Abstract
In colorectal cancer, the antitumorigenic guanylyl cyclase C (GCC) signalome is defective reflecting ligand deprivation from downregulation of endogenous hormone expression. Although the proximal intracellular mediators of that signal transduction system, including cyclic guanosine monophosphate (cGMP) and cGMP-dependent protein kinase (PKG), are well characterized, the functional significance of its distal effectors remain vague. Dysregulation of ligand-dependent GCC signaling through vasodilator-stimulated phosphoprotein (VASP), an actin-binding protein implicated in membrane protrusion dynamics, drastically reduced cGMP-dependent VASP phosphorylation levels in colorectal tumors from patients. Restoration of cGMP-dependent VASP phosphorylation by GCC agonists suppressed the number and length of locomotory (filopodia) and invasive (invadopodia) actin-based organelles in human colon cancer cells. Membrane organelle disassembly reflected specific phosphorylation of VASP Ser239, the cGMP/PKG preferred site, and rapid VASP removal from tumor cell protrusions. Importantly, VASP Ser239 phosphorylation inhibited the proteolytic function of invadopodia, reflected by suppression of the cancer cell ability to digest DQ-collagen IV embedded in Matrigel. These results demonstrate a previously unrecognized role for VASP Ser239 phosphorylation, a single intracellular biochemical reaction, as an effective mechanism which opposes tumor cell shape promoting colon cancer invasion and metastasis. Reconstitution of physiological cGMP circuitry through VASP, in turn, represents an attractive targeted approach for patients with colorectal cancer.
Keywords: Colorectal cancer, guanylyl cyclase C, cyclic GMP, vasodilator-stimulated phosphoprotein, invadopodia, filopodia
INTRODUCTION
Despite considerable advancements in elucidating pathognomonic genetic and molecular alterations, the causal mechanisms underlying initiation and progression of colorectal cancer, the third most common and deadly neoplasm in the western world, remain unknown. In the paracrine hormone hypothesis of colon cancer,1 tumorigenesis is characterized by a state of guanylyl cyclase C (GCC) ligand insufficiency, in which expression of endogenous hormones guanylin and uroguanylin, secreted by and activating GCC receptors on intestinal epithelial cells in an autocrine and paracrine fashion, is dramatically reduced following a yet unidentified event early during transformation.2–3 Ligand-induced GCC activation physiologically regulates intestinal fluid and electrolyte homeostasis through intracellular accumulation of the second messenger cyclic guanosine monophosphate (cGMP) and cGMP-dependent protein kinase (PKG) phosphorylation of ion channels.4 Activation of GCC also promotes the transition from the proliferative crypt phenotype to the differentiated colonocyte along intestinal mucosal surfaces by imposing cytostasis5 and oxidative metabolic reprogramming,6 effects presumably underlying GCC-mediated colorectal antitumorigenesis.7 Thus, the GCC ligandopenia associated with neoplastic transformation may promote colorectal carcinogenesis by producing a repressed GCC and cGMP pathway devoid of tumor suppressor activities. However, the exact consequences at the molecular level of the absence of GCC activation in colon cancer cells are unclear.
A poorly investigated target for the antitumorigenic GCC pathway is the vasodilator-stimulated phosphoprotein (VASP).8 A mammalian member of the highly conserved Ena/VASP family proteins, VASP acts as a molecular scaffold to bring together growing actin filaments with regulatory proteins at the cell leading edge.9 VASP controls cell spreading and migration by regulating the formation and stability of protrusive membrane structures driven by actin polymerization, including lamellipodia and filopodia, and the integrity of cell-matrix interactions at adhesion complexes.9 In that context, VASP exerts a pivotal role in filopodial dynamics, reflected by its localization at the filopodial tip complex where it promotes filopodia formation and elongation by recruiting the initial nanomachinery and imposing anti-capping pressure on actin filament barbed ends.10 Three critical domains mediates VASP/actin interactions:9 1) the Ena/VASP homology 1 (EVH1) domain at the N-terminus, which binds to proteins with poly-proline II helix, including vinculin and zyxin; 2) the central prolin-rich region, which binds to proteins containing SH3 and WW domains and the globular (G)-actin binding profilin, and 3) the EVH2 domain at the C-terminus, which binds to both G- and filamentous (F)-actin and mediates VASP oligomerization, thereby promoting F-actin bundling and stabilization. Importantly, VASP harbors 3 phosphorylation sites (Ser157, Ser239 and Thr278) which are targeted with different affinities by PKG, cAMP-dependent protein kinase and AMP-activated protein kinase, and modulate its localization and function.11–13 Phosphorylation of VASP Ser239, adjacent to the G-actin binding site in the EVH2 domain, is selectively regulated by cGMP-dependent signaling through PKG,11 an event that disrupts VASP anti-capping and filament-bundling activities and inhibits membrane protrusion formation.9, 14
While its engagement in migration and membrane protrusion dynamics in normal cells is well-established, a similar role for VASP Ser phosphorylation in cancer cells has not emerged. However, actin cytoskeletal remodeling at dynamic membrane regions determines oncogenic behaviors, from epithelial-to-mesenchymal transition to tissue invasion and metastasis.15–16 In principle, PKG-mediated VASP phosphorylation could represent one of the biochemical reactions of the antitumorigenic GCC/cGMP signalome.1 Here, human colon tumors exhibited reduced GCC ligand expression associated with depletion of cGMP-dependent VASP phosphorylation, indicating interruption of downstream signaling by the dysregulated GCC pathway. Restoration of cGMP-mediated phosphorylation of VASP Ser239 by ligand-induced GCC signaling suppressed the invasive cancer cell phenotype, with disassembly of protrusive membrane organelles (filopodia, invadopodia) and inability to promote DQ-collagen IV degradation. Together, these observations reveal a previously unrecognized suppressor of invasive membrane protrusions and therapeutic target, VASP Ser239 phosphorylation, for colorectal cancer.
MATERIAL AND METHODS
Reagents
ST was prepared as described,5 while 8-br-cGMP was obtained from Sigma-Aldrich (St. Louis, MO). Antibodies to human VASP, phosphorylated VASP at Ser157, GAPDH, villin and cortactin were from Santa Cruz Biotechnology (Santa Cruz, CA). Two anti-phoshorylated VASP at Ser239 antibodies were purchased, including clone 16C2 from Abcam (Cambridge, MA) and a rabbit antibody from Sigma-Aldrich. The anti-human guanylin antibody was from BioDesign International (Saco, ME), while the anti-human PKG was obtained from Assay Designs (Ann Arbor, MI). The antibody to the human GCC was obtained from Rockland Immunochemicals (Gilbertsville, PA). DQ-collagen IV, Alexa fluor 555 anti-rabbit IgG, Alexa fluor 633 anti-mouse IgG, Oregon Green 488 phalloidin and Slowfade Gold with DAPI were obtained from Invitrogen (Carlsbad, CA). Matrigel was from BD Bioscience (Bedford, MA), the general matrix metalloproteinase (MMP) inhibitor GM1006 from Calbiochem (San Diego, CA), and all the reagents for cell culture from Mediatech Inc. (Herndon, VA).
