Abstract
Objective
To determine the component costs of care to optimize treatment with limited resources.
Design
We used the Cost-Effectiveness of Preventing AIDS Complications Model of HIV disease and treatment to project life expectancy (LE) and both undiscounted and discounted lifetime costs (2010€).
Methods
We determined medical resource utilization among HIV-infected adults followed from 1998 to 2005 in Northern France. Monthly HIV costs were stratified by CD4 count. Costs of CD4, HIV RNA and genotype tests and antiretroviral therapy (ART) were derived from published literature. Model inputs from national data included mean age 38 years, mean initial CD4 count 372/µl, ART initiation at CD4 counts <350/µl, and ART regimen costs ranging from €760/month to €2,570/month.
Results
The model projected a mean undiscounted LE of 26.5 years and a lifetime undiscounted cost of €535,000/patient (€320,700 discounted); 73% of costs were ART-related. When patients presented to care with mean CD4 counts of 510/µl and initiated ART at CD4 counts <500/µl or HIV RNA >100,000 copies/ml, LE was 27.4 years and costs increased 1–2%, to €546,700 (€324,500 discounted). When we assumed introducing generic drugs would result in a 50% decline in first-line ART costs, lifetime costs decreased 4–6%, to €514,200 (€302,800 discounted).
Conclusions
As HIV disease is treated earlier with more efficacious drugs, survival and thus costs of care will continue to increase. The availability in high-income countries of widely-used antiretroviral drugs in generic form could reduce these costs.
Keywords: cost, France, HIV infection, simulation model
Introduction
Over the past two decades, survival among HIV-infected patients has increased substantially, largely due to the availability of more efficacious antiretroviral agents [1–3]. Guidelines have continued to move toward treating patients earlier in the course of disease [4,5], leading to increased use of both antiretroviral therapy (ART) and laboratory tests [6]. Recently updated HIV treatment guidelines in France and several other high-income countries [6,7] have also added newer and more expensive drugs to an increasing list of effective ART regimens. Despite the emergence of generic drugs, recommendations to reduce the frequency of laboratory tests, and efforts to replace inpatient hospitalizations with outpatient visits whenever possible [8], increased routine costs and improved survival are likely to have increased the lifetime cost of care for HIV-infected patients in the last decade, and may continue to do so in coming years.
As costs of care for HIV-infected patients rise, national policy makers must determine the most efficient and cost-effective methods for managing HIV disease. Estimates of the monthly and lifetime cost of care can help health policy makers understand the budget impact of HIV and allocate scarce resources. Although several studies have evaluated the overall costs of HIV care in Europe [9,10], an updated assessment that isolates cost components (e.g., ART, hospital visits, and laboratory tests) can aid in budget planning and treatment optimization. Our objective is to evaluate the current lifetime costs of medical care for HIV-infected adults, from the perspective of the healthcare system. We use a clinical database of patients followed in Tourcoing, France, and the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) Model of HIV disease. We also evaluate the impact of new antiretroviral drugs and ART initiation criteria on these costs.
Methods
Analytic framework
We estimated healthcare utilization among HIV-infected adults at different stages of disease using data collected at the Tourcoing AIDS Reference Center in Northern France [11–13]. Healthcare utilization was defined as the use of units of medical services, including inpatient and outpatient visits, laboratory tests, and drugs. We excluded costs of treatment among patients who were diagnosed but not in care. Health care utilization in this group was negligible from the healthcare system perspective. We applied a unit cost to each identified resource to develop disease stage-specific cost estimates. Disease stages were defined as chronic HIV with no history of or ongoing AIDS-defining disease (Stage 1); acute AIDS-defining disease, lasting from one month before to two months after diagnosis (Stage 2); chronic HIV with a history of AIDS-defining disease (Stage 3); and the month before death (Stage 4). We incorporated cost estimates as well as mainly French natural history and treatment characteristics into the CEPAC Model [4,10,14,15] to determine the individual time spent in each disease stage and project the lifetime direct medical cost of care for HIV-infected patients. Stage-specific cost and utilization estimates excluded the costs of antiretroviral drugs and CD4 count, HIV RNA and genotype tests. By including this information separately into the CEPAC Model, we were able to vary ART initiation criteria, the cost of newly available antiretroviral drugs, and laboratory testing frequencies in sensitivity analysis. We report life expectancies in projected years and lifetime medical costs in 2010€. Costs are reported both undiscounted and discounted at 3% per year [12].
Model overview
The CEPAC Model is a first-order state-transition Monte Carlo simulation of the natural history, clinical management and costs of HIV [14,16,17]. Patients transition monthly between “health states” characterized by current CD4 count, HIV RNA level, history of AIDS-defining diseases and costs. The model records the time spent in each health state, other clinical outcomes, and lifetime costs from entry into the simulation until death. Model specifications are described in detail in the Technical Appendix as well as in previous publications [10,14,15,18]. We ran 10 million HIV-infected patients through the CEPAC Model for each analysis. We used results for the entire cohort to estimate both individual life expectancy and average lifetime cost of care.
