Abstract
The maximum dosage of methotrexate (MTX) for treatment of rheumatoid arthritis (RA) formally approved in Japan is 8 mg/week. We intended to examine the efficacy and safety of MTX at dosages over 8 mg/week in Japanese rheumatoid arthritis patients using the large Institute of Rheumatology, Rheumatoid Arthritis (IORRA) cohort database. Among 9,122 patients registered in the IORRA database from the October 2000 survey to the October 2007 survey, 5,201 patients who had been treated with MTX were selected. We attempted to overcome the drawbacks innate to nonrandomized studies by using longitudinal analyses and multifactorial logistic regression analyses. Cross-sectional analysis of data obtained from the October 2007 survey indicated that dosages of MTX higher than 8 mg/week were used in 27.5% of patients treated with MTX. Longitudinal analyses based on data from three consecutive phases showed that final Disease Activity Score-28 (DAS28) values were significantly lower [n = 260, mean difference 0.563, 95% confidence interval (CI) 0.438–0.688, P < 2.2 × 10−22, two-sided paired t test] than initial values when MTX was increased from 8 mg/week or lower to over 8 mg/week. In addition, longitudinal analyses based on data from two consecutive phases indicated decreases in DAS28 values of 0.26 ± 1.04 (n = 690, P = 6.78 × 10−11, two-sided paired t test) when MTX dosages were increased from 8 mg/week or lower to over 8 mg/week, compared with decreases of 0.07 ± 0.89 (n = 2,125, P = 0.000307) when the dosage was maintained at 8 mg/week. The decreases in DAS28 values were significantly larger in the former than the latter (P = 2.27 × 10−6, two-sided unpaired t test). Concerning safety of MTX at dosages over 8 mg/week, we performed logistic regression analysis in which the objective variable was the existence or nonexistence of self-reported side-effects and the explanatory variable was the MTX dosage in the former phase, with adjustments made for age, sex, body mass index (BMI), steroid administration, folic acid administration, concomitant pulmonary diseases, and renal dysfunction. The results indicated that MTX dosages over 8 mg/week did not have any association with either severe or severe + moderate side-effects. These data regarding both efficacy and safety of MTX at dosages over 8 mg/week in Japanese RA patients would provide the basis for use of the drug at dosages currently not formally approved by the Japanese government.
Keywords: Efficacy, Methotrexate, Rheumatoid arthritis, Safety, Treatment
Introduction
Methotrexate (MTX) is known to be effective for treatment of rheumatoid arthritis (RA). Since the data from clinical trials have indicated that MTX suppresses pain and inflammation as well as progression of joint damage [1], it is now considered to be the standard drug for treatment of RA [2, 3].
Though MTX has already been approved for treatment of RA in Japan, the maximum dosage indicated by the Ministry of Health, Welfare, and Labor is only 8 mg/week, which is much lower than the dosage applied in recent clinical trials, where 15 to even 25 mg/week MTX was commenced [4–10]. Indeed, according to the Guidelines for the management of rheumatoid arthritis by the American College of Rheumatology (ACR) [11], the recommended dosages of MTX are 7.5–20 mg/week. To be noted, recent reports described that mortality decreased with long-term administration of MTX [12].
In the clinical trial performed by Kashiwazaki et al. [13], MTX was administered in weekly doses of 2–9 mg. Based on the results of this trial and some reports by Japanese rheumatologists [14], the maximum dosage was set at 8 mg/week in Japan.
It has been reported that the optimal dose of MTX differs between different patients [15]; however, antirheumatic effects are known to be dose dependent, and a higher effect can be expected at higher dose [11]. Some pieces of evidence have been obtained also in Japan that show the dose-dependent antirheumatic effects of MTX [16], and it is widely recognized that many Japanese RA patients require dosages higher than 8 mg/week.
So far, no published data have provided sufficient evidence for the efficacy and safety of MTX used at dosages over 8 mg/week for Japanese RA patients [13]. A standard solution to this issue would be to perform a new clinical trial for use of MTX at over 8 mg/week in Japanese RA patients as a new drug development process. However, it seems unrealistic to perform a clinical trial as a new drug development for dosages of the drug that are widely used by most Japanese rheumatologists.
Considering this circumstance, it is highly likely that efficacy and safety at higher dosages can be examined by analysis of data from real practice, particularly analysis of data from a highly valuable cohort study.
In the present study, we attempt to examine both efficacy and safety of use of MTX in Japanese RA patients at dosages over 8 mg/week using the IORRA cohort database. Great care was taken to avoid false conclusions by use of various statistical methods.
Patients and methods
Study cohort
The Institute of Rheumatology, Rheumatoid Arthritis (IORRA) cohort is formed of RA patients at the Institute of Rheumatology, Tokyo Women’s Medical University. It has been underway since October 2000, and patients who satisfied the revised classification criteria of the American Rheumatology Association [17] have been registered. Data collection is conducted twice a year from each patient after registration, and data have been obtained from 4,000–5,000 RA patients during each survey period. More specifically, data collection is conducted twice a year, i.e., in the period from April to May for one phase and from October to November for the other phase; however, for simplicity in this paper, we call these two annual phases as April and October, respectively. The phases of different years are numbered serially such that phase 1 denotes the time of the survey in October 2000.
About 99% of all RA outpatients who newly visited our institute were registered, and over 98% of them responded to a questionnaire (often by mail) in each phase since the establishment of the cohort. Thus, the cohort is nearly free from bias produced by selecting only specific parts of the patients from the outpatients; however, the problem that the participants are from a single facility in the Tokyo area of Japan still remains.
The data obtained from the IORRA cohort consist of the following three elements. The first element is formed of objectives evaluated by the physicians, including tender and swollen joint counts and global assessment measured by visual analog scale (VAS). The second component is the patient-reported information including VAS for pain, VAS for global assessment, physical function reported by the validated Japanese version of health assessment questionnaire (JHAQ) [18], height, weight, complications or comorbidity during the latest 6 months, and information about drugs from the patients during the period. To collect the information for the second element, each patient was given a questionnaire by the attending doctor, filled it at home, and then mailed it to our office within 2 weeks using a prestamped envelope. The third element consists of the laboratory data, which include C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), blood cell counts, blood chemistry, and other data obtained from blood and urine samples. Regarding safety data, information about side-effects is obtained by patient self-report. In the IORRA questionnaire, the term “side effects” but not “adverse events” is used. The data about side-effects derive from answers to questions about side-effects that occurred between the last survey and the current survey, but the exact time when the side-effect occurred cannot be determined. All data collected during each period are integrated into a single database for analysis.
Various articles have already been published using this database [16, 19–35]. In each phase, some patients dropped out and other new patients were registered. However, substantial proportions of the patients have been observed continuously for long periods. Written informed consent for the study was obtained from patients whenever data were collected.
Distribution of MTX dosage by cross-sectional analysis
The distribution of the weekly dosage of MTX was determined using data obtained in the October 2007 phase. During this phase, data were obtained from 5,257 patients.
Examination of associations between missing data and patient background for longitudinal studies of efficacy and safety of MTX at dosages higher than 8 mg/week
This analysis may be biased if the absence of dosage data is not independent of other events, because such associations may influence the results of analyses of associations between efficacy (safety) and MTX dosage. To analyze possible associations between the absence of dosage data and other events, we compared patient background between those with and without MTX dosage data. The Mann–Whitney test (for continuous variables) or Fisher’s exact test (for categorical variables) was conducted for each background factor.
Analysis of efficacy of increasing the MTX dosage from 8 mg/week or less to over 8 mg/week using data from three consecutive phases (“phase trios”)
For all longitudinal analyses in this study, data from patients who had fulfilled the following conditions were extracted from the IORRA database: (1) Patients registered in the database from October 2000 (the first survey) to October 2007 (the 15th survey). (2) Patients over 18 years of age at time of registration in IORRA. (3) Patients with records of being treated with MTX. (4) Data were excluded for periods when leflunomide, cyclosporine, tacrolimus or biologics was commenced. Among the total 9,122 patients, 5,201 fulfilled the conditions described above, and data were analyzed for those for whom MTX dosage data were not missing. Patient background characteristics were described at the phase when MTX was administered for the first time in patients who had been naïve to MTX, while the data of the initial visit to our institution were regarded as the baseline for analysis for patients who had been treated with MTX previously.