Clinical specimens and immunohistochemistry
Paraffin-embedded specimens from 7 patients (Supporting Information Table 1) with histologically-confirmed adenocarcinomas of the colo-rectum were obtained from the Department of Pathology, Anatomy and Cell Biology of Thomas Jefferson University (Philadelphia, PA) under a protocol approved by the Institutional Review Board and subjected to immunohistochemistry (IHC). Briefly, following deparaffinization and rehydratation (with xylene/ethanol/water washes), antigens in tissue sections (5 μm) were unmasked by two consecutive heating cycles (100°C for 5 min in 10 mM citric buffer, pH 6.0). Then, specimens were incubated overnight (4°C) with antibodies (1:100 dilution, unless otherwise indicated) against either human guanylin, GCC, PKG, VASP, and phosphorylated VASP at Ser157 or Ser239 followed by incubation with the respective secondary antibody and DAB substrate (Avidin-biotin kit; Vector Laboratory, Burlingame, CA). Staining intensity was quantified by a blinded pathologist on a 1 to 3 scale (1, low or absent; 2, medium; 3 high).
Cell culture
All the human colon carcinoma cell lines investigated were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and used within 6 months from resuscitation. Methods of cell authentication by ATCC include analyses of morphological and growth characteristics, isoenzymology and DNA profiles. T84 and HCT-116 human colon carcinoma cells were cultured (37°C, 5% CO2) with 10% fetal bovine serum in DMEM/F12 or DMEM, respectively. Cells (2–20 passages) were fed with fresh medium every third day and split when sub-confluent.
Plasmids and cell transductions
Full length VASP cDNA (Invitrogen, Carlsbad, CA) was sub-cloned into the Xho1-EcoR1 multiple cloning site of mouse stem cell virus (MSCV)-puro retroviral vector. Point mutations of VASP serines 157 (tcc → gcc) and 239 (agc → gcc) were performed employing the QuikChange XL Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) to generate VASP mutants with alanine substitutions at Ser157 (S157A), Ser239 (S239A) or both (AA). Green fluorescent protein (GFP)-VASP constructs were generated by sub-cloning wild type VASP or the VASP mutant S239A in-frame to the C-terminus of GFP using the pRetroQ-AcGFP-Cl vector (Stratagene). HEK 293T17 cells (from ATCC) were transfected with 1 μg of each purified MSCV construct plus 1 μg of the packaging vector pCl-Ampho employing Fugene transfection reagent (Roche, Basel, Switzerland). T84 cells were transduced (for 72 h) with viral supernatants supplemented with 4 μg/ml polybrene, then selected and maintained in 5 μg/ml puromycin. In this way, the following T84 clones stably-expressing the MSCV-driven gene were generated: VASP, VASP-S157A, VASP-S239A, VASP-AA, GFP, GFP-VASP and GFP-VASP-S239A.
Immunoblot analysis
Protein samples (40 μg in SDS loading buffer) were separated by electrophoresis on 10% acrylamide Tris-Glycine gels, transferred to nitrocellulose membranes, and probed (1:1,000 each) with rabbit polyclonal antibodies against VASP, phosphorylated VASP at Ser157, GAPDH, villin or mouse monoclonal antibody against phosphorylated VASP at Ser239 in TBS-Tween (5% milk) overnight at 4°C. After washing the primary antibody, membranes were probed with the appropriate secondary antibody (1:2,000) (SantaCruz Biotechnology) for 1 h at room temperature. Immunostained bands were imaged with a Kodak Image Station 4000R and quantified by densitometry.
Immunofluorescence and imaging
Filopodia dynamics were studied in cancer cells cultured on Lab-Tek glass microscope-chambered slides and treated for 2 h with PBS (control), the GCC ligand ST (1 μM) or the cGMP analog 8-br-cGMP (5 mM). Invadopodia were examined employing glass coverslips coated with 25 μl Matrigel and incubated at 37°C (10 min) to solidify. Here, cancer cells were plated drop-wise directly onto Matrigel, allowed to firmly adhere (1 h) and equilibrate (24 h) in their culturing medium, and finally treated (2 h) with PBS or ST (1 μM). Following treatments, cells were fixed (15 min) with ice-cold paraformaldehyde (4%, in PBS) and permeabilized (10 min) with 0.1% Triton X-100. Following overnight incubations (4°C; in PBS containing 2% bovine serum albumin) with mouse anti-cortactin and/or rabbit anti-VASP antibodies (1:100 each) and 5 units/ml Oregon Green 488 phalloidin, cells were incubated (60 min at room temperature) with Alexa fluor 633 anti-mouse IgG and/or Alexa fluor 555 anti-rabbit IgG. Specificity of antigen/antibody reactions was assessed employing the secondary antibody alone. Some preparations were counterstained with Slowfade Gold anti-fade reagent with DAPI to identify cell nuclei. Images (63x) were acquired with a confocal laser scanning microscope (LSM 510, Carl Zeiss, Jena, Germany) equipped with a monochrome CCD camera and pseudo-colored with image-analysis software.
For filopodia measurements, cells were analyzed by differential interference contrast (DIC) microscopy. Filopodial protrusions (≥5 μM) were examined on 8–10 random cell colonies per treatment (~50–100 filopodia/treatment) by quantifying the number (per cell colony perimeter) and length (the average distance from the cell base to the tip) of filopodia. The cancer cell colony perimeter (~264 ± 41 μM) was determined to ensure that results from colonies with different sizes could be directly compared. Measurements were performed using the Zeiss LSM Image Browser software (Carl Zeiss Microimaging, Thornwood, NY). For invadopodia analyses, the number and length of individual invadopodia (the baso-lateral actin-based membrane projections) per cancer cell were quantified (with the Zeiss LSM Image Browser software) on 8–10 random cells per treatment from 3-dimensional (3-D) reconstructions created from orthogonal X, Y and Z sections of complete Z-stack cell images (at 1 μm increments) of the confocal microscope. Finally, live cell microscopy was performed on culture dishes (fitted on a heated stage) of GFP-VASP (n=6) or GFP-VASP-S239A (n=7) cells by acquiring time-lapse images at 0, 1, 5, 10 and 15 min after treatment with the confocal microscope. Analysis of filopodia was performed with Image J (NIH, Bethesda, MD). The GFP-based fluorescent signal was quantified at filopodia tips (the distal of each filopodia), along the entire length of each filopodium and throughout the cell body, and normalized to the background fluorescence.
