Abstract
Aims
An association has been described between death from arrhythmia and early repolarization, an electrocardiogram pattern characterized by elevation of the QRS–ST junction (J-point). Little is known about this relationship in non-white populations. This study examines the relationship between J-point elevation (JPE) and sudden cardiac death (SCD) and whether this relationship differs by race or sex.
Methods and results
A total of 15 141 middle-aged subjects from the prospective, population-based Atherosclerosis Risk in Communities (ARIC) study were included in this analysis. The primary endpoint was physician-adjudicated SCD occurring from baseline (1987–1989) through December 2002, secondary endpoints were fatal and non-fatal coronary events and all-cause mortality occurring through December 2007. J-point elevation was defined as J-point amplitude ≥0.1 mV. Pre-specified subgroup analyses by sex and race were conducted. J-point elevation in any lead was present in 1866 subjects (12.3%). After adjustment for demographic, clinical, lifestyle, and laboratory variables, JPE was not significantly related to SCD in the overall sample [adjusted hazard ratio (HR), 1.23; 95% confidence interval (CI), 0.87–1.75]. However, significant interactions were present between race and JPE (P = 0.006) and between sex and JPE (P = 0.020). J-point elevation was significantly predictive of SCD in whites (adjusted HR, 2.03; 95% CI, 1.28–3.21) and in females (adjusted HR, 2.54; 95% CI, 1.34–4.82).
Conclusion
Our results suggest that JPE is associated with an increased risk of SCD in whites and in females, but not in blacks or males. Further studies are needed to clarify which subgroups of individuals with JPE are at increased risk for adverse cardiac events.
Keywords: Electrocardiography, Sudden cardiac death, J-point elevation, Epidemiology
Introduction
Sudden cardiac death (SCD) accounts for more than half of all deaths from cardiovascular disease.1–3 Coronary heart disease (CHD) underlies ∼80% of SCD cases, and SCD is the first manifestation of heart disease in 50% of these individuals.2,4 In most cases, SCD is thought to be caused by cardiac arrest from ventricular arrhythmia. Identification of individuals at increased risk of SCD may allow targeted prevention strategies.
Although traditionally viewed as benign, several case–control studies have suggested that the electrocardiogram (ECG) finding of early repolarization may be associated with the development of ventricular arrhythmia.5–7 Characterized by elevation of the QRS–ST junction (the J-point) above baseline on ECG, early repolarization is seen in 1–13% of people.8–12 Recently, two population-based studies showed that early repolarization was associated with an increased risk of death from cardiac causes.10,11 These are the only population-based studies on the topic of which we are aware, but they are limited by samples comprised predominantly of individuals of European descent. Importantly, blacks may have a higher prevalence of the early repolarization pattern and may be more likely to experience SCD.1,5,8,13,14
The early repolarization pattern has many morphological variants.15 A considerable degree of subjectivity is involved in the diagnosis of early repolarization, and no consensus criteria currently exist for its diagnosis. J-point elevation (JPE) is the central feature of the early repolarization pattern. Like early repolarization, it has been found in some studies to be more common in blacks.16,17 Examination of JPE specifically is advantageous in that it can be determined from computerized ECG coding programs and can thus provide a quantitative, objective measurement of ST-segment deviation, making it useful in large epidemiological studies.
The goals of this study were therefore to estimate the prevalence of JPE in a large middle-aged biracial cohort of US adults and to examine the association of JPE with risk of cardiac events and whether the prognostic significance of the finding varies by race or sex. Specifically, we investigated the association of JPE with SCD, fatal/non-fatal CHD events, and all-cause mortality.
Methods
Study population
The Atherosclerosis Risk in Communities (ARIC) study18 is a prospective, population-based cohort study designed to investigate the aetiology and natural history of cardiovascular disease. From 1987 to 1989, ARIC investigators used probability sampling to enroll 15 792 men and women aged 45–64 residing in four US communities: Jackson, MS; Washington County, MD; Forsyth County, NC; and the northwestern suburbs of Minneapolis, MN.
For the present analysis, we excluded 202 subjects for whom J-point amplitude data were missing or incomplete. We also excluded 604 subjects with a QRS complex duration of ≥120 ms in order to remove cases of bundle branch block, the Wolf–Parkinson–White syndrome, and idioventricular rhythm. Additionally, 48 subjects were excluded for race other than black or white. These exclusion counts are not mutually exclusive. After the above exclusions, 15 141 subjects remained for analysis.
Baseline measurements
At the baseline examination, a standard, resting, supine 12-lead ECG was obtained for each subject a minimum of 1 h after any smoking or caffeine ingestion. An electrode locator was used to determine and standardize the positioning of chest electrodes. Tracings were sent via a phone modem to be computer coded at the ARIC ECG Reading Center. All records with significant Minnesota Code19 findings as determined by the computer, as well as a random sample of tracings, were sent to the ECG coding centre to be visually coded. Discrepancies between the computer code and visual code were adjudicated by a senior coder. Later processing of the ECGs took place at EPICARE (Epidemiological Cardiology Research Center at Wake Forest University, Winston-Salem, NC, USA), where the 2001 version of the GE Marquette 12-SL program was used to automatically obtain ST amplitude at the J-point in relation to the isoelectric line.
