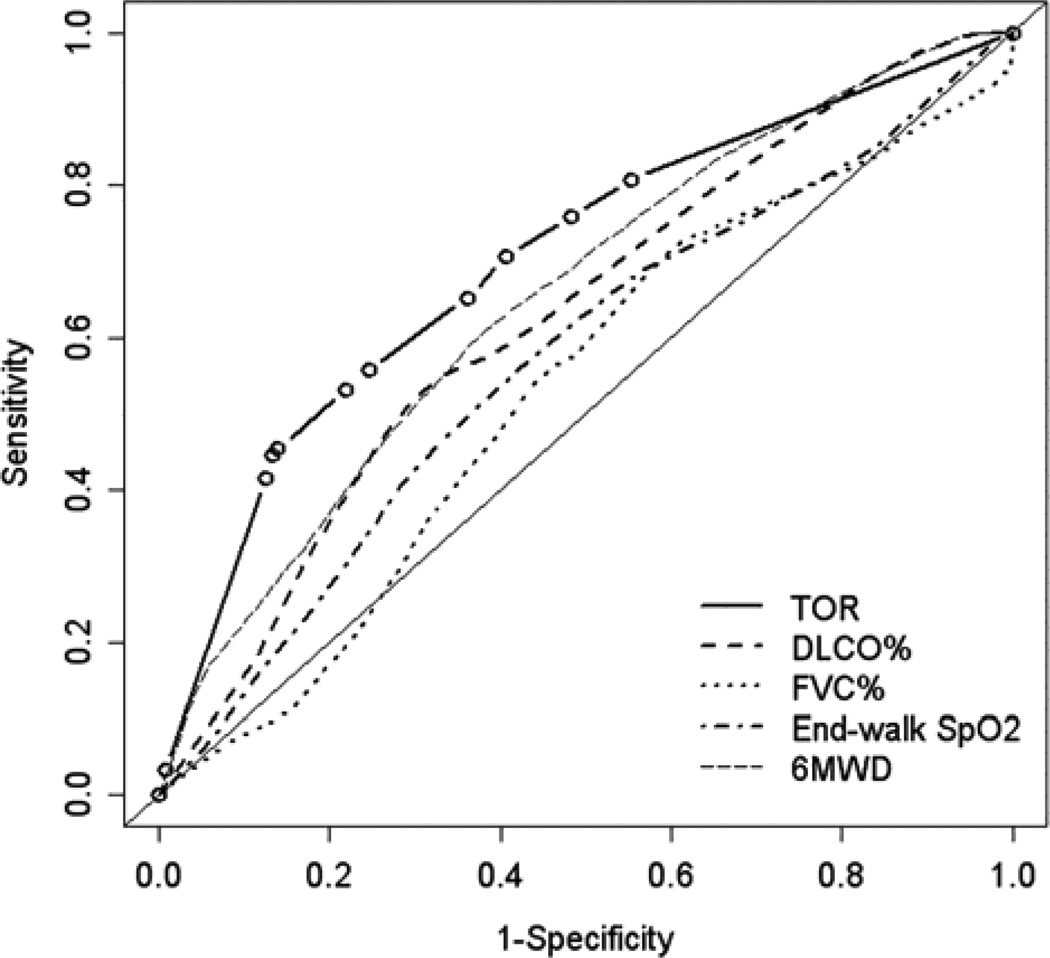
Time-dependent ROC curves for the prediction of death at 1 year by TOR and other established prognostic factors are shown in Figure 3A. The areas under the curves (AUCs) for TOR were 0.75 for death, similar to or greater than the AUCs for FVC, DLCO, 6MWD, and end-walk SpO2, which ranged from 0.60 to 0.70. A TOR of at least 1 L/min was 89% sensitive and 62% specific for death within 1 year, yielding positive and negative likelihood ratios of 2.34 and 0.18, respectively (Table 3). Table 3 shows the positive and negative predictive values for three TOR thresholds calculated using hypothetical low (10%), intermediate (50%), and high (90%) pre-test event probabilities. A TOR of 0 L/min combined with a low pre-test probability of death had a 98% negative predictive value at 1 year.
Figure 3.