DQ-collagen IV degradation
Glass coverslips were coated with 20 μl Matrigel containing 25 μg/ml DQ-collagen IV and incubated at 37°C (10 min) to solidify. Cancer cells were plated drop-wise directly onto these Matrigel scaffolds, allowed to firmly adhere (1 h), and treated (24 h) in their culturing medium with PBS or ST (1 μM). Then, cells were fixed in methanol (−20°C for 15 min) and processed by confocal microscopy to generate Z-stack images, as described for invadopodia analyses above. DQ-collagen IV degradation was quantified in 8–10 cells per treatment with the NIH Image J as the mean fluorescent intensity of the fluorescent cleavage product (from DQ-collagen IV) over the cell and pericellular area per cell area. Results were normalized to the background fluorescence.
Statistical analysis
Unless otherwise indicated, data are mean ± SEM of ≥3 independent experiments. Statistical analyses were performed with the Student’s t test.
RESULTS
VASP is an intracellular effector of the GCC pathway in colon cancer cells
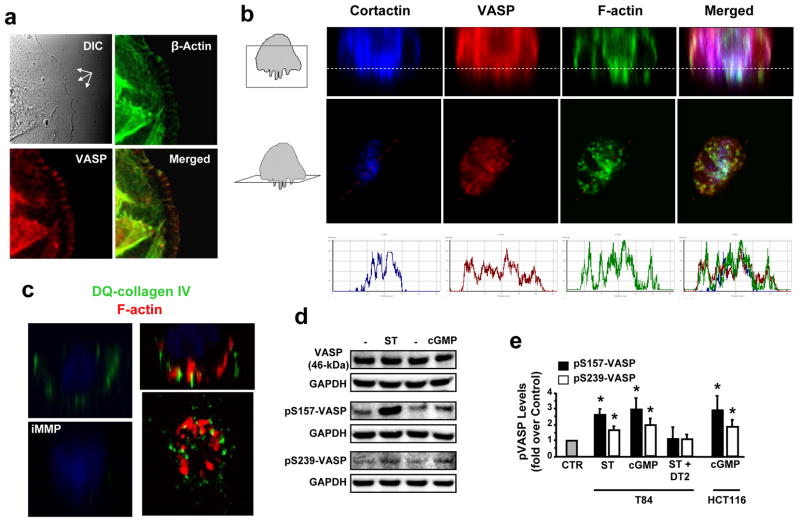
VASP is expressed in colon cancer cells (46-kDa band; Supporting Information Fig. 1a) and distributes to dynamic membrane domains, including cell leading edges (Fig. 1a) and invadopodia (Fig. 1b), where it colocalizes with cortactin and actin (Fig. 1b, right panels). Invadopodia identity was confirmed by assessing their ability to focally degrade DQ-collagen IV (Fig. 1c), a fluorogenic analog of the basement membrane component collagen IV. In addition, VASP is an intracellular target of ligand-dependent GCC signaling in human colon cancer cells,8 and the potent GCC agonist ST4 induced rapid phosphorylation of VASP at both Ser157 and Ser239 (Supporting Information Fig. 1b). ST effects on VASP were mimicked by the membrane-permeant cGMP analog 8-br-cGMP (Fig. 1d–e and Supporting Information Fig. 2a) and blocked by the selective PKG peptide inhibitor DT217 (Fig. 1e and Supporting Information Fig. 2b), confirming that ST induces VASP Ser phosphorylation by activating the GCC/cGMP/PKG pathway in human colon cancer cells.
Fig. 1.
VASP Ser phosphorylation is induced by GCC activation in colon cancer cells. (a) Cell leading edges (multi-arrows in upper-left panel) of T84 human colon carcinoma cells (on 2-D surfaces) were imaged (magnification, 63x) by DIC and confocal microscopy following immunofluorescent staining with phalloidin (green) and anti-VASP antibody (red). Yellow in merged image indicates F-actin and VASP colocalization at leading tips. (b) Representative confocal microscopy images of invadopodia from T84 cells grown on 3-D Matrigel scaffolds (Matrigel/air interfaces, dotted lines in top panels). Boxes in cartoons on left indicate visual planes in vertical (top panels) and horizontal (middle panels) cross-sections from Z-stack images. Cells were immunofluorescently stained for invadopodial marker cortactin (ref. 27) (blue), VASP (red) and F-actin (green). In merged images, white (upper panels) and coincidence of picks (bottom panels) from distinct fluorescent signals along lines depicted in middle panels indicate cortactin, VASP and F-actin colocalization. (c) Vertical or horizontal (bottom-right) T84 cell cross-sections of confocal microscopy images obtained as in b. Here, Matrigel contained DQ-collagen IV, which releases a fluorescent product (green) upon cleavage by extracellular proteases. Application of 60 nM of the broad MMP inhibitor GM6001 (iMMP) was employed to prevent DQ-collagen IV degradation by tumor cells (ref. 20) and serve as the negative control condition. Red in right panels reflects F-actin staining. (d) Immunoblots of a representative experiment with T84 cells, treated with the GCC ligand ST (1 μM, 5 min) or the cGMP analog 8-br-cGMP (5 mM, 30 min). pS157-VASP and pS239-VASP, VASP phosphorylated at Ser157 and Ser239, respectively; GAPDH, the loading control. (e) Bar graphs reflect densitometric quantification of immunobands, normalized to respective loading (GAPDH) and vehicle (PBS) controls (CTR), from experiments with T84 and HCT116 colon cancer cells treated as in d. Some conditions also received the peptide DT2 (5 μM, 30 min) to selectively and completely inhibit PKG-Iα activity (Ki, 12.5 nM; ref. 17). *, P < 0.05, versus respective PBS control.