J-point elevation was defined as a J-point amplitude of ≥0.1 mV (1 mm) in any lead. Left ventricular hypertrophy (LVH) was assessed by Cornell's voltage criteria.20
Body mass index (BMI) in kg/m2 was calculated based on measured height and weight. Fasting blood samples were analysed for lipid levels and chemistry. Diabetes was defined as a fasting glucose level of ≥126 mg/dL, a non-fasting level of ≥200 mg/dL, self-reported physician diagnosis of diabetes, or pharmacological treatment for diabetes. Information regarding race, smoking history, physical activity, family health history, and educational attainment was obtained through interviews. Physical activity was based on the reported level of sport activity using the Baecke physical activity questionnaire.21
Follow-up
The primary outcome was physician-adjudicated SCD. All cases previously categorized as fatal CHD that occurred in ARIC before 31 December 2002 were reviewed by a committee of physicians. Case information was sent separately to pairs of physician adjudicators using a standard coding form. When there was disagreement in the classification, the case was re-coded by paired investigators. If further disagreement occurred, it was resolved by discussion between these coders. After review of data from death certificates, informant interviews, physician questionnaires, coroner reports, and hospital discharge summaries, reviewers classified each CHD death as definite SCD, possible SCD, or non-sudden CHD death. Sudden cardiac death was defined as a sudden pulseless condition of cardiac origin in a previously stable individual. Because the ARIC study enrolled individuals aged 45–64 years at baseline, the SCD seen in this study will be primarily the atherosclerotic type, which accounts for the majority of the SCD burden.
In general, the adjudication committee made use of death within <1 h and within <24 h criteria for witnessed and unwitnessed events, respectively, but additionally used data on the circumstances of death and body position in classification. Only cases of definite SCD were included in the present analysis.
Secondary outcomes included fatal/non-fatal CHD events and all-cause mortality. A fatal/non-fatal CHD event was defined as a definite or probable myocardial infarction (MI) or definite CHD death.18 Events occurring between the baseline examination and 31 December 2007 were included in analysis of all outcomes with the exception of SCD, for which the end of follow-up was 31 December 2002.
Statistical analysis
We used Cox's proportional hazards models to obtain multivariate-adjusted hazard ratios (HRs) for all study outcomes for subjects with vs. those without JPE. We used an overall measure of JPE in any ECG lead and also grouped the leads into anterior (V1–V5), inferior (II, III, aVF), and lateral (I, aVL, V6) leads. Results are reported as HRs with 95% confidence intervals (CIs). Censoring occurred at the time of an event, death, loss to follow-up, or at the end of follow-up.
Potential covariates included in the initial model were: age, sex, race, BMI, heart rate, systolic blood pressure, smoking status, high-density lipoprotein level, low-density lipoprotein level, diabetes, level of physical activity, field centre, family history of premature CHD, LVH, serum electrolyte levels, and presence of major ECG abnormality on Minnesota Code.19 We also included data on prevalent CHD (including MI determined by self-report or ECG, self-report of heart or arterial surgery, coronary bypass, balloon angioplasty, coronary artery angioplasty) and on physician-diagnosed stroke, angina, and intermittent claudication as ascertained by the Rose questionnaire.22 The following cardiac medications were also initially included as covariates: digitalis, selective and non-selective β-blockers, calcium channel blockers, angiotensin-converting enzyme (ACE)-inhibitors, and antiarrhythmic drugs.
Variables found not to be related to JPE in bivariate analysis were dropped from the full model in a stepwise fashion, and the resulting reduced models were tested against the full model using the likelihood ratio test. Tested variables that did not significantly affect results were dropped from the model. Using this method, we created a set of reduced models fit to JPE in any lead as the main exposure and SCD as the outcome. This same set of covariates was then employed across all exposures and all outcomes in order to enhance comparability and reproducibility. Model 1 adjusts for demographic factors. Model 2 adjusts for demographic and clinical variables. Model 3 adjusts for demographic, clinical, lifestyle, and laboratory variables. The proportional hazards assumption was tested for each model using the test of the Schoenfeld residuals.
We also undertook pre-specified subgroup analyses by race and sex. We tested for the presence of interactions between race and JPE and between sex and JPE individually using the likelihood ratio test. When a significant interaction was present, we calculated HRs for the subgroups individually using coefficients from models with the pertinent interaction term(s) included. The effects of race and sex on the risk associated with JPE were also examined simultaneously. Finally, we examined the data for the presence of an interaction between personal history of CHD and JPE.
Statistical analyses were performed using STATA 11.0 (Stata Corp., College Station, TX, USA). All reported P-values are two-sided, with a P-value of <0.05 considered to indicate statistical significance.
This secondary analysis was exempted from full review by the Office of Human Research Ethics of the University of North Carolina at Chapel Hill.