For the analysis of the efficacy of increasing the dosage of MTX from 8 mg/week or less to over 8 mg/week, we analyzed data from three consecutive phases, which we call “a phase trio,” and that of two consecutive phases (“a phase pair”), as it is difficult to identify the exact time when the dosage was changed during the 6 months of each phase and to confirm the efficacy of increasing the dosage. Of the 5,201 patients who met the criteria, we first selected the cases for each of the four groups in Table 1A when the MTX dosages in the phase trio satisfied the following conditions: 8 mg/week or less at the phase before A, and over 8 mg/week at phase A and the phase after A (group T1); 8 mg/week or less at the phase before A, over 8 mg/week at phase A, and 8 mg/week or less at the phase after A (group T2); 8 mg/week or less at the phase before A, over 8 mg/week at phase A, and over 8 mg/week but staying at the same dosage as at phase A at the phase after A (group T3, which is also a part of group T1); 8 mg/week through the three consecutive phases (group T4). However, if treatments with steroid and folic acid were changed during the period, they were excluded from each of the groups. It is possible that multiple cases could be selected from a patient. If this occurred, the different cases from a patient reflect data for different time periods.
Table 1.
Definitions of the groups in the efficacy analysis
| Phase before A | MTX dosage at phase A (per week) | Phase after A | Number of trios | |
|---|---|---|---|---|
| (A) Phase trio | ||||
| Group T1 | 8 mg or less | Over 8 mg | Over 8 mg | 262 |
| Group T2 | 8 mg or less | Over 8 mg | 8 mg or less | 70 |
| Group T3 | 8 mg or less | Over 8 mg | Over 8 mg (same value as phase A) | 123 |
| Group T4 | 8 mg | 8 mg | 8 mg | 55 |
| Phase before A | MTX dosage at phase A (per week) | Number of pairs | ||
|---|---|---|---|---|
| (B) Phase pair | ||||
| Group P1 | 8 mg or less | Over 8 mg | 690 | |
| Group P2 | 8 mg | 8 mg | 2,125 | |
| Group P3 | Over 8 mg | Over 8 mg | 2,545 | |
There are 6-month intervals between consecutive phases
The primary and secondary endpoints were set as follows before performing the analyses:
Primary endpoint For phase trios that belong to group T1, we test whether the value of DAS28 recorded one phase after A (abbreviated as das2) is smaller than the value of DAS28 recorded one phase before A (abbreviated as das0). The paired t test (two-sided) is used.
Secondary endpoint 1 We test whether das2 is smaller than das0 in the union of group T1 and T2 (all phase trios in which the dosage of MTX had ever been increased over 8 mg/week). The paired t test (two-sided) is used.
Secondary endpoint 2 We test whether the value of subtracting das2 from das0 is larger in group T3 (a subset of phase trios in which MTX had been increased from 8 mg/week or less to over 8 mg/week and maintained until one phase after A) than in group T4. The unpaired t test (two-sided) is used.
Exploratory research For patients who belong to group T4, we test whether das2 is smaller than das0. The paired t test (two-sided) is used.
For each of the primary and secondary endpoints, the hypothesis that disease activity is decreased by increasing the MTX dosage from 8 mg/week or less to over 8 mg/week is supported when the result of the test is significant.
Analysis of efficacy of increasing the MTX dosage from 8 mg/week or less to over 8 mg/week using data from two consecutive phases (“phase pairs”)
We then analyze the efficacy of increasing the MTX dosage to over 8 mg/week using data from the following phase pairs (Table 1B): group P1 as the phase pairs in which the dosage of MTX was increased from 8 mg/week or less to over 8 mg/week, group P2 as the pairs in which the dosage was kept at 8 mg/week, and group P3 as the pairs in which the dosage was kept over 8 mg/week. We analyzed whether the values of DAS28 in phase A were lower than those in one phase before A for groups P1, P2, and P3 using the paired t test, and then tested whether the difference in DAS28 between phase A and one phase before phase A (das0–das1) was larger for group P1 than for group P2 using an unpaired t test.
Analysis of safety of MTX administered at dosages over 8 mg/week using data from two consecutive phases (“phase pairs”)
In this analysis, we assumed that a self-reported event in a phase reflects the dosage of MTX in the previous phase. Therefore, for a phase pair, we analyzed the relation between the dosage of MTX in the former phase and the self-reported side-effects in the latter phase. The phase pairs were stratified by MTX dosage in the former phase, and subdivided depending on whether the dosage was the same, increased, unknown or MTX was discontinued in the latter phase (Table 2). Examination of only phase pairs in which dosages of MTX were maintained would quite likely cause bias, because either discontinuation or dosage reduction of MTX may occur when side-effects are observed. Therefore, we used all phase pairs in Table 2 to analyze the relation between side-effects and the dosage of MTX.
Table 2.
Overview of data for safety analysis using phase pairs
| In the latter phase | MTX dosage (mg/week) in the former phase | 2–4 | Over 4–6 | Over 6–8 | 8 or lower | Over 8 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subtotal number of phase pairs | Number of phase pairs | % | Number of phase pairs | % | Number of phase pairs | % | Number of phase pairs | % | Number of phase pairs | % | |
| Dosage the same as the former | 17,495 | 5,249 | 56.0 | 5,052 | 55.3 | 4,273 | 57.3 | 14,574 | 56.1 | 2,921 | 54.2 |
| Dosage different from the former | 9,623 | 2,567 | 27.4 | 2,881 | 31.6 | 2,327 | 31.2 | 7,775 | 29.9 | 1,848 | 34.3 |
| Discontinuation | 1,417 | 655 | 7.0 | 345 | 3.8 | 228 | 3.1 | 1,228 | 4.7 | 189 | 3.5 |
| Data not available | 2,820 | 909 | 9.7 | 852 | 9.3 | 628 | 8.4 | 2,389 | 9.2 | 431 | 8.0 |
| Total | 31,355 | 9,380 | 100.0 | 9,130 | 100.0 | 7,456 | 100.0 | 25,966 | 100.0 | 5,389 | 100.0 |
We set the endpoints as below before the analysis. In this analysis, we addressed the question of whether side-effects occurred more frequently when the dosage of MTX exceeded 8 mg/week in comparison with cases where the dosage was 8 mg/week or less. Therefore, we performed logistic regression analysis in which the dependent variable was the existence or nonexistence of severe side-effects in the latter phase, the explanatory variable was the dosage of MTX in the former phase, and adjustments were made for age, sex, BMI, steroid administration, folic acid administration, pulmonary diseases, and renal dysfunction.
Primary endpoint We perform logistic regression analysis in which the dependent variable is the presence or absence of severe to moderate side-effects by MTX based on patient self-report, and the explanatory variable is whether dosage of MTX is over 8 mg/week or not.
Secondary endpoints 1–3 We perform logistic regression analysis in which the dependent variable is the presence or absence of severe side-effects by MTX, that by any drugs, and the presence or absence of severe to moderate side-effects by any drug based on patient self-report, respectively, and the explanatory variable is whether dosage of MTX is over 8 mg/week or not.
Analyses of efficacy of MTX at dosages over 8–16 mg/week by longitudinal study
We extracted phase pairs where the MTX dosages in two consecutive phases satisfied the following conditions: group E1 for phase pairs in which the dosage of MTX was increased from less than 16 mg/week to 16 mg or more per week, group E2 for 14 mg/week, group E3 for 12 mg/week, group E4 for 10 mg/week, and group E5 for pairs in which MTX was stable at dosage of 8 mg/week, respectively. We allowed the extraction of more than one phase pair from each patient. The endpoints were as follows:
Primary endpoint For group E1, the paired t test is used to examine whether the value of DAS28 in phase A is lower than the value in one phase before A.
Secondary endpoints 1–3 For each of group E2, E3, and E4, the paired t test is used to examine whether the value of DAS28 in phase A is lower than the value in one phase before A.
Secondary endpoints 4–7 The difference between the value of DAS28 in one phase before A and in phase A is denoted as das0–das1. The unpaired t test is used to examine whether the mean of das0–das1 for each of groups E1–E4 is larger than the mean of group E5.
Exploratory research For group E5, the paired t test is used to examine whether the value of DAS28 in phase A is lower than the value in one phase before A.
Analyses of safety of MTX at dosages over 8–16 mg/week by longitudinal study
In the analyses for dosages over 8–16 mg/week, we made the same assumption as in the previous analysis of the safety of MTX administered at dosages over 8 mg/week. We selected phase pairs divided into the following groups: group S1 for phase pairs in which the dosage of MTX was 16 mg/week or higher in the phase before A (phase A represents the phase in which an adverse event is reported), group S2 for those in which the dosage of MTX was from 14 to lower than 16 mg/week, group S3 for those in which it was from 12 to lower than 14 mg/week, group S4 for those in which it was from 10 to lower than 12 mg/week, and group S5 for those in which the dosage of MTX was 8 mg/week (Table 3). We performed logistic regression analysis in which the dependent variable was the existence or nonexistence of severe or moderate to severe side-effects in the latter phase, the explanatory variable was the dosage of MTX in the former phase, and adjustments were made for age, sex, BMI, steroid administration, folic acid administration, pulmonary diseases, renal dysfunction, and DAS28 at phase A.