GCC signaling through VASP is dysregulated in colon cancer
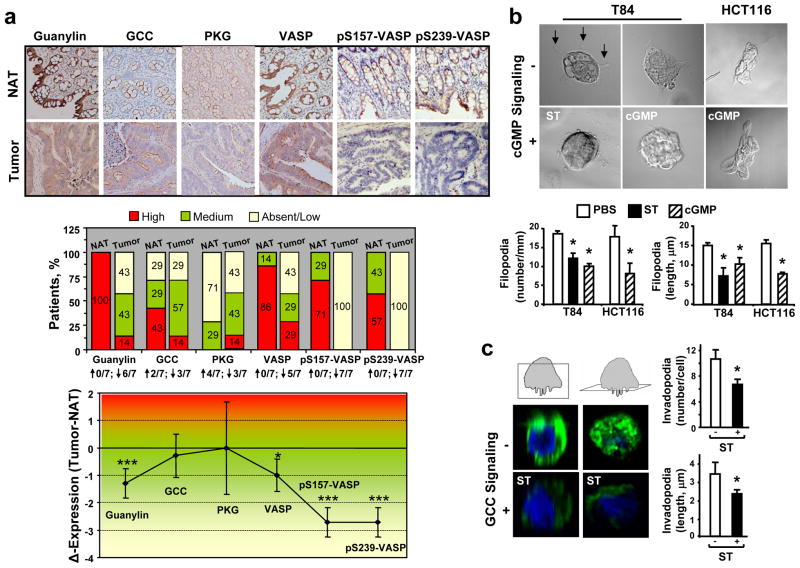
In the human colon, the GCC pathway is constitutively activated by the paracrine hormone guanylin, which induces cGMP- and PKG-dependent phosphorylation of specific substrates.1, 4 Accordingly, normal colonic mucosa from patients exhibited epithelial cells with VASP Ser157 and Ser239 phosphorylation in the context of an intact guanylin-VASP signaling axis (Fig. 2a, upper panel). However, following transformation and loss of endogenous ligand expression2–3 this GCC pathway becomes dysregulated (Fig. 2a, upper panel). While GCC and PKG exhibited mixed trends with no significant changes, expression of guanylin and all the VASP species were significantly reduced in colorectal adenocarcinomas compared to their respective normal counterparts (Fig. 2a). Of significance, VASP phosphorylation at Ser157 and Ser239 were the most compromised signals of the GCC pathway, reflected by their downregulation in all tumors examined (Fig. 2a, middle panel) and maximal loss of expression (Fig. 2a, lower panel). Thus, loss of VASP Ser phosphorylation in colon cancer reflects interruption of effective induction and transmission of GCC-VASP signaling, beyond the mere changes in VASP expression (Supporting Information Fig. 3).
Fig. 2.
GCC signaling through VASP is disrupted in colon cancer and its reconstitution in vitro suppresses dynamic membrane regions. (a, upper panel) Representative IHC images (magnification, 20x) of primary tumor (Tumor) and matched normal adjacent tissue (NAT) from 7 patients with colorectal cancer. Tissues were stained with the specific primary antibody (brown) and hematoxylin (blue, nuclei). The primary antibody for VASP phosphorylated at Ser239 was clone 16C2 from Abcam. (a, middle panel) Distribution (percentages indicated in boxes) of patients with different expression levels (by staining intensity, as per Material and Methods) of the GCC pathway components. The fraction of patients with upregulation (↑) or downregulation (↓) of the indicated protein in tumors compared to respective NATs is provided in bottom. (a, lower panel) Differential protein expression (the delta of staining intensity scores) in colorectal tumors compared to matched NATs. *, P < 0.05; ***, P < 0.005 of tumors versus NATs. pS157-VASP and pS239-VASP, VASP phosphorylated at Ser157 and Ser239, respectively. (b) Filopodia (arrows in upper-left slide) were imaged in cancer cell colonies by DIC. (c) Invadopodia were imaged by confocal microscopy in single T84 cells, immunofluorescently stained with 4′,6-diamidino-2-phenylindole (DAPI, for nuclei in blue) and phalloidin (for F-actin in green). Boxes in cartoons on top indicate visual planes in vertical (left panels) and horizontal (right panels) cross-sections from Z-stack images. In b-c, number and length of filopodia and invadopodia were quantified as described in Material and Methods, and results are shown in bar graphs. Treatments (2 h) were PBS (the vehicle control), ST (1 μM), or 8-bromo-cGMP (5 mM). *, P < 0.05, versus respective PBS control.
VASP Ser239 phosphorylation by GCC activation inhibits cancer cell shape mediating migration and invasion
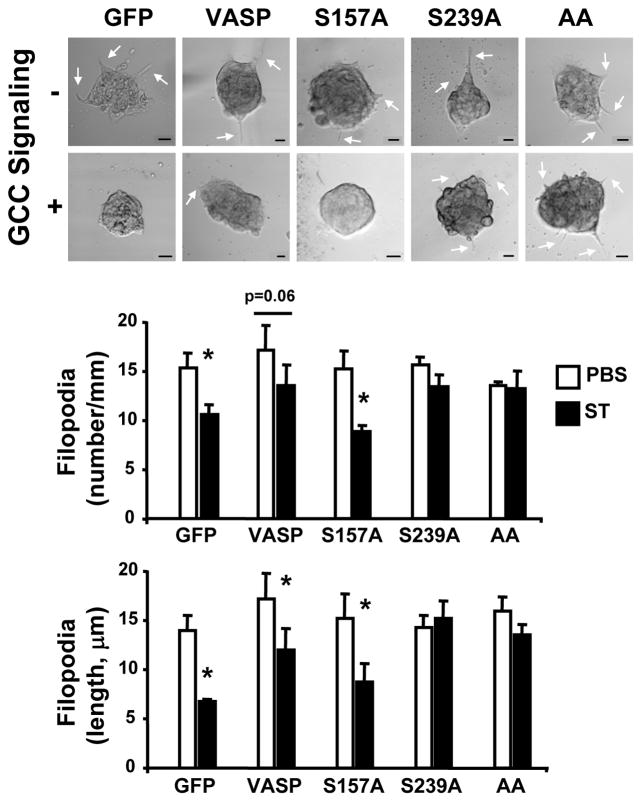
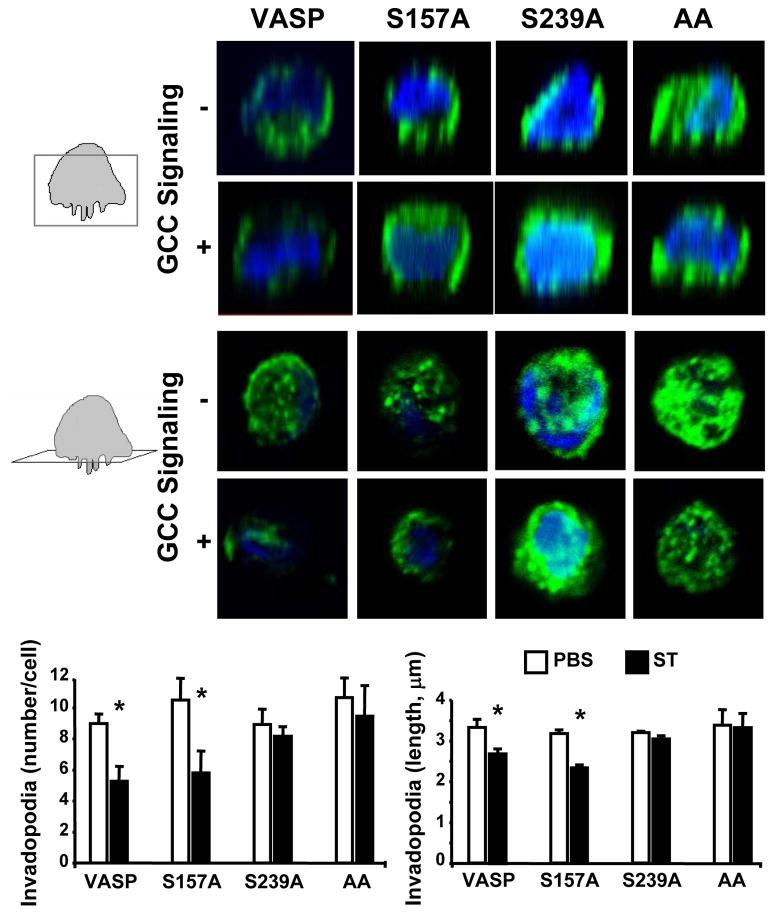