Cost and utilization of medical services
Study sample and data collection
We assigned healthcare costs to each “health state” using healthcare utilization data obtained from a clinical database of 1,775 HIV-infected adults who were followed at the Tourcoing AIDS Reference Center in France (January 1998 to December 2005). Details on this cohort have been published elsewhere [10,19]. We used 2004– 2005 utilization data for Stage 1 and 3 costs, to account for the rapid changes in ART that have occurred in the last two decades. Because the incidence of both AIDS-defining diseases and death has decreased considerably since 1996, our sample size was not large enough to stratify Stage 2 and 4 costs into two-year periods. We therefore used data from the entire follow-up period for Stage 2 and 4 costs.
We estimated the frequency and length of inpatient hospitalizations (>1 day), inpatient day-care visits (≤1 day, excluding overnight admissions), and outpatient visits, as well as utilization of laboratory tests (excluding CD4, HIV RNA and genotype tests) and clinical procedures, HIV- and AIDS-related disease prophylaxis and treatment, and treatment for hepatitis B virus (HBV), hepatitis C virus (HCV), and lipid and metabolic abnormalities.
Inpatient utilization and costs
We estimated inpatient visit costs using a macrocosting approach [12], in which we assigned a national average reimbursement rate derived from the French diagnosis-related group (DRG) classification system [20] to each inpatient stay. The DRG system classifies diseases into medically and economically homogenous groups. DRG costs from the French “Echelle Nationale de Coûts” [21,22] includes physician and nurse fees as well as mean cost of diagnosis, clinical procedures, laboratory tests, drugs dispensed during hospitalization, and hotel/overhead costs (details in Technical Appendix).
Outpatient utilization and costs
We derived the cost of outpatient visits using a microcosting approach [12], in which we enumerated and determined the cost of each resource utilized by patients, including physician fees, laboratory tests (excluding CD4 count, HIV RNA and genotype tests), and clinical procedures. Unit costs for laboratory tests are from the French “Nomenclature des actes de biologie médicale” [23], while unit costs of clinical procedures are from the French “Classification commune des actes médicaux” [24]. The clinical database recorded the type, but not the quantity, of medications prescribed at each visit. Outpatient medications included chemoprophylaxis and treatment for AIDS-defining diseases as well as treatment for HBV, HCV, hyperlipidemia and diabetes, but not ART (Technical Appendix, Table A1). When evaluating outpatient medication use, we assumed patients received non-ART medications in accordance with the French standard of care (see Technical Appendix) [6].
To determine costs for each stage of disease, we first multiplied resource utilization per patient per stage by unit cost per resource. After adding all healthcare costs incurred in each stage, we estimated per-person monthly costs by dividing stage costs by total per-person time spent in each stage. Finally, we determined mean cost per person-month in each stage. Details on the derivation of monthly costs by Stage are in the Technical Appendix.
CEPAC Model inputs
Simulated patient characteristics and disease progression
In the base case scenario, simulated patients had the same characteristics as the newly diagnosed French HIV-infected population in 2005 (Table 1) [25]. Mean age at entry into the model was 38 years (SD, 9). Mean initial CD4 count was 372/µl and 15% of patients presented to care with a history of AIDS-defining disease.
Table 1.
Summary of input parameters for the CEPAC model of HIV disease and treatment in France.
| Variable | Base case value | Sensitivity analysis rangea | Reference |
|---|---|---|---|
| Mean age at study entry, years (SD) | 38 (9) | — | [25] |
| % male | 62 | — | [25] |
| Mean CD4 at enrollment, cells/µl (SD) | 372 (257) | 97 – 510 | [54] |
| HIV RNA distribution at initiation of HIV care (copies/ml), % | |||
| > 100,000 | 18.8 | — | [54] |
| 30,001 – 100,000 | 21.1 | — | [54] |
| 10,001 – 30,000 | 15.2 | — | [54] |
| 3,001 – 10,000 | 13.9 | — | [54] |
| 501 – 3,000 | 11.2 | — | [54] |
| ≤500 | 19.8 | — | [54] |
| History of opportunistic disease at enrollment, % | 15 | — | [55] |
| Time interval between laboratory tests | |||
| CD4 count, months | 3 | 3–6 | [6] |
| HIV RNA, months | 3 | 3–6 | [6] |
| Genotype | Before any new regimen | — | [6] |
| Antiretroviral therapy efficacy, % HIV RNA level suppressed to <400 copies/ml at 24 weeks (mean increase in CD4 cell count at 48 weeks, cells/µl) |
|||
| TDF/FTC + EFV | 86 (190) | 76 – 96 | [56] |
| ATV/r + 2 NRTIs | 70b (110) | 63 – 83 | [57] |
| 3rd-linec | 58b (121) | 60 – 90 | [32,33,38,57] |
| 4th-linec | 65 (102d) | 50 – 75 | [34,35,39,58] |
| 5th-linec | 40 (121) | 20 – 50 | [36,37,59] |
| 6th-linec | 15 (45) | 10 – 40 | [36,37] |
| Protective effect of antiretroviral therapy on risk of AIDS-defining diseases and death, % |
46 | 0 – 73 | [60] |
| Antiretroviral therapy costs, 2010€/month | |||
| TDF/FTC+ EFV | 760 | 380 – 1,200 | [28,31] |
| ATV/r + 2 NRTIs | 990 | 500 – 990 | [28,31] |
| 3rd-line | 1,190 | 600 – 2,410 | [28,31] |
| 4th-line | 2,000 | 1,000 – 2,080 | [28,31] |
| 5th-line | 2,570 | 1,280 – 2,570 | [28,31] |
| 6th-line | 1,290 | 640 – 1,290 | [28,31] |
| Laboratory tests, 2010€/test | |||
| CD4 count | 22 | — | [23] |
| HIV RNA | 59 | — | [23] |
| Genotype | 297 | — | [23] |
ATV/r, ritonavir-boosted atazanavir; EFV, efavirenz; FTC, emtricitabine; NRTI: nucleoside reverse transcriptase inhibitor; SD, standard deviation;TDF, tenofovir.