Table 3.
Grouping for studies of associations between adverse events and dosages of MTX
| MTX dosage (per week) | Number of phase pairs | Fraction (%) of patients with severe adverse events | Fraction (%) of patients with severe or moderate adverse events | ||
|---|---|---|---|---|---|
| Phase before A | Phase A | ||||
| Group S1 | 16 mg or higher | Any | 192 | 3.28 | 13.06 |
| Group S2 | From 14 to lower than 16 mg | Any | 655 | 3.13 | 14.06 |
| Group S3 | From 12 to lower than 14 mg | Any | 1,488 | 4.43 | 14.81 |
| Group S4 | From 10 to lower than 12 mg | Any | 3,199 | 3.09 | 13.91 |
| Group S5 | 8 mg | Any | 5,574 | 3.34 | 14.47 |
Results
Distribution of MTX dosage by cross-sectional analysis
Among 5,257 patients whose data were in the database obtained during the October 2007 phase, the number of patients treated with MTX was 3,252 (61.9%). Figure 1 shows the distribution of the dosage of MTX in the 3,252 MTX-treated patients, in which dosages of MTX per patient were 7.54 ± 3.05 mg/week (mean ± standard deviation). Among them, 27.5% of patients were treated with MTX dosage higher than 8 mg/week, 11.5% higher than 12 mg/week, and 0.83% higher than 16 mg/week (Fig. 1).
Fig. 1.
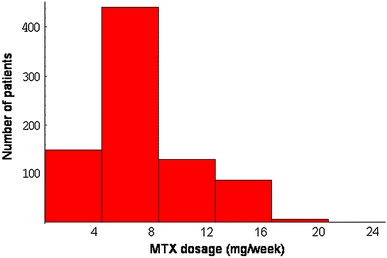
Histogram of MTX dosage in 3,252 patients (IORRA October 2007 survey)
Examination of associations between missing data and patient background for longitudinal studies of efficacy and safety of MTX at dosages higher than 8 mg/week
Table 4 presents the background of the 5,201 patients who matched conditions 1–4 in the “Patients and methods” section. Variables that showed significant differences between patients with and without dosage data after Bonferroni correction for multiple comparisons (P < 0.05/29 = 0.00172) included age at onset (P = 0.00000027), age (P = 0.000000000013), and folic acid dose (P = 0.00036) (Table 5). In addition, steroid (P = 0.0030) and bucillamine (P = 0.0036) showed relatively low P values after analysis of the association with missing dosage data, although the associations were not significant after correction for multiple comparisons.
Table 4.
Background of 5,201 patients in whom MTX was administered for the first time
| Variable | Number of missing data | Mean value (fraction) | Standard deviation | Median | 25 percentile | 75 percentile | Minimum value | Maximum value | |
|---|---|---|---|---|---|---|---|---|---|
| Sex | Male 0 and female 1 | 0 | 0.84 | ||||||
| Age at onset | (years) | 70 | 46.34 | 13.54 | 47 | 37 | 56 | 1 | 87 |
| Age | (years) | 0 | 55.46 | 12.78 | 57 | 48 | 65 | 18 | 89 |
| Duration of RA | (years) | 70 | 9.12 | 8.48 | 7 | 3 | 13 | 0 | 66 |
| Height | cm | 65 | 157.15 | 7.34 | 157 | 152 | 162 | 126 | 187 |
| Weight | kg | 91 | 52.92 | 9.14 | 52 | 47 | 58 | 25 | 97 |
| BMI | kg/m2 | 101 | 21.38 | 3.01 | 21.09 | 19.23 | 23.18 | 12.23 | 39.72 |
| DAS28–ESRa | 432 | 4.16 | 1.21 | 4.11 | 3.321 | 4.94 | 0.5015 | 8.644 | |
| JHAQb | 12 | 0.88 | 0.74 | 0.75 | 0.25 | 1.375 | 0 | 3 | |
| Number of tender joints | (45 joints) | 241 | 4.16 | 5.51 | 2 | 1 | 5 | 0 | 43 |
| Number of swollen joints | (45 joints) | 241 | 3.74 | 4.36 | 2 | 1 | 5 | 0 | 41 |
| Pain–VASc | 100 mm | 43 | 37.80 | 26.19 | 33 | 15 | 59 | 0 | 100 |
| GHd | 100 mm | 38 | 38.71 | 24.83 | 38 | 17 | 56 | 0 | 100 |
| Doctor–VASe | 100 mm | 71 | 25.23 | 19.19 | 21 | 10 | 35 | 0 | 100 |
| CRP | mg/dl | 190 | 1.68 | 2.14 | 0.9 | 0.3 | 2.2 | 0 | 27.5 |
| ESR | mm/h | 219 | 40.80 | 25.13 | 36.7 | 21 | 56.58 | 1.4 | 115.9 |
| RF | U/ml | 220 | 145.87 | 246.98 | 64 | 23 | 144 | 1 | 2940 |
| GPT | IU/l | 215 | 21.60 | 21.28 | 16 | 12 | 24 | 3 | 446 |
| GOT | IU/l | 218 | 21.52 | 13.09 | 18 | 15 | 24 | 5 | 253 |
| WBC | 1/mm3 | 282 | 7,645.72 | 2,295.12 | 7,300 | 6,000 | 9,000 | 2,400 | 20,300 |
| RF (±) | U/ml | 0 | 0.82 | ||||||
| NSAID | Fraction | 0 | 0.81 | ||||||
| Steroid | Fraction | 0 | 0.59 | ||||||
| MTX | Fraction | 0 | 1.00 | ||||||
| BUC | Fraction | 0 | 0.19 | ||||||
| SSZ | Fraction | 0 | 0.23 | ||||||
| DPC | Fraction | 0 | 0.05 | ||||||
| GST | Fraction | 0 | 0.06 | ||||||
| MTX dosage | mg/week | 132 | 5.25 | 2.26 | 4.4 | 4 | 6 | 2 | 18 |
| PSL dosagef | mg/day | 120 | 5.04 | 4.74 | 5 | 3 | 6 | 0.03 | 200 |
| Folic acid | Fraction | 0 | 0.12 |
RF rheumatoid factor, GPT glutamic pyruvic transaminase, GOT glutamic oxaloacetic transaminase, WBC white blood cell, NSAID nonsteroidal anti-inflammatory drug, BUC bucillamine, SSZ salazosulfapyridine, DPCd-penicillamine, GST gold sodium thiomalate
aDAS28-ESR DAS28 calculated using the value of erythrocyte sedimentation rate (ESR) (a score indicating RA activity proposed by the European League against Rheumatism; data of 28 joints are used to calculate the score)
bJHAQ answer to Japanese version of health assessment questionnaire (JHAQ, [18])
cPatient’s assessment of pain measured by visual analogue scale (VAS) represented by length (maximum 100 mm)
dPatient’s global assessment measured by VAS represented by length (maximum 100 mm)
ePhysician’s global assessment of disease activity measured by VAS represented by length (maximum 100 mm)
fDosage of steroid (in terms of prednisolone)
Table 5.