Results
Sample characteristics
Overall, the sample was 44.3% males and 26.9% blacks (Table 1). However, among subjects with JPE, 76.1% were males and 53.3% were blacks. Subjects with JPE also had lower BMI, lower heart rate, and higher blood pressure. They were more likely to be smokers (34.1 vs. 25.0%, P < 0.001), to have LVH (6.0 vs. 1.3%, P < 0.001), and to have a major Minnesota Code abnormality on ECG (14.5 vs. 8.6%, P < 0.001). Those with JPE were more likely to have a personal history of CHD or other vascular disease, but were less likely to have a family history of premature CHD. See Supplementary material online, Table S1, for baseline characteristics of subjects who subsequently experienced SCD compared with those who did not.
Table 1.
Baseline subject characteristics for overall sample and by J-point elevation status
| Subject characteristics | Overall sample (n = 15 141), mean (SD) or % | J-point elevation (n = 1866), mean (SD) or % | No J-point elevation (n = 13 275), mean (SD) or % | P-valuea |
|---|---|---|---|---|
| Age (years) | 54.1 (5.8) | 53.7 (5.8) | 54.1 (5.7) | 0.005 |
| Sex | ||||
| Male (%) | 44.3 | 76.1 | 39.8 | <0.001 |
| Female (%) | 55.7 | 23.9 | 60.2 | |
| Race | ||||
| White (%) | 73.1 | 46.7 | 76.8 | <0.001 |
| Black (%) | 26.9 | 53.3 | 23.1 | |
| Body mass index (kg/m2) | 27.7 (5.4) | 26.6 (4.7) | 27.9 (5.4) | <0.001 |
| Heart rate (b.p.m.) | 66.8 (10.4) | 64.7 (10.7) | 67.0 (10.3) | <0.001 |
| Systolic blood pressure (mmHg) | 121.2 (18.8) | 125.1 (21.9) | 120.7 (18.3) | <0.001 |
| Diastolic blood pressure (mmHg) | 73.7 (11.2) | 76.9 (12.9) | 73.3 (10.9) | <0.001 |
| Smoking status | ||||
| Current smoker (%) | 26.1 | 34.1 | 25.0 | <0.001 |
| Former smoker (%) | 32.1 | 33.5 | 31.9 | |
| Never smoker (%) | 41.8 | 32.4 | 43.1 | |
| Diabetic (%) | 11.9 | 13.7 | 11.6 | 0.008 |
| LVH by Cornell's criteria (%) | 1.9 | 6.0 | 1.3 | <0.001 |
| Cornell's voltage (µV) | 1216 (531) | 1388 (717) | 1192 (494) | <0.001 |
| Major abnormality on ECGb (%) | 9.5 | 14.5 | 8.6 | <0.001 |
| High-density lipoprotein (mg/dL) | 51.7 (17.1) | 51.4 (17.2) | 51.7 (17.1) | 0.42 |
| Low-density lipoprotein (mg/dL) | 137.4 (39.4) | 136.8 (40.6) | 137.5 (39.2) | 0.42 |
| Education | ||||
| Basic (%) | 23.6 | 31.7 | 22.4 | <0.001 |
| Intermediate (%) | 40.9 | 32.7 | 42.0 | |
| Advanced (%) | 35.6 | 35.6 | 35.6 | |
| Personal history of CHD (%) | 4.6 | 7.1 | 4.2 | <0.001 |
| History of stroke (%) | 1.7 | 2.6 | 1.6 | 0.001 |
| Angina (%) | 5.0 | 3.6 | 5.2 | 0.004 |
| Intermittent claudication (%) | 0.77 | 0.75 | 0.77 | 0.93 |
| Family history of premature CHD (%) | 10.3 | 7.6 | 10.6 | <0.001 |
| Cardiac medications (%) | ||||
| β-Blockers | 10.7 | 10.1 | 10.8 | 0.38 |
| Calcium channel blockers | 3.40 | 3.80 | 3.34 | 0.31 |
| Antiarrhythmics | 0.65 | 0.94 | 0.61 | 0.11 |
| Digitalis | 1.46 | 1.49 | 1.45 | 0.91 |
| ACE-inhibitors | 3.05 | 3.47 | 2.99 | 0.26 |
| Serum potassium (mmol/L) | 4.42 (0.48) | 4.41 (0.47) | 4.42 (0.48) | 0.55 |
| Serum sodium (mmol/L) | 141.0 (2.4) | 140.9 (2.5) | 141.0 (2.4) | 0.73 |
| Serum calcium (mg/dL) | 9.79 (0.43) | 9.81 (0.45) | 9.78 (0.43) | 0.003 |
| Serum magnesium (mg/dL) | 1.63 (0.16) | 1.62 (0.16) | 1.63 (0.16) | <0.001 |
aSignificance tests for comparisons by J-point elevation status based on two-sample t-test for continuous subject characteristics and Pearson's χ2 test for categorical subject characteristics.
bMajor ECG abnormality as defined by Minnesota Code.