See Technical Appendix for details on the endpoints used in the sensitivity analysis ranges.
at 48 weeks
Once patients start third-line therapy, genotype tests generally determine individualized regimens. ART lines 3–6 are therefore modeled as standard regimens with wide ranges of efficacy and costs, represented by various recent studies.
at 24 weeks
CD4 count-stratified mortality and incidence of AIDS-defining diseases were derived from two French cohorts [14,19]. Monthly decreases in CD4 count before patients initiate ART are from the Multicenter AIDS Cohort Study (MACS), the best long-term study to have followed disease progression in the pre-ART era [26].
Treatment characteristics
Simulated patients initiated ARTat CD4 counts <350/µl or at the onset of a severe AIDS-defining disease, as recommended by the national guidelines in 2005 [27]. Clinic visits, CD4 counts, and HIV RNA tests occurred every 3 months [6], while genotype tests were performed before the initiation of any new ART regimen, including the first. Patients could receive up to six sequential lines of ART (Table 1). They switched ART regimens upon observed failure, defined as two successive HIV RNA tests ≥500 copies/ml or an increase of ≥1 log10 copies/ml [6].
ART and laboratory costs
ART regimen costs were €760/month for first-line ART and ranged from €990/month to €2,570/month for subsequent regimens [28]. Individual antiretroviral drug costs are from the Tourcoing Hospital Pharmacy database and are reflective of costs throughout France. CD4, HIV RNA, and genotype test costs (Table 1) were included in the model separately from Stage costs, as discussed above [23].
Sensitivity analyses
We performed sensitivity analyses on major model parameters to determine their impact on the lifetime cost of care for HIV-infected patients. We first varied CD4 counts at initial presentation to care. We determined the average lifetime undiscounted cost and life expectancies of HIV-infected persons in France who presented to care early (defined as CD4 counts ≥200/µl and no AIDS-defining disease), with advanced disease (defined as CD4 counts <200/µl or AIDS-defining disease), and with base case characteristics [29]. We used these results to estimate the annual and lifetime cost of providing care to three cohorts of 7,360 patients – the estimated annual diagnosed number of HIV-infected patients in France [30] – presenting with the above-mentioned characteristics.
We evaluated the impact on life expectancy and costs of treating patients with newer and more expensive ART regimens, and of reducing the frequency of CD4 and HIV RNA tests. We reduced inpatient and day-care visits in response to the recent recommendations by the French Ministry of Health to replace inpatient hospitalizations with inpatient day-care visits, and to replace inpatient day-care visits with outpatient visits whenever possible [8]. We also increased ART efficacy and costs to account for the recent development of more effective antiretroviral drugs. French law sets the price of generic drugs to a maximum of 50% of brand-name prices [31]. We reduced the cost of ART by this amount to examine the impact of the future availability of generic drugs. We also evaluated alternative assumptions in areas where data were uncertain. In the “lowest virologic efficacy” scenario, the efficacies of ART regimens 3–6 corresponded to the lower bound of published efficacies, as in Table 1 [32– 37]. In the “highest efficacy” scenario, these efficacies corresponded to the upper bound of published efficacies [36–39].
Results
Monthly cost of care in the Tourcoing cohort
Stage 1 costs (excluding CD4, HIV RNA and genotype tests, and antiretroviral drugs) in the Tourcoing cohort ranged from €220/month (standard deviation [SD], €240) at CD4 counts >500/µl to €1,200/month (SD, €1,590) at CD4 counts ≤50/µl (Table 2). Stage 2 costs ranged from €6,180 (SD, €5,070) for fungal infections to €11,700 (SD, €5,610) for Pneumocystis jirovecii pneumonia (PCP). Stage 3 costs ranged from €270/month (SD, €330) at CD4 counts >500/µl to €1,070/month (SD, €1,190) at CD4 counts 51 –100/µl. Costs within one month of death (Stage 4) ranged from €7,400 (SD, €7,970) to €14,070 (SD, €8,000), depending on the cause of death.