Comparison of patients with and without MTX dosage data
| Variable | Patients with missing MTX dosage data (132) | Patients with MTX dosage data (5,069) | Difference of means | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number of missing data | Mean value (fraction) | Standard deviation | Number of missing data | Mean value (fraction) | Standard deviation | ||||
| Sex | Male 0 and female 1 | 0 | 0.82 | 0 | 0.84 | −0.02 | 0.55 | ||
| Age at onset | (years) | 3 | 52.44 | 13.30 | 67 | 46.18 | 13.51 | 6.26 | 0.00000027 |
| Age | (years) | 0 | 62.46 | 11.79 | 0 | 55.28 | 12.76 | 7.18 | 0.000000000013 |
| Duration of RA | (years) | 3 | 9.88 | 8.51 | 67 | 9.10 | 8.48 | 0.78 | 0.19 |
| Height | cm | 4 | 155.54 | 7.12 | 61 | 157.20 | 7.35 | −1.66 | 0.018 |
| Weight | kg | 4 | 52.62 | 8.49 | 87 | 52.92 | 9.15 | −0.31 | 0.76 |
| BMI | kg/m2 | 4 | 21.70 | 2.83 | 97 | 21.37 | 3.02 | 0.33 | 0.053 |
| DAS28–ESR | 17 | 4.35 | 1.22 | 415 | 4.15 | 1.21 | 0.20 | 0.10 | |
| JHAQ | 2 | 0.99 | 0.79 | 10 | 0.88 | 0.74 | 0.12 | 0.10 | |
| Number of tender joints | (45 joints) | 10 | 4.81 | 6.30 | 231 | 4.15 | 5.49 | 0.66 | 0.25 |
| Number of swollen joints | (45 joints) | 10 | 4.19 | 5.40 | 231 | 3.73 | 4.33 | 0.46 | 0.58 |
| Pain–VAS | 100 mm | 4 | 39.45 | 27.38 | 39 | 37.76 | 26.16 | 1.69 | 0.52 |
| GH | 100 mm | 5 | 39.54 | 25.52 | 33 | 38.69 | 24.81 | 0.85 | 0.78 |
| Doctor–VAS | 100 mm | 4 | 23.65 | 17.08 | 67 | 25.27 | 19.24 | −1.62 | 0.57 |
| CRP | mg/dl | 3 | 2.08 | 2.42 | 187 | 1.67 | 2.13 | 0.41 | 0.037 |
| ESR | mm/h | 4 | 46.41 | 27.92 | 215 | 40.66 | 25.04 | 5.76 | 0.029 |
| RF | U/ml | 3 | 142.82 | 228.65 | 217 | 145.95 | 247.47 | −3.13 | 0.65 |
| GPT | IU/l | 5 | 20.97 | 17.98 | 210 | 21.62 | 21.36 | −0.65 | 0.28 |
| GOT | IU/l | 5 | 22.65 | 12.30 | 213 | 21.49 | 13.11 | 1.15 | 0.19 |
| WBC | 1/mm3 | 8 | 7731.45 | 2,220.06 | 274 | 7,643.50 | 2,297.21 | 87.95 | 0.56 |
| RF (±) | U/ml | 0 | 0.81 | 0 | 0.82 | −0.01 | 0.73 | ||
| NSAID | Fraction | 0 | 0.73 | 0 | 0.81 | −0.08 | 0.033 | ||
| Steroid | Fraction | 0 | 0.46 | 0 | 0.59 | −0.13 | 0.0030 | ||
| BUC | Fraction | 0 | 0.10 | 0 | 0.20 | −0.10 | 0.0036 | ||
| SSZ | Fraction | 0 | 0.18 | 0 | 0.23 | −0.05 | 0.18 | ||
| DPC | Fraction | 0 | 0.08 | 0 | 0.05 | 0.02 | 0.23 | ||
| GST | Fraction | 0 | 0.07 | 0 | 0.06 | 0.01 | 0.56 | ||
| PSL dosage | mg/day | 8 | 5.48 | 2.23 | 112 | 5.03 | 4.77 | 0.45 | 0.0353 |
| Folic acid | Fraction | 0 | 0.03 | 0 | 0.12 | −0.09 | 0.00036 | ||
Abbreviations are explained in Table 4
By performing logistic regression analysis with missing and nonmissing event as the dependent variable and the explanatory variables mentioned above, we found that only age was significantly associated (P = 0.018) with missing dosage data whereas the associations with the other factors were not significant (detailed data not shown).
In any case, patients with missing dosage data constituted only about 3% of the patients in the dataset, and the absence of dosage data seems to have a small effect. However, results should be interpreted with care if a variable is strongly associated with age in the following consecutive analyses.
Analysis of efficacy of increasing the dosage of MTX from 8 mg/week or less to over 8 mg/week using data from three consecutive phases (“phase trios”)
Table 6 shows the background of the patients in each group of Table 1. It is clear that disease activity one phase before A in group T4 is lower than in group T1 or T3 at baseline; the mean value of DAS28 one phase before A in group T1 was 4.30 ± 1.21 (n = 260, mean ± SD), while it was 3.66 ± 1.23 (n = 55) for group T4 (P < 0.001, unpaired t test; two-sided) (Table 6). In addition, the mean CRP value one phase before A in group T1 was 2.00 ± 2.19 mg/dl (n = 256), while it was 1.00 ± 1.71 mg/dl (n = 52) for group T4 (P < 0.0005) (Table 6). Namely, the disease activity before increasing the dosage of MTX from 8 mg/week or less to over 8 mg/week was significantly higher than the disease activity for phase trios without such an increase. A similar difference was observed for the data in phase A. The mean DAS28 value in phase A was 4.00 ± 1.18 (n = 251) in group T1, while it was 3.49 ± 1.16 (n = 52) for group T4 (P < 0.005) (Table 6). As previously stated, age was significantly associated with missing MTX dosage data. We therefore compared age between groups T1 and T4. The mean age in group T1 was 53.33 ± 12.54 years (n = 262), while it was 53.85 ± 12.91 years (n = 55) in group T4 (Table 6), not significantly different (P = 0.785).
Table 6.
Background of patients for phase trios in Table 1A
| Variable | Group T1 (262) | Group T1 + group T2 (332) | Group T3 (123) | Group T4 (55) | P value (group T1 versus T4) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of missing data | Mean value (fraction) | Standard deviation | Number of missing data | Mean value (fraction) | Standard deviation | Number of missing data | Mean value (fraction) | Standard variation | Number of missing data | Mean value (fraction) | Standard variation | ||||
| MTX dosage | Phase before A | 0 | 7.27 | 1.13 | 0 | 7.04 | 1.33 | 0 | 7.18 | 1.22 | 0 | 8.00 | |||
| (mg/week) | Phase A | 0 | 10.08 | 1.56 | 0 | 10.23 | 1.81 | 0 | 10.49 | 1.24 | 0 | 8.00 | |||
| Phase after A | 0 | 11.00 | 1.85 | 0 | 10.03 | 2.61 | 0 | 10.49 | 1.24 | 0 | 8.00 | ||||
| DAS28–ESR | Phase before A | 2 | 4.30 | 1.21 | 2 | 4.23 | 1.21 | 2 | 4.16 | 1.25 | 0 | 3.66 | 1.23 | <0.001 | |
| Phase A | 11 | 4.00 | 1.18 | 16 | 3.93 | 1.18 | 8 | 3.67 | 1.20 | 3 | 3.49 | 1.16 | <0.005 | ||
| Phase after A | 15 | 3.74 | 1.13 | 18 | 3.71 | 1.12 | 12 | 3.62 | 1.19 | 2 | 3.41 | 1.24 | |||
| Folic acid | Phase A | 0 | 0.50 | 0 | 0.47 | 0 | 0.47 | 0 | 0.31 | ||||||
| PSL dosage (mg) | Phase A | 0 | 2.14 | 2.79 | 0 | 2.13 | 2.77 | 0 | 2.13 | 2.59 | 0 | 1.98 | 2.81 | ||
| Sex | Male: 0 and female: 1 | 0 | 0.82 | 0 | 0.83 | 0 | 0.83 | 0 | 0.84 | ||||||
| Age at onset | years | 3 | 43.37 | 13.00 | 4 | 43.68 | 13.06 | 0 | 43.15 | 13.05 | 0 | 42.89 | 14.31 | ||
| Age | years | 0 | 53.33 | 12.54 | 0 | 53.81 | 12.47 | 0 | 54.38 | 11.60 | 0 | 53.85 | 12.91 | ||
| Duration of RA | years | 3 | 10.05 | 7.59 | 4 | 10.20 | 7.89 | 0 | 11.23 | 8.00 | 0 | 10.96 | 8.23 | ||
| Height | cm | 6 | 158.41 | 7.46 | 8 | 158.09 | 7.36 | 3 | 158.15 | 7.13 | 0 | 158.45 | 8.00 | ||
| Weight | kg | 8 | 53.41 | 9.62 | 11 | 53.21 | 9.40 | 4 | 53.66 | 9.43 | 0 | 54.98 | 9.66 | ||
| BMI | kg/m2 | 8 | 21.20 | 2.97 | 11 | 21.22 | 2.94 | 4 | 21.39 | 3.27 | 0 | 21.84 | 2.96 | ||