Time-dependent receiver operating characteristic curves for the prediction of death within one year in the (A) derivation and (B) validation cohorts. The area under the curve (AUC) for the titrated oxygen requirement is 0.75 in the derivation cohort and 0.70 in the validation cohort. The AUC for the other four variables ranges from 0.60 to 0.70 in the derivation cohort and 0.53 to 0.65 in the validation cohort. Thin solid lines are lines of identity. TOR = titrated oxygen requirement; DLCO = diffusing capacity of carbon monoxide; FVC = forced vital capacity; SpO2 = oxyhemoglobin saturation measured by pulse oximetry; 6MWD = six-minute walk distance
Table 3.
Sensitivity, specificity, likelihood ratios, and predictive values of three titrated oxygen requirements for the prediction of one-year outcomes
| Pre-test probability 10% | Pre-test probability 50% | Pre-test probability 90% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | LR+ | LR− | PPV | NPV | PPV | NPV | PPV | NPV | |
| Derivation cohort | ||||||||||
| ≥1 L/min | 89% | 62% | 2.34 | 0.18 | 21% | 98% | 70% | 85% | 95% | 39% |
| ≥6 L/min | 44% | 89% | 4.00 | 0.63 | 31% | 93% | 80% | 61% | 97% | 15% |
| 15 L/min | 22% | 94% | 3.67 | 0.83 | 29% | 92% | 79% | 55% | 97% | 12% |
| Validation cohort | ||||||||||
| ≥1 L/min | 81% | 45% | 1.46 | 0.43 | 14% | 95% | 59% | 70% | 93% | 20% |
| ≥6 L/min | 55% | 86% | 3.96 | 0.52 | 31% | 95% | 80% | 66% | 97% | 18% |
| ≥15 L/min | 3% | 99.2% | 3.89 | 0.98 | 30% | 90% | 80% | 51% | 97% | 10% |
Abbreviation: LR+ = positive likelihood ratio, LR− = negative likelihood ratio, PPV = positive predictive value, NPV = negative predictive value.
Validation cohort
The validation cohort consisted of 151 patients: 46 patients with connective tissue disease-associated ILD, 19 with IPF (who were not included in the original cohort), 27 with idiopathic non-specific interstitial pneumonia, and 59 with other forms of ILD (14 with hypersensitivity pneumonitis, 15 with uncharacterized ILD, 11 with pulmonary sarcoidosis, 5 with lymphangioleiomyomatosis, 3 with ILD due to prior chemotherapy, 3 with alveolar proteinosis, 2 with asbestosis, 2 with silicosis, and 1 each with Hermansky-Pudlak syndrome, idiopathic pleuroparenchymal fibroelastosis, pulmonary langerhan’s cell histiocytosis, and neurofibromatosis). Associations of TOR with survival time and prediction of survival time in the validation cohort were similar but of smaller magnitude than the derivation cohort (Tables 2 and 3, Figures 2B, 3B, and E2). For example, the multivariable-adjusted hazard ratio for death was 1.10 (95% confidence interval 1.01 to 1.18, p = 0.02) in the validation cohort compared to 1.16 in the derivation cohort.
DISCUSSION
We have shown that the supplemental oxygen flow rate required to maintain an oxyhemoglobin saturation of 96% or greater at rest predicts one-year outcomes at least as well as established measures of lung mechanics (FVC), gas exchange (DLCO and end-walk SpO2), and exercise capacity (6MWD) in patients with IPF and other forms of ILD. Death was uncommon among those with a TOR of 0 L/min, and higher TOR values have greater specificity for the prediction of death. Six-minute walk testing using a supplemental oxygen flow rate determined by this novel titration protocol is safe.
The prognostic importance of supplemental oxygen requirements in ILD has previously been examined. Egan et al. used Organ Procurement and Transplantation Network (OPTN) data to examine the association between the “oxygen requirement at rest” and the risk of dying on the waiting list for lung transplantation among 608 ILD patients during the development of the lung allocation score [20]. Although these investigators ultimately included oxygen use in the waiting list urgency score of the lung allocation score, they reported that the supplemental oxygen flow rate was not significantly associated with a higher mortality rate in ILD in a multivariable-adjusted model [20]. The discrepancy between these results and ours may be due to a number of factors. First, we used a standardized assessment of oxygen requirements, rather than relying on flow rates reported by transplant center personnel, which could have been prone to misclassification bias. Second, since we did not restrict our analysis to those placed on the waiting list, our cohort may have been more heterogeneous with regard to disease severity, easing detection of an association with mortality. Third, the multivariable-adjusted model used in the LAS score may have included factors that explain the association between oxygen flow rate and mortality, such as pulmonary hypertension [21]. Only 80% of our study subjects underwent right heart catheterization, limiting our ability to examine pulmonary hypertension as a confounder. Even if pulmonary hypertension were to explain our findings, TOR would still be a clinically useful test, just as DLCO [4, 16, 17, 22, 23], end-walk SpO2 [4, 6], and heart rate recovery [24] remain practical clinical tests that are influenced by pulmonary hemodynamics.