Table 2.
Summary of resource utilization and costs in the Tourcoing AIDS Reference Center: inputs for the CEPAC model of HIV disease and treatment in France.
| Variable | Average monthly cost, 2010€ (SD)a | Time spent in stage (person-months) |
|---|---|---|
| Stage 1: No history of AIDS-defining event by CD4 count (cells/µl)b | ||
| >500 | 220 (240) | 290,226 |
| 351 – 500 | 290 (390) | 181,888 |
| 201 – 350 | 370 (590) | 51,898 |
| 101 – 200 | 810 (2,270) | 20,507 |
| 51 – 100 | 870 (930) | 5,240 |
| ≤50 | 1,200 (1,590) | 3,953 |
| Stage 2: Acute AIDS-defining diseasec | ||
| Pneumocystis jiroveci pneumonia | 11,700 (5,610) | 1,230 |
| Mycobacterium avium complex | 6,390 (2,900) | 160 |
| Toxoplasmic encephalitis | 9.420 (6,400) | 533 |
| Cytomegalovirus infection | 11,640 (3,780) | 359 |
| Candida esophagitis | 6,180 (5,070) | 2,297 |
| Other AIDS-defining event | 6,340 (6,890) | 8,963 |
| Stage 3: History of at least one AIDS-defining event by CD4 count (cells/µl)b | ||
| >500 | 270 (330) | 32,376 |
| 351 – 500 | 390 (850) | 29,649 |
| 201 – 350 | 760 (1,840) | 16,135 |
| 101 – 200 | 1,050 (2,230) | 9,828 |
| 51 – 100 | 1,070 (1,190) | 2,198 |
| ≤50 | 920 (580) | 2,200 |
| Stage 4: Month of deathc | ||
| No history of an AIDS-defining event | 7,400 (7,970) | 1,077 |
| ≤30 days after an AIDS-defining event | 14,070 (8,000) | 566 |
| >30 days after an AIDS-defining event | 11,750 (8,670) | 475 |
SD, standard deviation.
Stage-specific cost estimates excluded the costs of antiretroviral drugs and CD4 count, HIV RNA and genotype tests. These costs were added individually to the stage-specific costs to allow for variations in laboratory testing frequency, ART initiation criteria, and the cost of newly available antiretroviral drugs.
From the Tourcoing AIDS Reference Center, January 2004 – December 2005.
From the Tourcoing AIDS Reference Center, January 1998 – December 2005.
Life expectancy and costs using the CEPAC Model
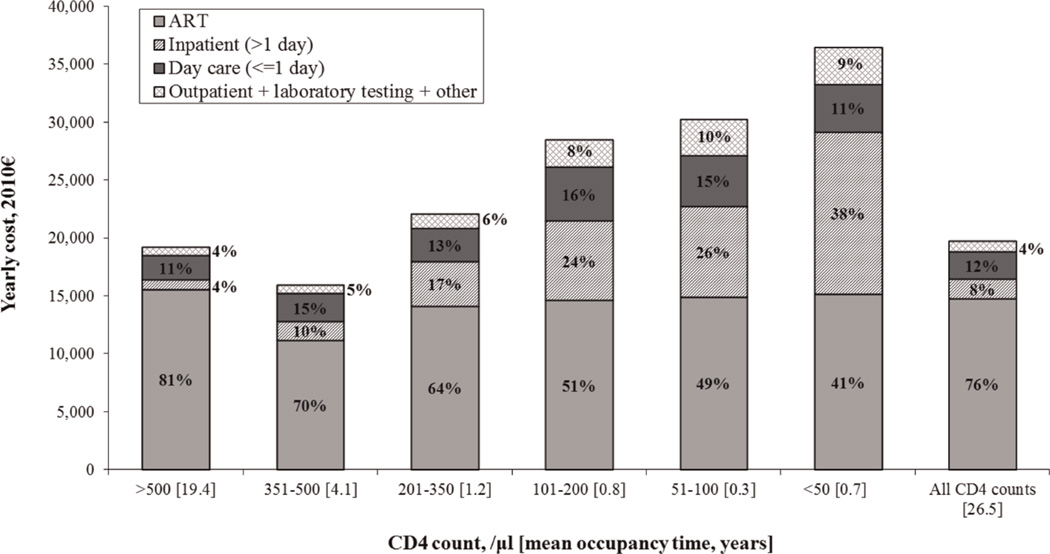
In the base case scenario, when mean CD4 count at presentation was 372/µl and patients initiated ART at CD4 counts <350/µl, projected life expectancy was 26.5 years and the lifetime cost of care was €535,000 (€320,700 discounted), or €20,170/year. On average, patients spent 55% of their lifetimes in Stage 1 and 73% with CD4 counts >500/µl, either before initiating ART or as a result of successful ART. Overall, 73% of lifetime costs and 76% of yearly costs were for ART (Fig. 1). On average, patients spent 18% of their time on ART (4.7 years) on first-line ART, 16% (4.6 years) on second-line ART, 13% (4.2 years) on third-line ART, 11% (4.0 years) on fourth-line ART, and 7% (2.6 years) on fifth-line ART. The 61% of patients who reached sixth-line ART remained on this regimen for an average of 14.1 years, or 34% of their total time on ART. Patients spent the largest fraction of their time on sixth-line ART, because they remained on it until death, even after observed failure. The average overall costs of each ART regimen were €43,100, €54,680, €60,250, €95,980, €80,030, and €219,730 for ART lines 1–6, respectively.