As VASP regulates membrane protrusion geometry18 and is present at cell leading edges and invadopodia (Fig. 1a–b), the functional consequences of ligand-dependent GCC signaling through VASP Ser phosphorylation on the membrane architecture of colon cancer cells were examined. GCC signaling through cGMP significantly reduced the number and length of migratory (filopodia; Fig. 2b) and invasive (invadopodia; Fig. 2c) actin-based protrusions. Filopodia identity was confirmed by specific immunostaining with the filopodia marker fascin (data not shown). Importantly, cGMP inhibitory effects were specifically mediated by induction of VASP Ser239 phosphorylation, as demonstrated by employing tumor cells stably expressing distinct VASP phosphomutants (Supporting Information Fig. 4a). Thus, cancer cells with VASP-S239A or VASP-AA, which contain point mutations at Ser239 and do not accept phosphorylation at that site (Supporting Information Fig. 4b), were resistant to GCC-mediated inhibition of filopodia (Fig. 3) and invadopodia (Fig. 4). In contrast, control cells expressing GFP or overexpressing VASP and tumor cells with the phosphomutant VASP-S157A, which harbors a point mutation at Ser157 only and does not accept phosphorylation at that site (Supporting Information Fig. 4b), were sensitive to suppression of filopodia (Fig. 3) and invadopodia (Fig. 4) upon ligand-dependent GCC signaling.
Fig. 3.
VASP Ser239 phosphorylation by GCC disrupts colon cancer cell filopodia. T84 cells were treated (with the PBS control or 1 μM ST to induce GCC signaling), imaged and analyzed for quantification of filopodia as in Fig. 2b. Cancer cells stably expressed the indicated MSCV-driven genes (see Material and Methods for details), including the controls GFP and VASP, and VASP-S157A (S157A), VASP-S239A (S239A) and VASP-AA (AA). Arrows in upper panels indicate representative filopodia. Bars, 10 μm. *, P < 0.05 versus respective control.
Fig. 4.
VASP Ser239 phosphorylation by GCC disrupts colon cancer cell invadopodia. T84 cells were treated (with the PBS control or 1 μM ST to induce GCC signaling), imaged and analyzed for quantification of invadopodia as in Fig. 2c. Cancer cells stably expressed the indicated MSCV-driven genes (see Material and Methods for details), including the control VASP, and VASP-S157A (S157A), VASP-S239A (S239A) and VASP-AA (AA). Boxes in cartoons on left of top images indicate visual planes in vertical (upper panels) and horizontal (bottom panels) cross-sections from Z-stacks. *, P < 0.05 versus respective control.
VASP Ser239 phosphorylation suppresses invasive membrane protrusions by inducing intracellular VASP redistribution
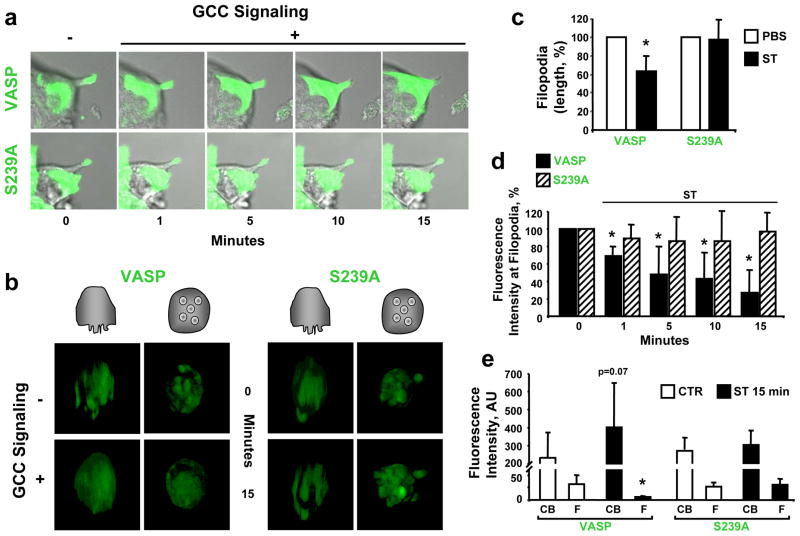
The molecular mechanism mediating inhibition of invasive cell shape by VASP Ser239 phosphorylation was investigated with live imaging microscopy in cells stably expressing GFP-VASP constructs (Fig. 5). Colon cancer cells with GFP-VASP, but not mutant cells expressing GFP-VASP-S239A, exhibited disassembly of filopodia (Fig. 5a) and invadopodia (Fig. 5b) upon ligand-dependent GCC activation. Protrusion retraction by GCC (Fig. 5c) reflected rapid removal of VASP from membrane projections (Fig. 5d), with a calculated half-life of 8.11 min (Supporting Information Fig. 5), and VASP redistribution to inner cell compartments away from membrane tips (Fig. 5e). However, the GFP-VASP-S239A mutant (resistant to Ser239 phosphorylation), was insensitive to GCC signaling (Fig. 5c), and retained its localization (Fig. 5d) and distribution (Fig. 5e) at locomotory membrane extensions, suggesting that VASP Ser239 phosphorylation represents a disassembly signal for invasive migratory organelles by uncoupling VASP from actin-based microregions at colon cancer cell membranes.
Fig. 5.