Prevalence of J-point elevation
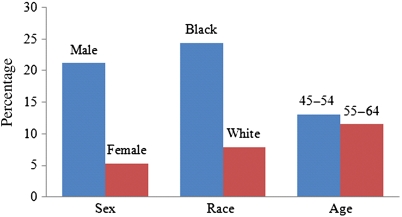
J-point elevation was present in 1866–15 141 subjects (12.3%). Elevation in the anterior leads (V1–V5) was found in 1767 subjects (11.7%). Elevation in the inferior leads (II, III, aVF) was found in 119 subjects (0.8%), and 92 subjects (0.6%) had elevation in the lateral leads (I, aVL, V6). Males were more likely than females to have JPE in at least one lead (21.2 vs. 5.3%, P < 0.001; Figure 1). Prevalence of JPE among blacks was higher than that among whites (24.4 vs. 7.9%, P < 0.001). J-point elevation was significantly more common among younger subjects (13.1%) than among older subjects (11.5%, P = 0.003).
Figure 1.
Prevalence of J-point elevation in various subgroups. P-values for comparisons between subgroups are all <0.05; significance tests based on Pearson's χ2 test.
Overall risk of death and cardiac outcomes
After a mean follow-up of 17 ± 4 years (13 ± 2 years for SCD), 3555 subjects (23.5%) died. Of these deaths, 237 (6.7%) were adjudicated as SCD. During follow-up, 1764 subjects experienced confirmed fatal or non-fatal CHD events. Of these events, 339 were fatal CHD, 105 were fatal MI, and 1320 were non-fatal MI.
In unadjusted analysis, subjects with JPE in any lead were ∼2.3 times more likely to suffer SCD than were subjects without JPE (adjusted HR, 2.28; 95% CI, 1.69–3.07; Table 2). Statistically significant relationships were also present between JPE in any lead and secondary outcomes in unadjusted analysis. After adjustment for race, sex, and age, JPE in any lead was no longer significantly related to SCD (adjusted HR, 1.31; 95% CI, 0.94–1.82), and HRs for secondary outcomes approached the null. Upon further adjustment for clinical, lifestyle, and laboratory variables in addition to demographic factors, these relationships remained non-significant. See Supplementary material online, Table S2, for data on the risk associated with the presence of JPE in the various lead groupings (anterior, lateral, and inferior).
Table 2.
Unadjusted and adjusted hazard ratios comparing subjects with J-point elevation in any lead to those without J-point elevation
| Sudden cardiac death HR (95% CI) | Fatal/non-fatal CHD HR (95% CI) | All-cause mortality HR (95% CI) | |
|---|---|---|---|
| Unadjusted model | 2.28 (1.69–3.07) | 1.46 (1.28–1.65) | 1.40 (1.28–1.53) |
| Model 1a | 1.31 (0.94–1.82)d,e | 1.06 (0.92–1.21)e | 1.05 (0.96–1.16) |
| Model 2b | 1.28 (0.90–1.82)d,e | 1.06 (0.92–1.23)e | 1.06 (0.96–1.18) |
| Model 3c | 1.23 (0.87–1.75)d,e | 1.03 (0.89–1.19)e | 1.02 (0.92–1.13) |
aCox's proportional hazards model, adjusted for age, sex, and race.
bCox's proportional hazards model, adjusted for age, sex, race, heart rate, systolic blood pressure, BMI, serum low-density lipoprotein, diabetes, presence of major ECG abnormality, Cornell's voltage for LVH, previous CHD, and history of angina or stroke.
cCox's proportional hazards model, adjusted for age, sex, race, heart rate, systolic blood pressure, BMI, serum low-density lipoprotein, diabetes, presence of major ECG abnormality, Cornell's voltage for LVH, previous CHD, history of angina or stroke, smoking status, physical activity, and serum potassium.
dSignificant race interaction present (see Table 3 for stratified HRs).
eSignificant sex interaction present (see Table 3 for stratified HRs).
Among subjects with JPE in any lead, a significant interaction was present between race and JPE for the primary outcome of SCD (Table 3). Whites with JPE had a higher risk of SCD (adjusted HR, 2.03; 95% CI, 1.28–3.21) than did whites without JPE. However, JPE did not confer an increased risk of SCD among blacks (adjusted HR, 0.82; 95% CI, 0.52–1.30). The ratio of HRs for whites compared with blacks was 2.46, indicating a large difference between these groups in the risk associated with JPE. The interaction between JPE and race was not significant for the secondary outcomes.
Table 3.