| JHAQ | 0 | 0.85 | 0.66 | 0 | 0.86 | 0.68 | 0 | 0.87 | 0.70 | 0 | 0.79 | 0.72 | |||
| Number of tender joints | 45 joints | 4 | 4.38 | 6.30 | 7 | 4.22 | 6.03 | 2 | 3.99 | 5.70 | 3 | 3.29 | 4.37 | ||
| Number of swollen joints | 45 joints | 4 | 4.22 | 4.50 | 7 | 4.05 | 4.37 | 2 | 4.26 | 4.33 | 3 | 2.83 | 3.25 | <0.01 | |
| Pain–VAS | 100 mm | 2 | 40.40 | 26.24 | 2 | 40.10 | 26.20 | 0 | 40.11 | 26.61 | 0 | 32.11 | 27.61 | ||
| GH | 100 mm | 1 | 39.85 | 23.96 | 1 | 40.01 | 24.15 | 0 | 38.48 | 24.09 | 0 | 32.09 | 24.93 | ||
| Doctor–VAS | 100 mm | 2 | 24.01 | 18.54 | 3 | 23.19 | 18.42 | 0 | 23.27 | 18.50 | 2 | 23.30 | 18.16 | ||
| CRP | mg/dl | 6 | 2.00 | 2.19 | 8 | 1.89 | 2.13 | 2 | 1.88 | 2.12 | 3 | 1.00 | 1.71 | <0.0005 | |
| ESR | mm/h | 7 | 45.51 | 24.02 | 10 | 44.52 | 24.49 | 3 | 43.55 | 24.62 | 4 | 29.30 | 19.22 | <10−6 | |
| RF | U/ml | 7 | 153.79 | 244.83 | 9 | 145.39 | 227.37 | 2 | 129.31 | 178.26 | 4 | 100.69 | 140.50 | ||
| GPT | IU/l | 7 | 22.45 | 19.43 | 9 | 22.16 | 18.27 | 3 | 24.58 | 23.31 | 4 | 28.78 | 21.96 | ||
| GOT | IU/l | 7 | 22.09 | 12.38 | 9 | 21.95 | 11.59 | 3 | 23.53 | 14.75 | 4 | 26.82 | 14.15 | <0.05 | |
| WBC | /mm3 | 7 | 7288.63 | 2334.61 | 10 | 7222.98 | 2,304.07 | 3 | 7,284.17 | 2,167.48 | 5 | 7,144.00 | 1,948.43 | ||
| RF (±) | 7 | 0.84 | 0.37 | 9 | 0.83 | 0.38 | 2 | 0.79 | 0.41 | 4 | 0.82 | 0.39 | |||
| NSAID | Fraction | 0 | 0.85 | 0 | 0.84 | 0 | 0.86 | 0 | 0.84 | ||||||
| Steroid | Fraction | 0 | 0.44 | 0 | 0.44 | 0 | 0.46 | 0 | 0.38 | ||||||
| BUC | Fraction | 0 | 0.18 | 0 | 0.17 | 0 | 0.17 | 0 | 0.16 | ||||||
| SSZ | Fraction | 0 | 0.24 | 0 | 0.25 | 0 | 0.20 | 0 | 0.16 | ||||||
| DPC | Fraction | 0 | 0.03 | 0 | 0.03 | 0 | 0.04 | 0 | 0.04 | ||||||
| GST | Fraction | 0 | 0.08 | 0 | 0.07 | 0 | 0.08 | 0 | 0.02 | ||||||
The results of the analyses using data for phase trios are summarized in Fig. 2. In each of the analyses with primary endpoint and secondary endpoint 1, we obtained a positive estimated value for das0–das2, and the test was significant.
Fig. 2.
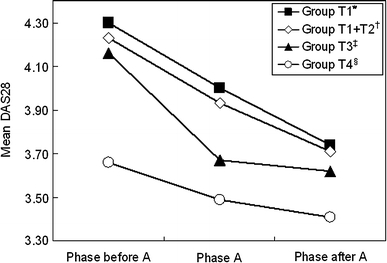
Results of longitudinal analyses for efficacy of MTX at dosages higher than 8 mg/week using data from three consecutive phases; changes in DAS28 values were examined in the 4 different groups in Table 1A. Asterisk Primary endpoint: Number of phase trios is 260. The difference of means is 0.563 [95% confidence interval (0.438, 0.688), t test statistic 8.88, P value <2.2 × 10−22]. Dagger Secondary endpoint 1: Number of phase trios is 330. The difference of means is 0.519 [95% confidence interval (0.408, 0.629), t test statistic 9.20, P value <2.2 × 10−22]. Double dagger Secondary endpoint 2: Number of phase trios is 121 versus 55. The difference of means is 0.287 [95% confidence interval (−0.05, 0.38), t test statistic 1.67, P value 0.096]. Section symbol Exploratory research: Number of phase trios is 55. The difference of means is 0.238 [95% confidence interval (−0.010, 0.487), t test statistic 1.92, P value 0.06]
In contrast, for the phase trios in which dosages of MTX were maintained at 8 mg/week (group T4), there were no significant differences between the DAS28 values between one phase before A and one phase after A (P = 0.060, Fig. 2).
For differences between the DAS28 value for one phase before A and one phase after A (das0–das2), we tested whether the mean value for group T3 was different from the mean value for group T4 (secondary endpoint 2). The mean value of das0–das2 for group T3 tended to be larger than for group T4, but the difference was not significant (P = 0.096) (Fig. 2).
The above-mentioned results indicate that RA activity is decreased by increasing the dosage of MTX from 8 mg/week or less to over 8 mg/week. In contrast, RA activity did not significantly decrease when the dosage of 8 mg/week was maintained.
Analysis of efficacy of increasing the dosage of MTX from 8 mg/week or less to over 8 mg/week using data from two consecutive phases (“phase pairs”)
The DAS28 values in phase A were lower than those one phase before A for any of groups P1, P2, and P3 (Fig. 3, see Table 1B for group definition). Thus, for group P1 (n = 690), the DAS values (das0) one phase before A were 4.21 ± 1.17 and those (das1) in phase A were 3.95 ± 1.20 (P = 6.78 × 10−11), 3.62 ± 1.17 and 3.55 ± 1.18 (P = 0.000307) for group P2 (n = 2125), and 3.65 ± 1.14 and 3.54 ± 1.16 (P = 2.19 × 10−10) for group P3 (n = 2545), respectively.
Fig. 3.
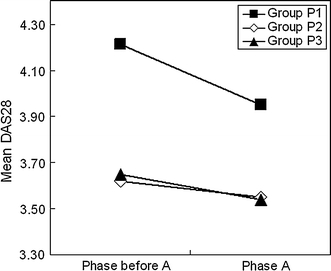
Results of longitudinal analysis for efficacy of MTX at dosages higher than 8 mg/week using data from two consecutive phases; changes in DAS28 values were examined in the three different groups in Table 1B. Test of the difference between DAS28 decrease in group P1 and that in group P2: P = 2.27 × 10−6
The decrease in DAS28 for group P1 from one phase before A to phase A was more remarkable than in group P2. Thus, the values of das0–das1 for group P1 and group P2 were 0.26 ± 1.04 and 0.07 ± 0.89, respectively, and the difference was significant (Fig. 3) (P = 2.27 × 10−6).
The above results indicate that increase in the dosage of MTX from 8 mg/week or less to over 8 mg/week suppresses RA activity more effectively than keeping the dosage at 8 mg/week or lower.
Analysis of safety of MTX administered at dosages over 8 mg/week using data from two consecutive phases (“phase pairs”)
Of the extracted pairs, in 54–58% the MTX dosage in the latter was equal to the dosage in the former (Table 2); in the other pairs, the dosage was changed, administration was stopped or data were missing in the latter phase. The fraction of the phase pairs without dosage data in the latter phase was less than 10% (Table 2). Among the phase pairs whose dosages of MTX in the former phase were over 8 mg/week, the fractions of discontinuation and missing data in the latter phase were 3.5% and 8.0%, respectively (Table 2). These fractions were not larger than those in the other phase pairs with lower dosages of MTX. Thus, among the phase pairs with other dosages of MTX, the fractions of discontinuation and missing data in the latter phase were 3.1–7.0% and 8.4–9.7%, respectively (Table 2).
Results of the analyses for the primary endpoint and the secondary endpoints 1–3 indicated that no statistical significant associations were present between the dosage of MTX (over 8 mg/week or not) and the presence or the absence of self-reported side-effects (Table 7).
Table 7.