Our results suggest that both TOR and 6MWD are each independently associated a higher risk of death in IPF. In contrast to previous studies [4, 6], however, we did not detect an association between end-walk SpO2 and the risk of death, possibly due to the administration of higher concentrations of oxygen during walk testing using our protocol. Our approach was to measure and account for supplemental oxygen use, walk distance, and desaturation in the same survival models, permitting an independent examination of the impact of each factor. Future studies may help elucidate ideal walk testing practices in order to maximize our ability to predict future events.
Practices of referral, listing, and prioritization for lung transplantation might be improved by standardizing the measurement and reporting of supplemental oxygen use. Current guidelines recommend that all patients with IPF be referred for evaluation for lung transplantation at the time of diagnosis. If our findings are validated in future studies, it may be reasonable to incorporate a TOR threshold in referral guidelines. It may also be appropriate to implement our titration protocol at lung transplant centers to standardize reporting of supplemental oxygen use.
It may be that measures of gas exchange (e.g., TOR, end-walk SpO2, and DLCO) are better predictors of outcome than measures of lung mechanics (e.g., reduced FVC) [4, 6, 10, 25]. TOR, in particular, may have certain strengths that merit its continued investigation and perhaps clinical use. For example, in contrast to pulmonary function testing and six-minute walk testing, TOR is inexpensive (and in many settings cost free) and quick (often < 3 minutes). TOR also standardizes a widespread clinical practice of gauging disease severity by oxygen requirement. Clinical trialists should consider including TOR as potential surrogate endpoints in studies of novel therapies.
Our findings were somewhat weaker in the validation cohort compared to the derivation cohort. This may be a result of the inclusion of participants with a variety of different ILDs in the validation cohort despite our attempt to control for diagnosis using statistical methods. In addition, the non-IPF diagnoses comprising the validation cohort typically have lower mortality rates than IPF, possibly limiting our ability to detect small differences in mortality rates. Finally, the IPF cohort was prospectively enrolled, while the validation cohort was assembled retrospectively, which may have introduced selection and information bias.
Our study has several limitations. First, we limited our sampling frame to a single tertiary care referral center with a lung transplant program, possibly selecting for those with more severe disease. Nevertheless, we were able to identify a subset with low rates of death and lung transplantation. We also excluded those who did not complete an oxygen titration study at our center, a group characterized by older age and lower lung function. Application of our findings to those with advanced disease should therefore be done cautiously and should be integrated with clinical judgment. Second, since priority for lung transplantation in the United States is in part based on oxygen requirements, we were careful to avoid treating transplantation as an event of interest, and instead chose to censor upon lung transplantation. However, informative censoring may have limited our ability to examine death as an isolated event. Third, a number of the factors that influence the oxyhemoglobin desaturation curve, such as arterial pH, 2,3-DBG, and body temperature, could have introduced “noise” into our study. Since this measurement error is unlikely to be differential by the risk of death, such error would tend to weaken the results. Therefore, the actual relationship may be even stronger than that shown. Fourth, our six-minute walk protocol varies from that suggested by the American Thoracic Society guidelines and from other centers’ published experience [4, 6, 10]. Differences in exercise test results between our study and others should therefore be interpreted with care. Our oxygen titration protocol may provide a useful means to standardize six-minute walk testing across centers.
In summary, we developed a simple, safe, inexpensive oxygen titration study that standardizes the administration of supplemental oxygen during six-minute walk testing. The oxygen flow rate required to reach a resting oxyhemoglobin saturation of at least 96% may aid in identifying those at high and low risk of death. Our findings support the hypothesis that impaired gas exchange is a clinically useful measure of disease severity in IPF.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institutes of Health Grants HL086714, RR024156, the Robert Wood Johnson Physician Faculty Scholars Program, and the Herbert and Florence Irving Scholar Award. This publication was made possible by grant number KL2 RR024156 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at the NCRR Website. Information on Re-engineering the Clinical Research Enterprise can be obtained from the NIH Roadmap website.