Multivariate-adjusted hazard ratiosa comparing subjects with J-point elevation in any lead to subjects without J-point elevation, stratified by race and by sex
| Sudden cardiac death HR (95% CI) | Fatal/non-fatal CHD HR (95% CI) | All-cause mortality HR (95% CI) | |
|---|---|---|---|
| Race | |||
| White | 2.03 (1.28–3.21)b | 1.16 (0.95–1.40) | 1.10 (0.95–1.28) |
| Black | 0.82 (0.52–1.30)b | 0.91 (0.74–1.12) | 0.96 (0.83–1.10) |
| Ratio of hazard ratios | 2.46 | 1.27 | 1.15 |
| Sex | |||
| Female | 2.54 (1.34–4.82)b | 1.47 (1.10–1.96)b | 0.96 (0.77–1.19) |
| Male | 1.02 (0.69–1.50)b | 0.94 (0.80–1.11)b | 1.04 (0.92–1.16) |
| Ratio of hazard ratios | 2.50 | 1.55 | 0.92 |
aBased on Model 3: Cox's proportional hazards model, adjusted for age, sex, race, heart rate, systolic blood pressure, BMI, serum low-density lipoprotein, diabetes, presence of major ECG abnormality, Cornell's voltage for LVH, previous CHD, history of angina or stroke, smoking status, physical activity, and serum potassium. For the analysis by race, Model 3 included an interaction term for the significant interaction between J-point elevation status and race. For the analysis by sex, Model 3 included an interaction term for the significant interaction between J-point elevation status and sex.
bP-value for interaction <0.05.
A significant interaction between sex and JPE was also present. J-point elevation was associated with significantly increased risk of SCD in females (adjusted HR, 2.54; 95% CI, 1.34–4.82). However, JPE was not a marker of increased risk of SCD in males (adjusted HR, 1.02; 95% CI, 0.69–1.50). The ratio of HRs for females compared with males for SCD was 2.50, indicating that JPE connotes a much greater risk of SCD in females than it does in males. J-point elevation was also associated with a significantly increased risk of fatal/non-fatal CHD events in females, but not in males. No significant interaction was found between sex and JPE for all-cause mortality. Absolute event rates can be found in Table 4.
Table 4.
Sudden cardiac death event rates, overall, and stratified by race and by sex
| Total subjects in stratum | J-point elevation # (%) | SCD events among exposed | No J-point elevation # (%) | SCD events among unexposed | |
|---|---|---|---|---|---|
| Overall | 15 141 | 1866 (12.3) | 53 | 13 275 (87.7) | 181 |
| Race | |||||
| White | 11 068 | 871 (7.9) | 24 | 10 197 (92.1) | 113 |
| Black | 4073 | 995 (24.4) | 32 | 3078 (75.6) | 68 |
| Sex | |||||
| Female | 8434 | 446 (5.3) | 13 | 7988 (94.7) | 62 |
| Male | 6707 | 1420 (21.2) | 43 | 5287 (78.8) | 119 |
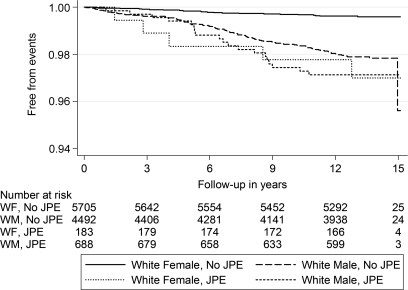
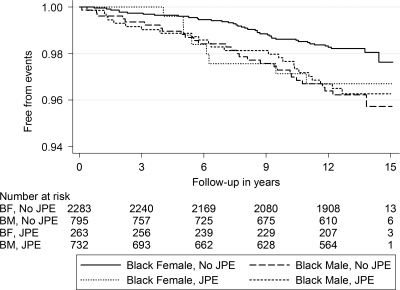
Given that the relationship between JPE and SCD differed based on race and sex, the four subgroups (white males, white females, black males, and black females) were examined separately. White females were found to have a significantly increased risk of SCD when JPE was present (adjusted HR, 8.77; 95% CI, 3.19–24.13; Table 5). Figures 2 and 3 show the Kaplan–Meier curves for SCD in subjects with JPE stratified by race and sex simultaneously.
Table 5.
Multivariate-adjusted hazard ratiosa comparing subjects with J-point elevation in any lead to subjects without J-point elevation, stratified by race and sex simultaneously
| White males HR (95% CI) (n = 5180) | White females HR (95% CI) (n = 5888) | Black males HR (95% CI) (n = 1527) | Black females HR (95% CI) (n = 2546) | |
|---|---|---|---|---|
| Sudden cardiac death | 1.51 (0.91–2.52) | 8.77 (3.19–24.13) | 0.82 (0.47–1.47) | 1.33 (0.59–3.01) |
| Fatal/non-fatal CHD | 1.11 (0.90–1.37) | 1.32 (0.77–2.25) | 0.80 (0.62–1.04) | 1.47 (1.03–2.09) |
| All-cause mortality | 1.14 (0.97–1.35) | 0.93 (0.61–1.39) | 0.94 (0.79–1.12) | 0.96 (0.74–1.25) |
aBased on Model 3: Cox's proportional hazards model, adjusted for age, sex, race, heart rate, systolic blood pressure, BMI, serum low-density lipoprotein, diabetes, presence of major ECG abnormality, Cornell's voltage for LVH, previous CHD, history of angina or stroke, smoking status, physical activity, serum potassium, an interaction term between J-point elevation status and race, and an interaction term between J-point elevation status and sex.
Figure 2.