Results of longitudinal safety analysis using phase pairs
| Fraction (%) of patients who reported adverse events in the group with dosage of 8 mg/week or lower | Fraction (%) of patients who reported adverse events in the group with dosage over 8 mg/week | Regression coefficientb | P valuec | |
|---|---|---|---|---|
| Primary endpointa | 4.88 | 5.16 | −0.0351 | 0.63 |
| Secondary endpoint 1 | 0.92 | 1.10 | 0.119 | 0.45 |
| Secondary endpoint 2 | 3.66 | 4.02 | -0.0096 | 0.91 |
| Secondary endpoint 3 | 14.60 | 16.90 | 0.0384 | 0.38 |
aSee text for the definitions of the endpoints
bMaximum-likelihood estimate of the slope in the logistic regression analysis
cP value of the test for the null hypothesis that the slope is zero
Analysis of efficacy of MTX at dosages over 8–16 mg/week by longitudinal study
For groups E1, E2, E3, E4, and E5, the numbers of phase pairs extracted were 62, 140, 267, 499, and 2,125, respectively (Fig. 4). Concerning the primary endpoint, das0 values were 4.26 ± 1.17 while das1 values were 3.86 ± 1.20 for group E1. The mean value of das1 was significantly lower than that of das0 (difference between means of 0.399, P = 0.00063) (Fig. 4). Moreover, concerning the secondary endpoints 1–3, the average value of DAS28 in phase A was lower than that one phase before A in each of group E2, E3, and E4 (differences of 0.268–0.449, P = 2.08 × 10−8, P = 3.27 × 10−12, P = 1.41 × 10−9, respectively, Fig. 4).
Fig. 4.
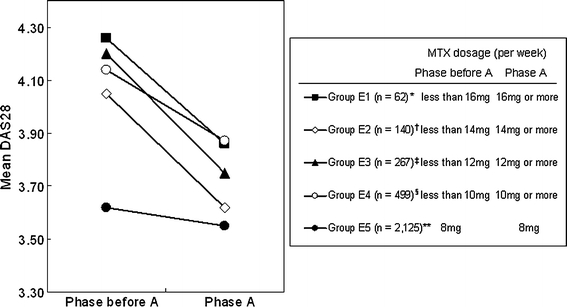
Results of longitudinal analysis of efficacy of increasing MTX dosage from lower than 10–16 to 10–16 mg/week or higher; changes in DAS28 values were examined in 5 different groups. Asterisk difference of means is 0.399 [95% confidence interval (0.178, 0.621), P value <0.00063]. Difference from group E5 is 0.329 (P value 0.0041). Dagger difference of means is 0.430 [95% confidence interval (0.287, 0.572), P value <2.08 × 10−8]. Difference from group E5 is 0.360 (P value 0.0000036). Double dagger difference of means is 0.449 [95% confidence interval (0.328, 0.569), P value <3.27 × 10−12]. Difference from group E5 is 0.379 (P value 1.37 × 10−10). Section symbol difference of means is 0.268 [95% confidence interval (0.183, 0.353), P value <1.41 × 10−9]. Difference from group E5 is 0.198 (P value 0.000012). Double asterisk difference of means is 0.070 [95% confidence interval (0.032, 0.108), P value <0.00031]
In addition, concerning the exploratory research, the average value of DAS28 in phase A was also significantly lower (difference of 0.07) than one phase before A even in group E5. Though the difference of the average DAS28 was the least in group E5 among all the groups, the result of the test was significant, probably because of the large sample size (P = 0.00031, Fig. 4). Next, concerning the secondary endpoints 4–7, we performed unpaired t tests to examine whether the mean of das0–das1 for group E5 was different from each of the means for the other groups.
We found that the mean decrease (das0–das1) in DAS28 for each of group E1, E2, E3, and E4 was significantly larger than that for group E5 (Fig. 4). For instance, in the comparison between group E1 and group E5, the difference of the mean value of das0–das1 was 0.329 [P = 0.0041, unpaired t test (two-sided)].
These data indicate that increases in the dosage of MTX from 10 mg/week or less to over 10 mg/week, from 12 mg/week or less to over 12 mg/week, from 14 mg/week or less to over 14 mg/week, and from 16 mg/week or less to over 16 mg/week led to significant reductions in DAS28. In addition, the size of the average decrease in DAS28 for each of those groups was significantly larger than that in the group in which the dosage of MTX was maintained at 8 mg/week.
Analysis of safety of MTX at dosages over 8–16 mg/week by longitudinal study
The fractions of patients with severe side-effects based on self-report were 3.09–4.43%, and the fractions of patients with severe or moderate side-effects based on self-reports were 13.06–14.81% (Table 3). Unexpectedly, the fraction of patients with side-effects was lower among patients who received MTX at 16 mg/week or higher than in those whose dosage of MTX was kept at 8 mg/week. These results probably reflect the tendency for physicians to administer lower dosages of MTX to higher-risk patients, while they administer higher dosages to lower-risk patients. Moreover, it is easily imaginable that physicians tend to maintain MTX dosages in higher-risk patients and tend to increase the dosages of MTX in lower-risk patients.
Based on the above notion, we performed logistic regression analysis in which adjustment was made for age, BMI, steroid administration, folic acid administration, history of pulmonary diseases, history of renal dysfunction, and DAS28 value one phase before A, which may be recognized as risks by physicians. In the logistic regression, the dependent variable was the presence or absence of severe side-effects or severe to moderate side-effects, and explanatory variables were group S1 versus group S5, group S2 versus group S5, group S3 versus group S5, and group S4 versus group S5 (as levels 0 and 1), in addition to the above-mentioned eight variables.
Table 8 presents the results of the logistic regression analysis. In the case where the dependent variable of the model was presence or absence of severe side-effects, three variables, i.e. steroid administration (P = 0.000041), history of pulmonary diseases (P = 2.16 × 10−7), and DAS28 in phase A (P = 0.000024), were significantly associated. In the case where the dependent variable was severe to moderate side-effects, BMI (P = 0.002) and folic acid administration (P = 2.42 × 10−9) were positively associated with the presence of side-effects, in addition to steroid administration (P = 3.39 × 10−15), history of pulmonary diseases (P = 9.55 × 10−6), and DAS28 in phase A (P = 2.92 × 10−9). Though it is reasonable from the medical point of view that steroid administration, leanness, history of pulmonary diseases, and higher disease activity were positively associated with side-effects, it is hard to understand why folic acid administration tends to cause side-effects. The positive association between folic acid administration and side-effects probably reflects the tendency for this drug to be administered when side-effects take place.
Table 8.
Logistic regression analysis for study of association between adverse events and dosage of MTX over 8 mg/week
| Variable | Estimated value of slope | Odds ratio | Lower bound (95% CI) | Upper bound (95% CI) | Significance (P value) |
|---|---|---|---|---|---|
| (A) Severe side-effect | |||||
| Cutoff | −4.2640 | 0.014 | 0.005 | 0.040 | 9.02E−16 |
| Group S1 versus group S5 | −0.0122 | 0.988 | 0.428 | 2.280 | 0.977 |
| Group S2 versus group S5 | 0.2976 | 1.347 | 0.882 | 2.057 | 0.169 |
| Group S3 versus group S5 | −0.0421 | 0.959 | 0.679 | 1.355 | 0.811 |
| Group S4 versus group S5 | −0.0179 | 0.982 | 0.761 | 1.268 | 0.891 |
| Sex | −0.1624 | 0.850 | 0.631 | 1.146 | 0.287 |
| Age | −0.0031 | 0.997 | 0.988 | 1.006 | 0.499 |
| BMI | 0.0029 | 1.003 | 0.967 | 1.040 | 0.874 |
| Steroid | 0.5566 | 1.745 | 1.337 | 2.277 | 0.0000412 |
| Folic acid | 0.1394 | 1.150 | 0.921 | 1.435 | 0.218 |
| Pulmonary diseases | 1.1545 | 3.172 | 2.051 | 4.908 | 2.16E−07 |
| Renal dysfunction | −0.5538 | 0.575 | 0.078 | 4.253 | 0.588 |
| DAS28 at phase A | 0.1901 | 1.209 | 1.107 | 1.321 | 0.000024 |
| (B) Severe + moderate side-effect | |||||
| Cutoff | −2.0443 | 0.129 | 0.074 | 0.226 | 5.51E−13 |
| Group S1 versus group S5 | 0.0279 | 1.028 | 0.662 | 1.597 | 0.901 |
| Group S2 versus group S5 | 0.1100 | 1.116 | 0.875 | 1.424 | 0.376 |
| Group S3 versus group S5 | 0.0685 | 1.071 | 0.897 | 1.278 | 0.448 |
| Group S4 versus group S5 | 0.0733 | 1.076 | 0.942 | 1.230 | 0.282 |
| Sex | 0.0312 | 1.032 | 0.872 | 1.220 | 0.716 |
| Age | −0.0035 | 0.996 | 0.992 | 1.001 | 0.152 |
| BMI | −0.0311 | 0.969 | 0.950 | 0.989 | 0.002 |
| Steroid | 0.5381 | 1.713 | 1.498 | 1.958 | 3.39E−15 |
| Folic acid | 0.3595 | 1.433 | 1.273 | 1.612 | 2.42E−09 |
| Pulmonary diseases | 0.6902 | 1.994 | 1.469 | 2.707 | 9.55E−06 |
| Renal dysfunction | 0.3250 | 1.384 | 0.625 | 3.066 | 0.423 |
| DAS28 at phase A | 0.1438 | 1.155 | 1.101 | 1.211 | 2.92E−09 |
Importantly, however, there was no association between the presence of severe side-effects or the presence of severe to moderate side-effects and the difference between group S5 and any of groups S1–S4. To summarize, we found no evidence that the safety of MTX administered at dosages of 10, 12, 14 or 16 mg/week or more was lower than the safety of MTX administered at the dosage of 8 mg/week.