VASP Ser239 phosphorylation induces membrane protrusion retraction in colon cancer cells. Fluorescently-tagged VASP constructs, GFP-VASP (VASP) and GFP-VASP-S239A (S239A), were stably expressed in T84 cells as described in Material and Methods. GCC signaling was induced with ST (1 μM), while PBS was the vehicle control. (a) Representative time course of a live cell experiment analyzing filopodial kinetics by confocal microscopy, performed as detailed in Material and Methods. Photographs reflect merged DIC and confocal microscopy images. (b) Representative 3-D projections from live imaging (confocal microscopy) analyzing invadopodia in single tumor cells plated on Matrigel scaffolds. Cartoons on top indicate visual vertical (left panels) and basolateral en face (right panels) perspectives of 3-D reconstructions from Z-stack images. (c) The length of filopodia following treatments for 15 min was quantified as in Fig. 2b and expressed as percentages of respective PBS controls. (d) Quantification of GFP-tagged VASP signals at filopodia tips from experiments shown in a. Results are percentages of respective time 0 (before ST administration) controls. (e) Quantification of GFP-tagged VASP signals in cell bodies (CB) and filopodia (F) before (CTR, time 0 control) and after ST (15 min) treatments, as shown in a. AU, arbitrary units. *, P < 0.05 versus respective control.
VASP Ser239 phosphorylation prevents DQ-collagen IV degradation by colon cancer cells
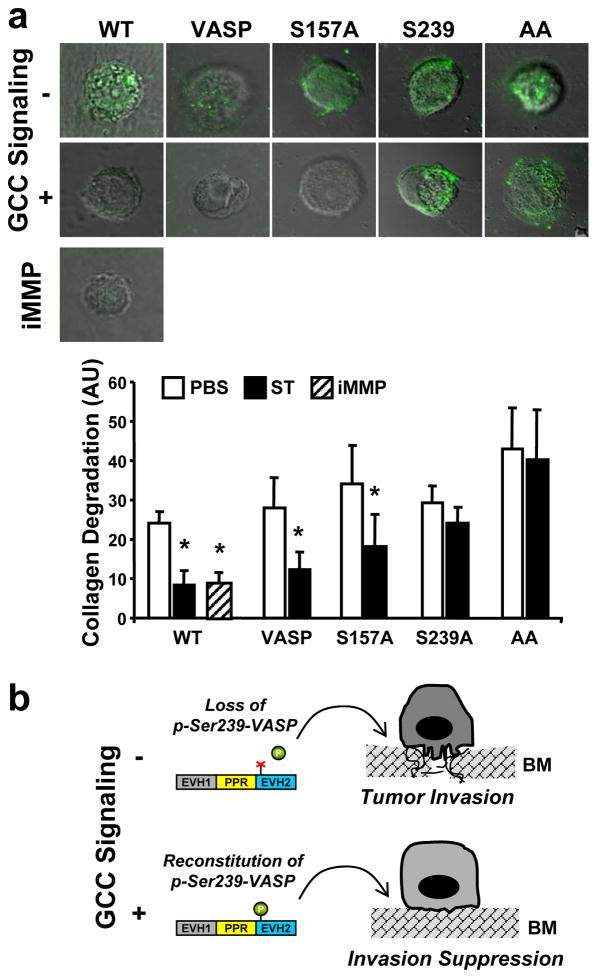
Metastatic cancer dissemination requires degradation and remodeling of the surrounding environment,19 a malignant attribute conferred to colon cancer cells by their invadopodia (Fig. 1c). Strikingly, activation of GCC signaling through VASP abolished the cancer ability to degrade DQ-collagen IV, with similar magnitude to that of a general inhibitor of MMPs (Fig. 6a), enzymes mediating tumor matrix degradation.20 However, colon cancer cells with VASP-S239A or VASP-AA, but not VASP-S157A, did continue to proteolytically digest DQ-collagen IV following ligand-dependent GCC signaling (Fig. 6a), indicating that VASP Ser239 phosphorylation mediates arrest of invadopodia-mediated proteolysis by the GCC pathway. Thus, VASP Ser239 phosphorylation is a unique biochemical reaction that suppresses membrane protrusions promoting tumor invasion in colorectal cancer (Fig. 6b).
Fig. 6.
VASP Ser239 phosphorylation inhibits the cleavage of DQ-collagen IV by colon cancer cells. (a) T84 cells stably-expressing the indicated MSCV-driven genes (VASP, the control; VASP-S157A, S157A; VASP-S239A, S239A; VASP-AA, AA) were treated for 24 h with PBS, 1 μM ST or 60 nM of the broad MMP inhibitor GM6001 (iMMP), imaged and analyzed by confocal microscopy for quantification of DQ-collagen IV degradation as detailed in Material and Methods. Photographs (top) are merged DIC and confocal microscopy images. Results (bottom) are expressed as arbitrary units (AU) of the fluorescent signal from the cleaved collagen product. WT, wild-type T84 cells. *, P < 0.05, versus respective PBS control. (b) Proposed role of VASP in colorectal carcinogenesis. During tumor progression, VASP phosphorylation at Ser239 (p-Ser239-VASP) is reduced, reflecting a dysregulated GCC pathway with interruption of cGMP-mediated signaling, which promotes the formation of migratory membrane protrusions and cancer cell invasion. Reconstitution of GCC and cGMP signaling, in turn, provides a unique therapeutic opportunity to restrain the invasive tumor cell shape and prevent metastasis.
DISCUSSION
The antitumorigenic GCC signalome is deregulated during colorectal transformation reflecting loss of endogenous ligand expression.1–3 Present findings reveal that this alteration affects a cGMP-dependent intracellular pathway comprising VASP as the distal effector, with subsequent silencing of an important biochemical reaction, VASP Ser239 phosphorylation, which opposes malignant cell shape. Interruption of GCC-VASP signaling, in turn, results in specific depletion of the VASP phosphospecies, which represent previously undescribed biomarkers for GCC deregulation and tumorigenesis in colon cancer. Conversely, restoration of GCC signaling by ligand replacement therapy may provide a rationale strategy for reconstituting cGMP-VASP circuitry and preventing metastasis. Indeed, ligand-dependent GCC activation compromises motility and metastasis of colon cancer cells,21 presumably by targeting VASP signaling suppressing invasive membrane protrusions (Fig. 6b) described herein.