Kaplan–Meier survival curves for sudden cardiac death in white subjects with and without J-point elevation, stratified by sex.
Figure 3.
Kaplan–Meier survival curves for sudden cardiac death in black subjects with and without J-point elevation, stratified by sex.
No significant interaction between personal history of CHD and JPE was found for any of the outcomes, meaning that the risk for cardiac events associated with JPE did not differ significantly between those with and those without a personal history of CHD.
Discussion
Our study suggests that JPE in any lead in whites and in females is associated with an increased risk of SCD. This finding has, to our knowledge, not been previously discussed in the literature. A trend towards increased risk associated with JPE in whites and females was observed across the secondary outcomes, though the differences between groups were not uniformly significant.
Several explanations could account for the observed difference in risk between groups. The possibility exists that subtle physiological differences are responsible for the discrepancies in risk. Similarly, JPE could be a single phenotypic manifestation of a diverse array of genotypes.23 These genotypic variations may be differentially distributed between various racial groups. Some genotypes may increase risk of poor outcomes, whereas others may be completely benign while producing similar ECG findings. Additionally, the possibility exists that in some patients JPE may indicate underlying structural heart disease. Some instances of JPE may be due not to early repolarization, but instead to delayed depolarization, similar to that seen in periinfarction block.24 This pattern can be seen in the setting of prior MI, LVH, latent ischemic heart disease, and other forms of structural heart disease, all of which could themselves be risk factors for SCD. Although we controlled for the presence of major ECG abnormalities, which should include most instances of periinfarction block, this point is worth noting.
Alternatively, race- and sex-based differences could be attributable to the use of a single ECG cut-off across both races and sexes. The normal distributions of various ECG findings were originally obtained from samples of white males. We have since become aware of the need to use different cut-offs for ‘abnormality’ based on the characteristics of the individual patient. For example, clinicians now often use different cut-offs for defining LVH in males and females. Several studies have shown that the LVH criteria that are used clinically are of limited utility in blacks.25,26 Perhaps, we are seeing risk discrepancies not due to differences in underlying pathophysiology, but because of a need for more accurate race- and sex-based norms. It is possible that a certain degree of JPE is a normal finding in blacks and in males, but that the presence of JPE in a female or a white individual may indicate a more pronounced underlying abnormality, whether it be structural or electrical. This concept makes intuitive sense given the fact that JPE is more prevalent in blacks and in males, but was not shown in our study to be associated with an increased risk of SCD in these groups.
Consistent with past studies that examined the closely related early repolarization pattern,10,17 our study found that JPE was associated with male sex, black race, younger age, and lower heart rate. Several case–control studies have reported a higher prevalence of early repolarization among survivors of idiopathic ventricular fibrillation (VF).5,6 Another case–control study found that JPE was more common among survivors of primary VF.7 A more recent population-based study found that early repolarization in the inferior leads, defined as JPE ≥ 0.1 mV, was a marker of increased risk of death from cardiac causes and of sudden death from arrhythmia.10 Finally, a case–cohort study conducted in Germany showed an increased risk of cardiovascular mortality in individuals manifesting the early repolarization pattern.11
In all of these studies, race was either not reported or predominantly white. No subgroup analyses based on sex were reported. The sex- and race-based differences present in our study were not obvious upon examination of the overall HRs, but during the pre-specified subgroup analysis by race and sex, these relationships became evident. Even though these interactions were not uniformly significant across all outcomes, there was a trend towards increased risk among whites and females. The largely consistent directionality of these effects decreases the probability that the results were due to chance.
In this study, we examined the ECG finding of JPE, defined as a J-point amplitude ≥0.1 mV in any lead. Although JPE is closely related to early repolarization, they are not equivalent. J-point elevation can be present even in the absence of the early repolarization pattern, but early repolarization cannot be diagnosed without its central feature of JPE.27 This partially explains why the exposure prevalence in this study (12.3%) was higher than that observed in most previous studies, though a recent population based study found a 13.1% prevalence of early repolarization.8–11 Additionally, we included leads V1–V3, whereas these leads are frequently omitted in studies of early repolarization in an effort to exclude subjects with Brugada's syndrome.5,14,28 However, Brugada's syndrome has also been reported in the inferior leads.29,30 Because we wished to examine the prognostic significance of JPE in any lead regardless of the aetiology behind this elevation, leads V1–V3 were not excluded.
In addition to differences in the studied exposure, differences in cohort characteristics between this study and previous studies may explain the discrepancies in results. If JPE does indeed connote increased risk in whites but not in blacks, then it is not surprising that a cohort which was likely to be largely white showed a significant overall relationship.10 The fact that our cohort is 27% black is a strength of the study in terms of its generalizability, but may mask a differential effect based on race when analysis is not stratified.
Another strength of the study is the process used for ascertainment of SCD events. The SCD outcome was ascertained through a strict adjudication process, providing a more accurate definition of SCD than is often available in epidemiological studies. Limits to the retrospective adjudication process certainly exist; however, we are confident that the adjudication process provided a more valid and reliable measure of SCD than that provided by the often-used time-based definitions.