Fig. 1. Components of annual cost of HIV care by CD4 count.
Annual costs were higher when simulated patients had CD4 counts >500/µl than when they were 351–500/µl, because the majority of patients with CD4 counts >500/µl were on antiretroviral therapy (ART).
The average yearly cost of care was €36,540 among patients with CD4 counts ≤50/µl. This cost consisted predominantly of ART (41%) and inpatient hospitalizations (38%) (Fig. 1). At CD4 counts >500/µl, the average yearly cost was €19,240, ofwhich 81% was ART-related. At CD4 counts 351–500/µl, the average yearly cost was €15,970, ofwhich 70% was ART-related. Yearly costs were higher in the former group, because a larger proportion of patients in the CD4 count >500/µl group were on ART (Fig. 1).
Sensitivity analyses: impact on lifetime cost
Timing of presentation to care
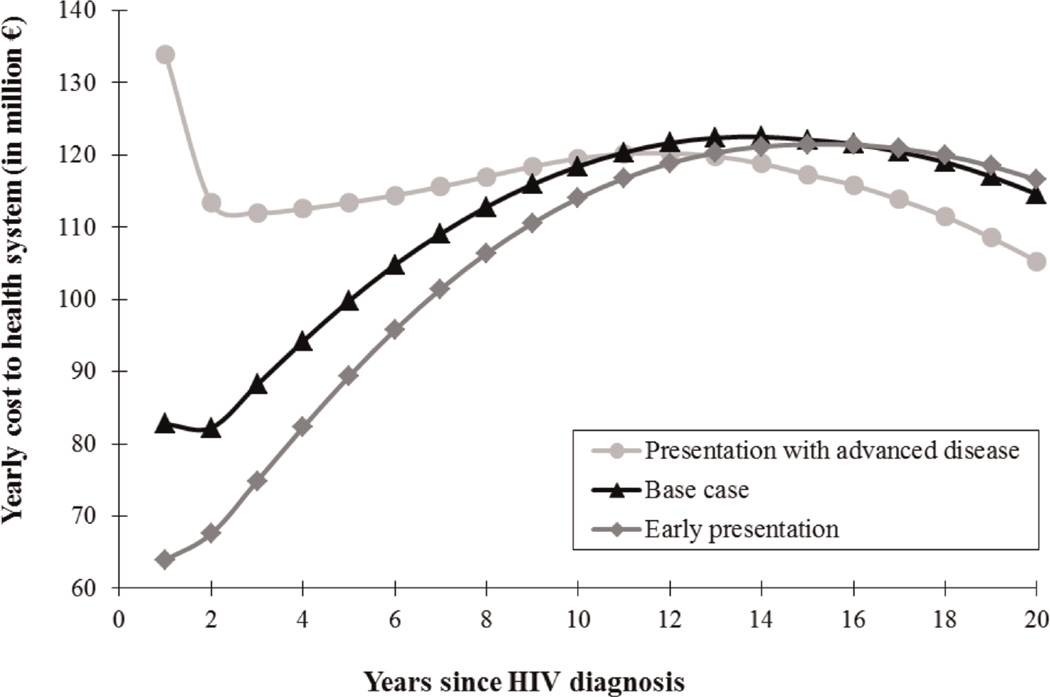
Life expectancy was highest, at 27.5 years, when patients presented to care early (mean CD4 count, 510/µl). Undiscounted lifetime costs for these patients were €534,800/person (Table 3). Life expectancy and undiscounted lifetime costs were lowest for patients presenting with advanced disease (mean CD4 count, 97/µl), at 23.8 years and €513,200. Figure 2 shows the annual undiscounted costs of providing care to three hypothetical cohorts of 7,360 HIV-infected persons presenting to care at the different disease stages described above [30]. These simulated patients initiated ARTwhen their CD4 counts were observed to be <350/µl, and were followed for 20 years. The cost of providing care to a population of 7,360 patients in the first year after HIV diagnosis was €134 million when all patients presented with advanced disease [29]. In this population, annual costs to the health system were highest in the first year of care. Annual costs decreased substantially in the following two years. For the first 12 years of care, however, annual costs to the health system in this population were higher than among patients who presented to care early.