Membrane protrusion extension principally reflects the balance of antagonistic forces at actin filament barbed ends imposing persistence (anti-capping proteins) or arrest (capping proteins) of actin polymerization.10 VASP is a critical anti-capping protein that initiates and maintains dynamic membrane regions by orchestrating a cytoskeletal assembly-line promoting simultaneous F-actin elongation and bundling.10, 22 In this way, Ena/VASP family members control migration and membrane protrusion formation underlying important physio(patho)logical processes, from wound-healing, platelet aggregation and neural development9 to inflammation, cancer spreading and metastasis.9, 23–24 Accordingly, expression of Ena/VASP proteins positively correlates with disease progression in patients with lung25 or breast26 cancer, suggesting their involvement in carcinogenesis and metastasis. The present study underscores the critical role of VASP in organizing cancer functions associated with the malignant membrane shape. In that context, although with unclear activities VASP is a molecular component of invadopodia,27 cancer cell projections mediating focal matrix proteolysis. Here, colon cancer cells required VASP for invadopodia stability and function, indicating that VASP maintains the structural integrity of these invasive protrusions. Indeed, intracellular VASP redistribution and removal from protrusive tips, upon GCC-dependent phosphorylation, destabilizes actin-driven membrane extensions (Fig. 5) by presumably depleting actin filament plus-ends of VASP-mediated anti-capping forces,22 resulting in protrusion retraction from the persistent actin retrograde flow. Importantly, invadopodia represent attractive antimetastatic targets since they function as critical hubs of invasive cell behavior, spatially and temporally organizing the activities of actin nucleators and regulators (Arp2/3, N-WASP, cortactin), signaling enzymes (Src, Erk1/Erk2, FAK), adhesive receptors (integrins β1/β3, CD44), and proteases (MT1-MMP, MMP2, MMP9).27–28 Thus, the ability of cGMP-dependent VASP phosphorylation to dismantle invadopodia in colon cancer cells (Fig. 4) offers an innovative antiinvadopodial strategy to be exploited against tumor metastasis in patients.
Tumor suppressors inhibiting metastatic cell dissemination are scarce. Their critical value resides in the potential to serve as biomarkers to improve clinical staging and targets to reduce cancer mortality. VASP Ser239 phosphorylation, a simple intracellular biochemical reaction, could represent a unique invasion suppressor with sophisticated regulatory dynamics, an inducible mechanism embedded into signal transduction networks shaping tumor cell metastasis. Further, inhibition of metastasis by VASP Ser239 phosphorylation could be a general strategy, as VASP is ubiquitously expressed across human tissues.9 Of significance, cGMP-dependent VASP Ser157 phosphorylation does not appear to affect the invasive phenotype of colon cancer cells (Figs. 3, 4 and 6). Since VASP Ser239 is selectively regulated by PKG-dependent phosphorylation,9, 14 while VASP Ser157 is a more promiscuous site phosphorylated by PKG, cAMP-dependent protein kinase, and protein kinase C-dependent and -independent mechanisms,11, 29 present observations suggest a unique role of the GCC/cGMP/PKG pathway in suppressing colorectal cancer invasion through VASP Ser239. Reduced levels of VASP Ser157 phosphorylation in colon cancer (Fig. 2a), in turn, may reflect more complex deregulation of tumor intracellular networks, including cGMP-dependent and -independent pathways, probably affecting carcinogenetic processes beyond GCC-regulated invasive cell shape, a model currently being explored in this laboratory.
Present findings also support the existence of a biological divergence between cGMP and cAMP signaling on Ena/VASP activities, an established paradigm in neurons where the cAMP-VASP axis mediates attractive guidance cues by increasing the number and length of filopodia, while the cGMP/PKG/VASP pathway promotes repulsive guidance cues.30–31 However, the precise consequences of cyclic nucleotide signaling through VASP on the actin cytoskeleton appear to be cell and tissue specific, as demonstrated by identical cGMP and cAMP inhibitory actions on platelet aggregation, reflecting respective VASP phosphorylation mechanisms.32 Regardless, VASP Ser239 phosphorylation induced by cGMP is emerging as a pivotal mechanism mediating VASP redistribution and disassembly of VASP-regulated membrane protrusions, including filopodia and lamellipodia.14, 33–34 In that context, Ser239 is strategically located within the EVH2 domain of VASP, immediately adjacent to the G-actin binding site, which localizes VASP at filopodial tips and coordinates the transfer of profilin-bound G-actin to the barbed end of the elongating F-actin filament.33 Phosphorylation of VASP Ser239 interferes with EVH2-dependent processes at membrane leading edges, destabilizing VASP-actin interactions and organelle protrusive dynamics.14, 33 Moreover, VASP Ser239, but not VASP Ser157, phosphorylation inhibits VASP-driven F-actin polymerization.13 This study demonstrates VASP Ser239 phosphorylation suppresses the invasive actin cytoskeleton of tumor cells and represents an unappreciated prototype to be exploited in innovative diagnostic and therapeutic approaches for colorectal cancer. Specifically, ligand-dependent GCC signaling may prevent colon cancer metastasis by neutralizing oncogenic VASP functions at the invasive cancer front.
Supplementary Material
Acknowledgments
This work was supported by grants to GMP from NIH (R03CA133950) and Elsa U. Pardee Foundation, and to SAW from NIH (CA75123, CA95026, RC1CA112147). The National Institute of Health specifically disclaims responsibility for any analyses, interpretations or conclusions. SAW is the Samuel M.V. Hamilton Professor of Medicine, and a paid consultant to Merck and the Chair (uncompensated) of the Scientific Advisory Board of Targeted Diagnostics and Therapeutics, Inc. GMP receives research salary support from Merck.
List of Principal Abbreviations
- cGMP
cyclic guanosine monophosphate
- EVH1
Ena/VASP homology 1
- EVH2
Ena/VASP homology 2
- F-actin
filamentous actin
- G-actin
globular actin
- GFP
green fluorescent protein
- GCC
guanylyl cyclase C
- IHC
immunohistochemistry
- MMP
matrix metalloproteinase
- PKG
cGMP-dependent protein kinase
- VASP
vasodilator-stimulated phosphoprotein
Footnotes
All the other authors have no conflict of interest to disclose.
Impact Statement: The manuscript demonstrates VASP Ser239 phosphorylation opposes malignant cell shape promoting invasion in colon cancer cells. Induction of VASP Ser239 phosphorylation may represent an innovative intervention to prevent tumor progression and metastasis in patients with colorectal cancer.