A further strength of this study is the integrity of the J-point amplitude data. The J-point amplitude measurement is based on a computer algorithm that produces highly repeatable measurements and eliminates inter-reader variability. The use of the computerized measurements also virtually removes intra-reader variability with a repeatability of 100% and variability of 0%. This contrasts with studies of early repolarization in which the subjective judgement of the ECG reader is a factor and varying definitions of early repolarization are used between studies.27
Despite the large size of the cohort, relatively few SCD events occurred in our study. In addition, few subjects manifested JPE in the inferior and lateral ECG leads. Therefore, very few SCD events occurred in these groups. Estimates of the effects in these lead groupings are somewhat unstable, and thus, it is difficult to draw conclusions about patterns in these lead groupings. In addition, this study included only individuals between the ages of 45 and 64 years. Therefore, caution must be exercised in extending conclusions to individuals outside this age range.
Our study shows that JPE may be an important marker of risk for SCD in some populations. A link appears to exist between JPE and ventricular arrhythmia, but this increased risk is likely to be confined to certain subgroups. The trends found based on sex and race in the present study merit further investigation. Future research must also elucidate which other factors modulate the risk associated with JPE in order to delineate which subgroups of individuals with JPE are at significantly increased risk of SCD. Ultimately, we must understand why these differences exist in order to target strategies to prevent SCD in these higher-risk individuals.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (grant numbers N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022).
Conflict of interest: none declared.
Acknowledgements
The authors thank Anna Kucharska-Newton, PhD, and Charles Campbell for their assistance in data set preparation and management.
References
- 1.Zheng Z, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. doi:10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 2.Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. 2010;7:216–225. doi: 10.1038/nrcardio.2010.3. doi:10.1038/nrcardio.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salonen JT. Primary prevention of sudden coronary death: a community-based program in North Karelia, Finland. Ann N Y Acad Sci. 1982;382:423–437. doi: 10.1111/j.1749-6632.1982.tb55235.x. doi:10.1111/j.1749-6632.1982.tb55235.x. [DOI] [PubMed] [Google Scholar]
- 4.Chugh SS, Reinier K, Teodorescu C, Evanado A, Kehr E, Al Samara M, Mariani R, Gunson K, Jui J. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–228. doi: 10.1016/j.pcad.2008.06.003. doi:10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haissaguerre M, Derval N, Sacher F, Jesel L, Diesenhofer I, de Roy L, Pasquie J, Nogami A, Babuty D, Yli-Mayry S, De Chillou C, Scanu P, Mabo P, Matsuo S, Probst V, Le Scouarnec S, Defaye P, Schlaepfer J, Rostock T, Lacroix D, Lamaison D, Lavergne T, Aizawa Y, Englund A, Anselme F, O'Neill M, Hocini M, Lim KT, Knecht S, Veenhuyzen GD, Bordacher P, Chauvin M, Jais P, Coureau G, Chene G, Klein GJ, Clementy J. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. doi:10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 6.Nam GB, Kim YH, Antzelevitch C. Augmentation of J waves and electrical storms in patients with early repolarization. N Engl J Med. 2008;358:2078–2079. doi: 10.1056/NEJMc0708182. doi:10.1056/NEJMc0708182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosso R, Kogan E, Belhassen B, Rozovski U, Scheinman MM, Zeltser D, Halkin A, Steinvil A, Heller K, Glikson M, Katz A, Viskin S. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: incidence and clinical significance. J Am Coll Cardiol. 2008;52:1231–1238. doi: 10.1016/j.jacc.2008.07.010. doi:10.1016/j.jacc.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Klatsky AL, Oehm R, Cooper RA, Udaltsova N, Armstrong MA. The early repolarization normal variant electrocardiogram: correlates and consequences. Am J Med. 2003;115:171–177. doi: 10.1016/s0002-9343(03)00355-3. doi:10.1016/S0002-9343(03)00355-3. [DOI] [PubMed] [Google Scholar]