Table 3.
Projected life expectancy and lifetime costs of care for HIV-infected adults in France.
| Undiscounted | Discounted | |||
|---|---|---|---|---|
| Life expectancy, years (SD) | Annual cost, 2010€ | Lifetime cost, 2010€ (SD) | Lifetime cost, 2010€ (SD) | |
| Base case | 26.5 (14.0) | 20,170 | 535,000 (308,000) | 320,700 (146,200) |
| Sensitivity analyses | ||||
| Timing of presentation to care | ||||
| Presentation with advanced disease (mean CD4 count, 97/µl)a | 23.8 (14.2) | 21,600 | 513,200 (314,200) | 322,500 (154,600) |
| Early presentation (mean CD4 count, 510/µ1) | 27.5 (13.9) | 19,440 | 534,800 (307,100) | 313,000 (144,600) |
| Probability of virologic suppression after six months of ART regimen | ||||
| Third-line, 90%; fourth-line, 75% | 27.9 (14.7) | 22,700 | 634,000 (369,400) | 374,700 (177,200) |
| “Lowest efficacy” scenariob | 25.6 (13.5) | 19,890 | 509,100 (293,400) | 309,200 (139,600) |
| “Highest efficacy” scenarioc | 28.1 (14.7) | 20,280 | 569,200 (329,400) | 332,400 (153,000) |
| ART cost | ||||
| Raltegravir-based first-line ART: €1,200d | 26.5 (14.0) | 21,080 | 559,100 (312,500) | 341,300 (149,300) |
| First-line ART reduced by 50%e | 26.5 (14.0) | 19,380 | 514,200 (305,200) | 302,800 (145,000) |
| All lines of ART reduced by 50%f | 26.5 (14.0) | 12,790 | 339,400 (1 87,000) | 203,800 (87,200) |
| ART initiation criteria | ||||
| Expanded ART initiation criteriag | 26.4 (13.9) | 20,560 | 543,400 (308,300) | 329,000 (146,800) |
| Immediateh | 26.4 (13.6) | 20,970 | 554,000 (306,300) | 339,200 (145,600) |
| Early presentation + expanded ART initiation criteria | 27.4 (13.8) | 19,960 | 546,700 (307,400) | 324,500 (145,500) |
| Protective effect of ART on morbidity and mortality (base case: 46%) | ||||
| None | 22.6 (13.7) | 20,440 | 461,100 (306,400) | 287,800 (153,900) |
| 73% | 30.2 (14.1) | 20,350 | 615,000 (318,400) | 353,300 (142,000) |
| Outpatient visit and laboratory test frequencies | ||||
| 25% decrease in outpatient visits; CD4 and HIV RNA tests every 4 months | 26.5 (14.0) | 20,150 | 534,500 (307,700) | 320,400 (146,100) |
| 50% decrease in outpatient visits; CD4 and HIV RNA tests every 6 months | 26.5 (14.0) | 20,130 | 534,000 (307,400) | 320,100 (146,000) |
ART, antiretroviral therapy; SD, standard deviation.
ln the cohort followed at the Tourcoing AIDS Reference Center from 1 998 to 2005, the mean CD4 count of patients presenting to care with advanced disease, defined as CD4 counts <200/µl or AIDS-defining disease [29], was 97/µl. Undiscounted lifetime costs are similar in the base case and early presentation groups, but discounted costs are €7,700 higher in the base case group. This substantial difference may be explained by the earlier deaths and thus decreased discounting of expensive end-of-life costs in the base case.
In the “lowest efficacy” scenario, the efficacies of ART regimens 3–6 correspond to the lower bound of published efficacies, as in Table 1 [32]–[37].
ln the “highest efficacy” scenario, the efficacy of ART regimens 3–6 correspond to the upper bound of published efficacies [36–39].
This is the cost of tenofovir + emtricitabine + raltegravir. This regimen may become the standard first-line ART regimen in the near future [7].
Expanded ART initiation criteria: CD4 count <500/µl, HIV RNA >100,000 copies/ml, or AIDS-defining disease.
Immediate: ART initiation at presentation to care, regardless of CD4 count and HIV RNA.
Fig. 2. Distribution of annual undiscounted cost to the health system of providing care to a cohort of 7,360 persons recently diagnosed with HIV, by disease stage.
This figure shows results for three cohorts of simulated patients: (a) Presentation with advanced disease: patients presenting to care with advanced disease, defined as CD4 counts <200/µl or AIDS-defining disease (mean CD4 count, 97/µl); lifetime cost; lifetime cost was €513,200 (€322,500 discounted). (b) Base case: patients presenting to care with base case characteristics, similar to those of patients presenting to care in France in 2005 (mean CD4 count, 372/µl); lifetime cost was €535,000 (€320,700 discounted). (c) Early presentation: patients presenting to care early (mean CD4 count, 510/µl); lifetime cost was €534,800 (€313,000 discounted). Simulated patients in each of these cohorts initiated antiretroviral therapy (ART) at CD4 counts <350/µl or severe AIDS-defining disease. Undiscounted lifetime costs are similar in the base case and early disease groups, but discounted costs are €7,700 higher in the base case group. This substantial difference may be explained by the earlier deaths and thus decreased discounting of expensive end-of-life costs in the base case.