Discussion
In the present study, we examined whether dosages of MTX over 8 mg/week are beneficial from the viewpoints of both efficacy and safety using cohort data from the IORRA database.
There are benefits and drawbacks in examining efficacy and safety by analysis of such cohort data rather than clinical trial data. The drawbacks include the following problems: (a) both physicians and patients were aware of the contents of the treatments, (b) this is not a controlled trial in which placebo was used, and (c) no randomization was done. On the other hand, it has benefits that a randomized controlled trial does not possess. Firstly, it can provide data from a larger number of patients for longer time periods. In cohort-based studies, data such as rare side-effects or long-term effects may be obtained. For example, rare side-effects from MTX include pneumonitis and myelosuppression, both of which are hard to analyze in a randomized controlled trial with only a small sample size for a short period of time. In the present study in which the sample size is larger and the observation periods are longer, rare but severe side-effects including such events may be analyzed. Secondly, data from a cohort study reflect routine medical practice rather than an artificial situation. In a randomized controlled trial, the sample often reflects only a small proportion of the entire patients because of strict enrolment criteria applied to make the targets as uniform as possible and the results of the test as clear as possible. Such drawbacks of randomized controlled trials may be weaker in cohort studies. Thirdly, the ethical problem is smaller in cohort studies than in randomized controlled trials. In the latter case, use of placebo and randomization may force patients to undergo inappropriate treatments.
When simple associations were tested between dosages of MTX and disease activity, higher dosages were associated with higher disease activity. This is as expected but would be a strong confounder in the present study. Similarly, higher dosage was associated with lower frequency of side-effects. This is also as expected but would also be a strong confounder. Those associations, of course, reflect the attitudes of physicians to adjust dosages of MTX according to disease activity, concomitant medical conditions, and side-effects. We attempted to overcome such drawbacks of nonrandomized studies by using longitudinal analyses and multifactorial logistic regression analyses.
Our longitudinal analyses based on phase trios and phase pairs indicated that increase in the dosage of MTX from 8 mg/week or lower to over 8 mg/week decreased RA activity compared with cases where MTX dosages were maintained. Concerning safety, we found no significant increases in the frequencies of severe or severe + moderate side-effects by the increase in the dosage of MTX from 8 mg/week or lower to over 8 mg/week.
The results of the present study indicate that 27.5% of patients treated with MTX were administered dosages over 8 mg/week in the Institute of Rheumatology, Tokyo Women’s Medical University. Although many Japanese rheumatologists are using MTX at dosages over 8 mg/week, this is not officially approved. Therefore, in clinical trials whose data are to be included in documents for new drug approval by the Japanese government, the 8 mg/week restriction is applied. Thus, even in recent clinical studies in which the effects of biological agents were tested with or without MTX, dosages of MTX not more than 8 mg/week were used [36, 37].
Hashiramoto et al. [38] recently reported the results from a prospective study in which 8 mg/week MTX was used. They concluded that such a low dosage of MTX appeared to be favorable; however, it is clearly insufficient and cannot halt progression of rheumatic joint destruction.
MTX has been approved for use in patients with juvenile idiopathic arthritis (JIA) by the Japanese government. This approval was granted without data from clinical trials sponsored by the pharmaceutical company; rather, it was achieved by collecting necessary information through ongoing efforts (including collection and analysis of information about approval status in other countries, adequate evidence from the literature, implementation of a clinical use survey in Japan, etc.) [16, 19–35]. Curiously, the maximum dosage (10 mg/m2/week) was set on the basis of pharmacokinetic data from children, rather than relying on the dosing method and dose for adults. Since the average body surface area of the Japanese men is around 1.68 m2, some children with JIA can take much higher doses of MTX than formally approved for adult RA patients.
Since insufficient evidence has been provided for the efficacy and safety of MTX dosages over 8 mg/week in Japanese RA patients, the results of the present study will serve as the basis for Japanese rheumatologists to use MTX at dosages over 8 mg/week.
Acknowledgment
We thank the Japanese College of Rheumatology (JCR) for continuous support of this study.
Conflict of interest The IORRA study is supported by a research grant from 37 pharmaceutical companies: Abbott Japan Co., Ltd., Asahikasei Kuraray Medical Co., Ltd., Asahikasei Pharma Corporation, Astellas Pharma Inc., AstraZeneca K.K., Banyu Pharmaceutical Co., Ltd., Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Daiichi Fine Chemical Co., Ltd., Daiichi Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Eisai Co., Ltd., GlaxoSmithKline K.K., Janssen Pharmaceutical K.K. Japan Tobacco Inc., Kaken Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Mitsubishi Chemical Medience Corporation, Mitsubishi Tanabe Pharma Corporation, Mochida Pharmaceutical Co., Ltd., Mundiphama K.K., Nippon Chemiphar Co., Ltd., Nippon Shinyaku Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc. Sanofi-Aventis K.K, Santen Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Sekisui Medical Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Company Limited, Teijin Pharma Limited, Torii Pharmaceutical Co., Ltd., UCB Japan Co., Ltd., and Zeria Pharmaceutical Co., Ltd. S.M. has received lecture fees and/or honoraria from Abbott Japan, Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd., Eisai Co. Ltd., Mitsubishi Tanabe Pharma Corporation, Santen Pharmaceutical Co. Ltd., and Takeda Pharmaceutical Company Limited. H.Y. has received lecture/consulting fees from Abbott Japan, Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Hoffmann-La Roche, Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Pfizer Japan Inc., and Takeda Pharmaceutical Company Limited. The other authors have declared no conflicts of interest.
References
- 1.Weinblatt ME, Polisson R, Blotner SD, Sosman JL, Aliabadi P, Baker N, et al. The effects of drug therapy on radiographic progression of rheumatoid arthritis: results of a 36-week randomized trial comparing methotrexate and auranofin. Arthritis Rheum. 1993;36:613–619. doi: 10.1002/art.1780360507. [DOI] [PubMed] [Google Scholar]
- 2.Cannella AC, O’Dell JR. Is there still a role for traditional disease-modifying antirheumatic drugs (DMARDs) in rheumatoid arthritis? Curr Opin Rheumatol. 2003;15:185–192. doi: 10.1097/00002281-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Yazici Y, Sokka T, Kautiainen H, Swearingen C, Kulman I, Pincus T. Long term safety of methotrexate in routine clinical care: discontinuation is unusual and rarely the result of laboratory abnormalities. Ann Rheum Dis. 2005;64:207–211. doi: 10.1136/ard.2004.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 5.Mease PJ, Revicki DA, Szechinski J, Greenwald M, Kivitz A, Barile-Fabris L, et al. Improved health-related quality of life for patients with active rheumatoid arthritis receiving rituximab: results of the dose-ranging assessment: international clinical evaluation of rituximab in rheumatoid arthritis (DANCER) trial. J Rheumatol. 2008;35:20–30. [PubMed] [Google Scholar]
- 6.Bijl AE, Goekoop-Ruiterman YP, Vries-Bouwstra JK, Ten Wolde S, Han KH, Krugten MV, et al. Infliximab and methotrexate as induction therapy in patients with early rheumatoid arthritis. Arthritis Rheum. 2007;56:2129–2134. doi: 10.1002/art.22718. [DOI] [PubMed] [Google Scholar]