References
- 1.Pitari GM, Li P, Lin JE, Zuzga D, Gibbons AV, Snook AE, Schulz S, Waldman SA. The paracrine hormone hypothesis of colorectal cancer. Clin Pharmacol Ther. 2007;82:441–7. doi: 10.1038/sj.clpt.6100325. [DOI] [PubMed] [Google Scholar]
- 2.Steinbrecher KA, Tuohy TM, Heppner Goss K, Scott MC, Witte DP, Groden J, Cohen MB. Expression of guanylin is downregulated in mouse and human intestinal adenomas. Biochem Biophys Res Commun. 2000;273:225–30. doi: 10.1006/bbrc.2000.2917. [DOI] [PubMed] [Google Scholar]
- 3.Notterman DA, Alon U, Sierk AJ, Levine AJ. Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res. 2001;61:3124–30. [PubMed] [Google Scholar]
- 4.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 5.Pitari GM, Di Guglielmo MD, Park J, Schulz S, Waldman SA. Guanylyl cyclase C agonists regulate progression through the cell cycle of human colon carcinoma cells. Proc Natl Acad Sci U S A. 2001;98:7846–51. doi: 10.1073/pnas.141124698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin JE, Li P, Snook AE, Schulz S, Dasgupta A, Hyslop TM, Gibbons AV, Marszlowicz G, Pitari GM, Waldman SA. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology. 2010;138:241–54. doi: 10.1053/j.gastro.2009.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li P, Schulz S, Bombonati A, Palazzo JP, Hyslop TM, Xu Y, Barab AA, Siracusa LD, Pitari GM, Waldman SA. Guanylyl cyclase C suppresses intestinal tumorigenesis by restricting proliferation and maintaining genomic integrity. Gastroenterology. 2007;133:599–607. doi: 10.1053/j.gastro.2007.05.052. [DOI] [PubMed] [Google Scholar]
- 8.Deguchi A, Soh JW, Li H, Pamukcu R, Thompson WJ, Weinstein IB. Vasodilator-stimulated phosphoprotein (VASP) phosphorylation provides a biomarker for the action of exisulind and related agents that activate protein kinase G. Mol Cancer Ther. 2002;1:803–9. [PubMed] [Google Scholar]
- 9.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–64. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 10.Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Lamellipodial versus filopodial mode of the actin nanomachinery: pivotal role of the filament barbed end. Cell. 2004;118:363–73. doi: 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem. 1994;269:14509–17. [PubMed] [Google Scholar]
- 12.Blume C, Benz PM, Walter U, Ha J, Kemp BE, Renne T. AMP-activated protein kinase impairs endothelial actin cytoskeleton assembly by phosphorylating vasodilator-stimulated phosphoprotein. J Biol Chem. 2007;282:4601–12. doi: 10.1074/jbc.M608866200. [DOI] [PubMed] [Google Scholar]
- 13.Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, Schuh K, Gertler F, Munzel T, Renne T. Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J Cell Sci. 2009;122:3954–65. doi: 10.1242/jcs.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay SL, Ramsey S, Aitchison M, Renne T, Evans TJ. Modulation of lamellipodial structure and dynamics by NO-dependent phosphorylation of VASP Ser239. J Cell Sci. 2007;120:3011–21. doi: 10.1242/jcs.003061. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–52. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dostmann WR, Taylor MS, Nickl CK, Brayden JE, Frank R, Tegge WJ. Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Ialpha inhibit NO-induced cerebral dilation. Proc Natl Acad Sci U S A. 2000;97:14772–7. doi: 10.1073/pnas.97.26.14772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bear JE, Gertler FB. Ena/VASP: towards resolving a pointed controversy at the barbed end. J Cell Sci. 2009;122:1947–53. doi: 10.1242/jcs.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 20.Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–17. doi: 10.1016/j.tcb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Lubbe WJ, Zuzga DS, Zhou Z, Fu W, Pelta-Heller J, Muschel RJ, Waldman SA, Pitari GM. Guanylyl cyclase C prevents colon cancer metastasis by regulating tumor epithelial cell matrix metalloproteinase-9. Cancer Res. 2009;69:3529–36. doi: 10.1158/0008-5472.CAN-09-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Applewhite DA, Barzik M, Kojima S, Svitkina TM, Gertler FB, Borisy GG. Ena/VASP proteins have an anti-capping independent function in filopodia formation. Mol Biol Cell. 2007;18:2579–91. doi: 10.1091/mbc.E06-11-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, Wang Y, Goswami S, Wyckoff JB, Lauffenburger DA, Sahai E, Condeelis JS, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell. 2008;15:813–28. doi: 10.1016/j.devcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han G, Fan B, Zhang Y, Zhou X, Wang Y, Dong H, Wei Y, Sun S, Hu M, Zhang J, Wei L. Positive regulation of migration and invasion by vasodilator-stimulated phosphoprotein via Rac1 pathway in human breast cancer cells. Oncol Rep. 2008;20:929–39. [PubMed] [Google Scholar]
- 25.Dertsiz L, Ozbilim G, Kayisli Y, Gokhan GA, Demircan A, Kayisli UA. Differential expression of VASP in normal lung tissue and lung adenocarcinomas. Thorax. 2005;60:576–81. doi: 10.1136/thx.2004.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu LD, Zou HF, Zhan SX, Cao KM. EVL (Ena/VASP-like) expression is up-regulated in human breast cancer and its relative expression level is correlated with clinical stages. Oncol Rep. 2008;19:1015–20. [PubMed] [Google Scholar]
- 27.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 28.Stylli SS, Kaye AH, Lock P. Invadopodia: at the cutting edge of tumour invasion. J Clin Neurosci. 2008;15:725–37. doi: 10.1016/j.jocn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Wentworth JK, Pula G, Poole AW. Vasodilator-stimulated phosphoprotein (VASP) is phosphorylated on Ser157 by protein kinase C-dependent and -independent mechanisms in thrombin-stimulated human platelets. Biochem J. 2006;393:555–64. doi: 10.1042/BJ20050796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebrand C, Dent EW, Strasser GA, Lanier LM, Krause M, Svitkina TM, Borisy GG, Gertler FB. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron. 2004;42:37–49. doi: 10.1016/s0896-6273(04)00108-4. [DOI] [PubMed] [Google Scholar]
- 31.Gomez TM, Robles E. The great escape; phosphorylation of Ena/VASP by PKA promotes filopodial formation. Neuron. 2004;42:1–3. doi: 10.1016/s0896-6273(04)00188-6. [DOI] [PubMed] [Google Scholar]
- 32.Aszodi A, Pfeifer A, Ahmad M, Glauner M, Zhou XH, Ny L, Andersson KE, Kehrel B, Offermanns S, Fassler R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J. 1999;18:37–48. doi: 10.1093/emboj/18.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barzik M, Kotova TI, Higgs HN, Hazelwood L, Hanein D, Gertler FB, Schafer DA. Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins. J Biol Chem. 2005;280:28653–62. doi: 10.1074/jbc.M503957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yaroslavskiy BB, Zhang Y, Kalla SE, Garcia Palacios V, Sharrow AC, Li Y, Zaidi M, Wu C, Blair HC. NO-dependent osteoclast motility: reliance on cGMP-dependent protein kinase I and VASP. J Cell Sci. 2005;118:5479–87. doi: 10.1242/jcs.02655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.