Newer ART regimens
Changes in ARTefficacy and costs had the greatest impact on lifetime costs (Table 3). When third-line ART consisted of raltegravir, etravirine and ritonavir-boosted darunavir (six-month probability of virologic suppression, 90%) [38] and fourth-line ART consisted of etravirine, ritonavir-boosted darunavir and enfuvirtide (six-month probability of virologic suppression, 75%) [31,35], both life expectancy and costs increased. In this scenario, life expectancy increased from 26.5 to 27.9 years and undiscounted lifetime costs increased 17–18% to €634,000 (€374,700 discounted). Of these costs, 77% were for ARTand 7% were for hospitalizations. When we substituted first-line efavirenz with raltegravir and increased the cost but not the efficacy of the regimen, lifetime cost increased 4–6% to €559,100/patient (€341,300 discounted). Of these costs, 74% were for ARTand 8% were for inpatient hospitalizations. Lifetime costs decreased 4–6% when we reduced the cost of first-line ART alone by 50% (to €514,200 undiscounted; €302,800 discounted), to examine the impact of the future availability of generic drugs [31]. Of these costs, 72% were for ART and 9% were for inpatient hospitalizations. They decreased 36–37% when we reduced the cost of all antiretroviral medications by the same amount (€339,400 undiscounted; €203,800 discounted) [31]. Of these costs, 58% were for ARTand 13% were for inpatient hospitalizations.
Other sensitivity analyses
Variations in ART initiation criteria had a smaller impact on the results. When patients presented to care with a mean CD4 count of 372/µl and initiated ART at CD4 counts <500/µl or HIV RNA >100,000 copies/ml, life expectancy remained the same but lifetime costs increased 2–3%, from €535,000 to €543,400 (€329,000 dis counted). Of these costs, 74% were for ARTand 8% were for inpatient hospitalizations. When patients presented early (mean CD4 count, 510/µl) and initiated ART at CD4 counts <500/µl or HIV RNA >100,000 copies/ml, life expectancy increased by almost one year to 27.4 years compared to the base case, and lifetime costs increased 1–2%, to €546,700 (€324,500 discounted). Of these costs, 76% were for ART and 8% were for inpatient hospitalizations.
Modifications in inpatient day-care, inpatient hospitalization and outpatient utilization had little impact on total costs (data not shown). Changes in laboratory test utilization had little impact on results (Table 3).
Discussion
Using cost and resource utilization data from a national database and a widely published HIV simulation model, we projected survival and costs of HIV care. Although it is difficult to compare studies on the lifetime costs of HIV directly, due to differences in costing methodology, our results are consistent with those reported by Schackman et al. in 2006, as well as other studies [40]. Our study demonstrates that total lifetime costs of care have increased, but that the overall annual costs of care have remained remarkably stable over time. The main driver of the increase in total lifetime costs of care is the dramatic improvement in survival generated by both earlier ART initiation and more efficacious treatment. In 2002, we used the same database and the same model to estimate the undiscounted lifetime cost of HIV care at €382,000 (€264,200 discounted) over a lifetime of 16.4 years, or €23,200/year (inflation-adjusted to 2010€) [10]. In our current study, we found a projected life expectancy of 26.5 years and a lifetime cost of care of €535,000 (€320,700 discounted), or €20,170/year. This finding is consistent with other published cost analyses. In 2008, for instance, Krentz et al. estimated the cost of medical care for HIV-infected patients in the Southern Alberta Cohort over a nine-year period. They found that annual costs of care had remained relatively stable for most HIV patients, except those with CD4 counts <75 cells/mL [41]. Some studies have stated that, although overall annual costs have remained similar throughout the combination ART era, the cost distribution has changed, with a progressive decline in inpatient costs and increase in ART costs [42]. This is not consistent with studies that were published more recently, however [41].