- 7.Wessels JA, Kooij SM, le Cessie S, Kievit W, Barerra P, Allaart CF, et al. A clinical pharmacogenetic model to predict the efficacy of methotrexate monotherapy in recent-onset rheumatoid arthritis. Arthritis Rheum. 2007;56:1765–1775. doi: 10.1002/art.22640. [DOI] [PubMed] [Google Scholar]
- 8.Kooij SM, Vries-Bouwstra JK, Goekoop-Ruiterman YP, Zeben D, Kerstens PJ, Gerards AH, et al. Limited efficacy of conventional DMARDs after initial methotrexate failure in patients with recent onset rheumatoid arthritis treated according to the disease activity score. Ann Rheum Dis. 2007;66:1356–1362. doi: 10.1136/ard.2006.066662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heijde D, Klareskog L, Rodriguez-Valverde V, Codreanu C, Bolosiu H, Melo-Gomes J, et al. Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from theTEMPO study, a double-blind, randomized trial. Arthritis Rheum. 2006;54:1063–1074. doi: 10.1002/art.21655. [DOI] [PubMed] [Google Scholar]
- 10.Goekoop-Ruiterman YP, Vries-Bouwstra JK, Allaart CF, Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381–3390. doi: 10.1002/art.21405. [DOI] [PubMed] [Google Scholar]
- 11.American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46:328–46. [DOI] [PubMed]
- 12.Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 13.Kashiwazaki S, Ichikawa Y, Sugawara S, Nagaya I, Kawai S, Hakota M, et al. Determination of the clinical optimal dose of L-377 (methotrexate capsule) for the treatment of rheumatoid arthritis. Drug Evaluation. 1996;16:437–458. [Google Scholar]
- 14.Akahoshi T, Ueda H, Kashiwazaki S. Immunosuppressive drugs in the treatment of rheumatoid Arthritis (in Japanese) Saishin Igaku. 1989;44:1918–1925. [Google Scholar]
- 15.Ranganathan P, McLeod HL. Methotrexate pharmacogenetics: the first step toward individualized therapy in rheumatoid arthritis. Arthritis Rheum. 2006;54:1366–1377. doi: 10.1002/art.21762. [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka H, Inoue E, Tanaka E, Nakajima A, Taniguchi A, Terai C, et al. Influence of methotrexate dose on its efficacy and safety in rheumatoid arthritis patients: evidence based on the variety of prescribing approaches among practicing Japanese rheumatologists in a single institute-based large observational cohort (IORRA) Mod Rheumatol. 2007;17:98–105. doi: 10.1007/s10165-006-0546-7. [DOI] [PubMed] [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda Y, Singh G, Yamanaka H, Tanaka E, Urano W, Taniguchi A, et al. Validation of a Japanese version of the Stanford Health Assessment Questionnaire in 3, 763 patients with rheumatoid arthritis. Arthritis Rheum. 2003;49:784–788. doi: 10.1002/art.11465. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima A, Inoue E, Tanaka E, Singh G, Sato E, Hoshi D, et al. Mortality and cause of death in Japanese patients with rheumatoid arthritis based on a large observational cohort, IORRA. Scand J Rheumatol. 2010 (Epub ahead of print PMID: 20476859). [DOI] [PubMed]
- 20.Yamada T, Nakajima A, Inoue E, Tanaka E, Taniguchi A, Momohara S, et al. Incidence of malignancy in Japanese patients with rheumatoid arthritis. Rheumatol Int. 2010 May 16 (Epub ahead of print PMID: 20473757). [DOI] [PubMed]
- 21.Shidara K, Hoshi D, Inoue E, Yamada T, Nakajima A, Taniguchi A, et al. Incidence of and risk factors for interstitial pneumonia in patients with rheumatoid arthritis in a large Japanese observational cohort, IORRA. Mod Rheumatol. 2010;20:280–286. doi: 10.1007/s10165-010-0280-z. [DOI] [PubMed] [Google Scholar]
- 22.Momohara S, Inoue E, Ikari K, Kawamura K, Tsukahara S, Iwamoto T, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA) Ann Rheum Dis. 2010;69:312–313. doi: 10.1136/ard.2009.107599. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka E, Inoue E, Mannalithara A, Bennett M, Kamitsuji S, Taniguchi A, et al. Medical care costs of patient with rheumatoid arthritis during the prebiologics period in Japan: a large prospective observational cohort study. Mod Rheumatol. 2010;20:46–53. doi: 10.1007/s10165-009-0236-3. [DOI] [PubMed] [Google Scholar]
- 24.Iikuni N, Sato E, Hoshi M, Inoue E, Taniguchi A, Hara M, et al. The influence of sex on patients with rheumatoid arthritis in a large observational cohort. J Rheumatol. 2009;36:508–511. doi: 10.3899/jrheum.080724. [DOI] [PubMed] [Google Scholar]
- 25.Momohara S, Ikari K, Mochizuki T, Kawamura K, Tsukahara S, Toki H, et al. Declining use of synovectomy surgery for patients with rheumatoid arthritis in Japan. Ann Rheum Dis. 2009;68:291–292. doi: 10.1136/ard.2008.087940. [DOI] [PubMed] [Google Scholar]
- 26.Furuya T, Kotake S, Inoue E, Nanke Y, Yago T, Hara M, et al. Risk factors associated with incident fractures in Japanese men with rheumatoid arthritis: a prospective observational cohort study. J Bone Miner Metab. 2008;26:499–505. doi: 10.1007/s00774-007-0836-y. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka E, Mannalithara A, Inoue E, Hara M, Tomatsu T, Kamatani N, et al. Efficient management of rheumatoid arthritis significantly reduces long-term functional disability. Ann Rheum Dis. 2008;67:1153–1158. doi: 10.1136/ard.2007.072751. [DOI] [PubMed] [Google Scholar]
- 28.Shinozaki M, Inoue E, Nakajima A, Hara M, Tomatsu T, Kamatani N, et al. Elevation of serum matrix metalloproteinase-3 as a predictive marker for the long-term disability of rheumatoid arthritis patients in a prospective observational cohort IORRA. Mod Rheumatol. 2007;17:403–408. doi: 10.1007/s10165-007-0608-5. [DOI] [PubMed] [Google Scholar]
- 29.Yamanaka H, Inoue E, Singh G, Tanaka E, Nakajima A, Taniguchi A, et al. Improvement of disease activity of rheumatoid arthritis patients from 2000 to 2006 in a large observational cohort study IORRA in Japan. Mod Rheumatol. 2007;17:283–289. doi: 10.1007/s10165-007-0587-6. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto H, Koizumi K, Kamitsuji S, Inoue E, Hara M, Tomatsu T, et al. Beneficial action of statins in patients with rheumatoid arthritis in a large observational cohort. J Rheumatol. 2007;34:964–968. [PubMed] [Google Scholar]
- 31.Iikuni N, Nakajima A, Inoue E, Tanaka E, Okamoto H, Hara M, et al. What’s in season for rheumatoid arthritis patients? Seasonal fluctuations in disease activity. Rheumatology (Oxford) 2007;46:846–848. doi: 10.1093/rheumatology/kel414. [DOI] [PubMed] [Google Scholar]
- 32.Iwatani M, Inoue E, Nakamura T, Nakajima A, Hara M, Tomatsu T, et al. Efficacy profile of bucillamine in rheumatoid arthritis patients in a large observational cohort study, IORRA. Mod Rheumatol. 2006;16:376–380. doi: 10.1007/s10165-006-0527-x. [DOI] [PubMed] [Google Scholar]
- 33.Furuya T, Kotake S, Inoue E, Nanke Y, Yago T, Kobashigawa T, et al. Risk factors associated with incident clinical vertebral and nonvertebral fractures in Japanese women with rheumatoid arthritis: a prospective 54-month observational study. J Rheumatol. 2007;34:303–310. [PubMed] [Google Scholar]
- 34.Tanaka E, Inoue E, Kawaguchi Y, Tomatsu T, Yamanaka H, Hara M, et al. Acceptability and usefulness of mizoribine in the management of rheumatoid arthritis in methotrexate-refractory patients and elderly patients, based on analysis of data from a large-scale observational cohort study. Mod Rheumatol. 2006;16:214–219. doi: 10.1007/s10165-006-0487-1. [DOI] [PubMed] [Google Scholar]
- 35.Nakajima A, Kamitsuji S, Saito A, Tanaka E, Nishimura K, Horikawa N, et al. Disability and patient’s appraisal of general health contribute to depressed mood in rheumatoid arthritis in a large clinical study in Japan. Mod Rheumatol. 2006;16:151–157. doi: 10.1007/s10165-006-0475-5. [DOI] [PubMed] [Google Scholar]
- 36.Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19:12–19. doi: 10.1007/s10165-008-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kameda H, Ueki Y, Saito K, Nagaoka S, Hidaka T, Atsumi T, et al. Etanercept (ETN) with methotrexate (MTX) is better than ETN monotherapy in patients with active rheumatoid arthritis despite MTX therapy: a randomized trial. Mod Rheumatol. 2010 (Epub ahead of print PMID: 20574649). [DOI] [PubMed]
- 38.Hashiramoto A, Shiozawa K, Tanaka Y, Yamane T, Murata M, Tanaka C, et al. Prospective study of methotrexate treatment for rheumatoid arthritis treated legitimately according to the government recommended 8 mg/week dose. Mod Rheumatol. 2009;19:637–642. doi: 10.1007/s10165-009-0205-x. [DOI] [PubMed] [Google Scholar]