- 9.Mehta M, Jain AC, Mehta A. Early repolarization. Clin Cardiol. 1999;22:59–65. doi: 10.1002/clc.4960220203. doi:10.1002/clc.4960220203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. doi:10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 11.Sinner MF, Reinhard W, Muller M, Beckmann BM, Martens E, Perz S, Pfeufer A, Winogradow J, Stark K, Meisinger C, Wichmann HE, Peters A, Riegger GAJ, Steinbeck G, Hengstenberg C, Kääb S. Association of early repolarization pattern on ECG with risk of cardiac and all-cause mortality: a population-based prospective cohort study (MONICA/KORA) PLoS Med. 2010;7:e1000314. doi: 10.1371/journal.pmed.1000314. doi:10.1371/journal.pmed.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antzelevitch C, Yan GX. J wave syndromes. Heart Rhythm. 2010;7:549–558. doi: 10.1016/j.hrthm.2009.12.006. doi:10.1016/j.hrthm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman MJ. RS-T segment elevation in mid- and left precordial leads as a normal variant. Am Heart J. 1953;46:817–820. doi: 10.1016/0002-8703(53)90080-5. doi:10.1016/0002-8703(53)90080-5. [DOI] [PubMed] [Google Scholar]
- 14.Wellens HJ. Early repolarization revisited. N Engl J Med. 2008;358:2063–2065. doi: 10.1056/NEJMe0801060. doi:10.1056/NEJMe0801060. [DOI] [PubMed] [Google Scholar]
- 15.Boineau JP. The early repolarization variant—an electrocardiographic enigma with both QRS and J-STT anomalies. J Electrocardiol. 2007;40:3.e1–3.e10. doi: 10.1016/j.jelectrocard.2006.05.001. doi:10.1016/j.jelectrocard.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Vitelli LL, Crow RS, Shahar E, Hutchinson RG, Rautaharju PM, Folsom AR. Electrocardiographic findings in a healthy biracial population. Atherosclerosis Risk in Communities (ARIC) study investigators. Am J Cardiol. 1998;81:453–459. doi: 10.1016/s0002-9149(97)00937-5. doi:10.1016/S0002-9149(97)00937-5. [DOI] [PubMed] [Google Scholar]
- 17.Reddy VK, Gapstur SM, Prineas R, Colangelo LA, Ouyang P, Kadish AH. Ethnic differences in ST height in the Multi-Ethnic Study of Atherosclerosis. Ann Noninvasive Electrocardiol. 2008;13:341–351. doi: 10.1111/j.1542-474X.2008.00252.x. doi:10.1111/j.1542-474X.2008.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Prineas RJ, Crow RS, Zhang J. The Minnesota Code Manual of Electrocardiographic Findings. 2nd ed. London: Springer-Verlag; 2010. [Google Scholar]
- 20.Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex-specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75:565–572. doi: 10.1161/01.cir.75.3.565. doi:10.1161/01.CIR.75.3.565. [DOI] [PubMed] [Google Scholar]
- 21.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 22.Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular Survey Methods. World Health Organization Monograph Series No. 56. 2nd ed. Geneva: World Health Organization; 1982. [PubMed] [Google Scholar]
- 23.Myerburg RJ, Castellanos A. Early repolarization and sudden cardiac arrest: theme or variation on a theme? Nat Clin Pract Cardiovasc Med. 2008;5:760–761. doi: 10.1038/ncpcardio1354. doi:10.1038/ncpcardio1354. [DOI] [PubMed] [Google Scholar]
- 24.Castle CH, Keane WM. Electrocardiographic ‘peri-infarction block’. A clinical and pathologic correlation. Circulation. 1965;31:403–408. doi: 10.1161/01.cir.31.3.403. [DOI] [PubMed] [Google Scholar]
- 25.Lee DK, Marantz PR, Devereux RB, Kligfield P, Alderman MH. Left ventricular hypertrophy in black and white hypertensives. Standard electrocardiographic criteria overestimate racial differences in prevalence. JAMA. 1992;267:3294–3299. doi:10.1001/jama.267.24.3294. [PubMed] [Google Scholar]
- 26.Gottdiener JS, Reda DJ, Materson BJ, Massie BM, Notargiacomo A, Hamburger RJ, Williams DW, Henderson WG. Importance of obesity, race and age to the cardiac structural and functional effects of hypertension. The Department of Veterans Affairs Cooperative Study Group on Antihypertensive Agents. J Am Coll Cardiol. 1994;24:1492–1498. doi: 10.1016/0735-1097(94)90145-7. doi:10.1016/0735-1097(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 27.Rosso R, Adler A, Halkin A, Viskin S. Risk of sudden death among young individuals with J-waves and early repolarization: putting the evidence into perspective. Heart Rhythm. 2011;8:923–929. doi: 10.1016/j.hrthm.2011.01.037. doi:10.1016/j.hrthm.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 28.Wilde AAM, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Corrado D, Hauer RNW, Kass RS, Nademanee K, Priori SG, Towbin A for the Study Group on the Molecular Basis of Arrhythmias of the European Society of Cardiology . Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–2519. doi: 10.1161/01.cir.0000034169.45752.4a. doi:10.1161/01.CIR.0000034169.45752.4A. [DOI] [PubMed] [Google Scholar]
- 29.Riera AR, Ferreira C, Schapachnik E, Sanches PC, Moffa PJ. Brugada syndrome with atypical ECG: downsloping ST-segment elevation in inferior leads. J Electrocardiol. 2004;37:101–104. doi: 10.1016/j.jelectrocard.2004.01.002. doi:10.1016/j.jelectrocard.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Ueyama T, Shimizu A, Esato M, Kanemoto M, Kametani R, Sawa A, Suzuki S, Matsuzaki M. A case of a concealed type of Brugada syndrome with a J wave and mild ST-segment elevation in the inferolateral leads. J Electrocardiol. 2007;40:39–42. doi: 10.1016/j.jelectrocard.2006.05.006. doi:10.1016/j.jelectrocard.2006.05.006. [DOI] [PubMed] [Google Scholar]