REFERENCES
- 1.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 2.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 3.King TE, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JAJ, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty KR, Andrei AC, Murray S, Fraley C, Colby TV, Travis WD, Lama V, Kazerooni EA, Gross BH, Toews GB, Martinez FJ. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med. 2006;174:803–809. doi: 10.1164/rccm.200604-488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164:1171–1181. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 6.Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long Q, Murray S, Kazerooni EA, Gross BH, Lynch JP, 3rd, Martinez FJ. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:1084–1090. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]
- 7.Kawut SM, O'Shea MK, Bartels MN, Wilt JS, Sonett JR, Arcasoy SM. Exercise testing determines survival in patients with diffuse parenchymal lung disease evaluated for lung transplantation. Respir Med. 2005;99:1431–1439. doi: 10.1016/j.rmed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Hallstrand TS, Boitano LJ, Johnson WC, Spada CA, Hayes JG, Raghu G. The timed walk test as a measure of severity and survival in idiopathic pulmonary fibrosis. Eur Respir J. 2005;25:96–103. doi: 10.1183/09031936.04.00137203. [DOI] [PubMed] [Google Scholar]
- 9.Lederer DJ, Arcasoy SM, Wilt JS, D'Ovidio F, Sonett JR, Kawut SM. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:659–664. doi: 10.1164/rccm.200604-520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lettieri CJ, Nathan SD, Browning RF, Barnett SD, Ahmad S, Shorr AF. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med. 2006;100:1734–1741. doi: 10.1016/j.rmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Fell CD, Liu LX, Motika C, Kazerooni EA, Gross BH, Travis WD, Colby TV, Murray S, Toews GB, Martinez FJ, Flaherty KR. The prognostic value of cardiopulmonary exercise testing in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:402–407. doi: 10.1164/rccm.200802-241OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bye PT, Anderson SD, Woolcock AJ, Young IH, Alison JA. Bicycle endurance performance of patients with interstitial lung disease breathing air and oxygen. Am Rev Respir Dis. 1982;126:1005–1012. doi: 10.1164/arrd.1982.126.6.1005. [DOI] [PubMed] [Google Scholar]
- 13.Harris-Eze AO, Sridhar G, Clemens RE, Gallagher CG, Marciniuk DD. Oxygen improves maximal exercise performance in interstitial lung disease. Am J Respir Crit Care Med. 1994;150:1616–1622. doi: 10.1164/ajrccm.150.6.7952624. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society. ATS Statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz DA, Helmers RA, Galvin JR, Van Fossen DS, Frees KL, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149:450–454. doi: 10.1164/ajrccm.149.2.8306044. [DOI] [PubMed] [Google Scholar]
- 16.Collard HR, King TE, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 17.Latsi PI, du Bois RM, Nicholson AG, Colby TV, Bisirtzoglou D, Nikolakopoulou A, Veeraraghavan S, Hansell DM, Wells AU. Fibrotic idiopathic interstitial pneumonia: the prognostic value of longitudinal functional trends. Am J Respir Crit Care Med. 2003;168:531–537. doi: 10.1164/rccm.200210-1245OC. [DOI] [PubMed] [Google Scholar]
- 18.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 19.Heagerty PJ, Lumley T, Pepe MS. Time dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–344. doi: 10.1111/j.0006-341x.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 20.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA, Grover FL. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 21.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 22.Flaherty KR, Mumford JA, Murray S, Kazerooni EA, Gross BH, Colby TV, Travis WD, Flint A, Toews GB, Lynch JP, 3rd, Martinez FJ. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168:543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 23.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, Ryu JH. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128:2393–2399. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 24.Swigris JJ, Swick J, Wamboldt FS, Sprunger D, du Bois R, Fischer A, Cosgrove GP, Frankel SK, Fernandez-Perez ER, Kervitsky D, Brown KK. Heart rate recovery after 6-min walk test predicts survival in patients with idiopathic pulmonary fibrosis. Chest. 2009;136:841–848. doi: 10.1378/chest.09-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mogulkoc N, Brutsche MH, Bishop PW, Greaves SM, Horrocks AW, Egan JJ. Pulmonary function in idiopathic pulmonary fibrosis and referral for lung transplantation. Am J Respir Crit Care Med. 2001;164:103–108. doi: 10.1164/ajrccm.164.1.2007077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.