It is notable that almost three quarters of the cost of care for HIV-infected patients is for antiretroviral drugs, consistent with previous analyses [40]. Potential changes in the cost of ART will therefore have a considerable impact on results. The United States Department of Health and Human Services, for instance, recently added raltegravir-based regimens as one of the preferred options for ART-naïve patients [7]. When we replaced efavirenz with raltegravir in first-line ART, the lifetime cost of care increased. Although raltegravir-based first-line ART has been shown to be a well-tolerated and effective alternative to efavirenz in ART-naïve patients [43], a less expensive generic form of efavirenz will likely become available soon [7]. When we assumed using generic efavirenz reduced first-line ART costs by 50% [31], undiscounted lifetime costs decreased only slightly, perhaps because we did not simultaneously consider generic drugs for the five subsequent ART regimens. This scenario is realistic, because the older drugs used in first-line regimens are close to or past their 20-year patent protection period. Although the use of generics in first-line ART makes only a small difference at the individual level, it would play an important role in budget planning at the population level. As an increasing number of safe and effective first- and second-line HIV medications becomes available and resources for care become increasingly constrained in high-income settings, national and international policy makers may need to consider drug prices when selecting preferred regimens, similar to the process for selecting cardiovascular and other chronic disease treatments in low-income settings [44]. Moreover, as recommendations for earlier ART initiation increase the market for antiretroviral drugs [6,7], lower drug prices could make earlier care more feasible.
Delayed entry into HIV care resulted in reduced life expectancy and higher immediate costs after treatment initiation. This finding is consistent with results recently reported by Fleishman et. al. in the US. They demonstrated that after 7–8 years of care, HIV-infected patients who initiated care late had substantially higher medical costs than patients who were diagnosed and started care earlier [45]. Interventions such as routine HIV screening, which was recently recommended in France [46], could reduce the number of patients initiating care with advanced HIV. We recently demonstrated that this strategy would be both effective and cost-effective in France [47].
The United States Department of Health and Human Services and the French Ministry of Health also recently recommended that HIV-infected patients initiate ART with CD4 counts <500/µl [5–7]. The current model-based analysis suggests that modifying treatment initiation criteria may have little impact on life expectancy under current conditions, because a substantial proportion of patients continue to present to care with initial CD4 counts <350/µl [29,48,49].
Although this analysis aims to inform budget planning and HIV financing policy in France, we realize that most global HIV policy decisions are directed toward low- and middle-income countries (LMIC), and that budget impact analyses in these settings are necessary. While overall costs of care are lower in LMIC, the distribution of costs is also very different. For example, because most HIV treatment programs in LMIC use cheaper centrally-sourced generic antiretroviral drugs, laboratory monitoring costs play a larger role in overall costs than in high-income settings like France. Findings, particularly the impact of introducing generics and earlier ART initiation, will therefore likely be different in LMIC settings [18].
This analysis has several limitations. First, the clinical database we used to estimate cost of care was not from a national sample and may not be representative of other areas of France. However, since treatment guidelines are the same across France [6], standards of care are probably similar, and our results are likely generalizable to the rest of the country. Second, in calculating the monthly cost of care by disease stage, we did not consider the cost of care for cancer treatments that occur outside our HIV inpatient and outpatient clinics, palliative care, or home healthcare received outside of the Tourcoing AIDS Reference Center. For example, we did not include utilization data for patients with oncological diseases other than Kaposi’s sarcoma who were treated at other hospitals and specialty clinics. Third, to estimate the life expectancy and lifetime cost of HIV disease we used a simulation model that relies on multiple assumptions. The long-term efficacy of some ART regimens and the durability of their virologic and immunologic benefits are not fully known, because randomized trials generally report short-term outcomes. However, the results were only minimally sensitive to variations in ART efficacy. Finally, we note the mounting evidence pointing to the preventive benefits of ART in reducing HIV transmission [50]. Since this analysis restricts attention to the impact of treatment on the individual infected patient, it does not account for any life expectancy gains or resource savings that might accrue from preventing the further spread of infection. Our findings may therefore underestimate the health benefits and overestimate the costs of expanding ART initiation criteria.
Although the financial impact of care and treatment of HIV-infected individuals continues to grow, few studies worldwide have estimated the lifetime cost of treating HIV disease with current regimens [9]. Even when estimates are available [51–53], the limited period of time during which cost and utilization data are collected makes it difficult to extrapolate future expenditures. With current clinical and cost data, we projected the lifetime cost of treating HIV disease in France. We showed that earlier presentation to care reduces annual costs to the health system in the first decade of treatment, and also improves survival, thus increasing ARTutilization costs in this group in later years. We found that lower antiretroviral costs associated with the availability of generic drugs could dramatically reduce individual lifetime costs in developed countries. Policy makers can use these projections to prioritize HIV-related health interventions and to ensure that sufficient resources are committed to HIV care.
Acknowledgements
The authors gratefully acknowledge the assistance of the clinicians whoe participated in data collection at the Tourcoing AIDS Reference Center, as well as Naishin Fu, Jennifer Chu, John Chiosi, Erin Rhode and Adam Stoler from the Cost-Effectiveness of Preventing AIDS Complications group. We are indebted to Sylvie Vandamme, Marie Marien, and Sandrine Dassoneville for their assistance with data management.
Financial support: Supported by Sidaction; the Agence National de Recherches sur le SIDA et les hepatitis virales (ANRS); the National Institute of Allergy and Infectious Diseases (R37 AI042006, K24 AI062476); and the National Institute on Drug Abuse (R01 DA015612). None of the funders were involved in designing the study, collecting, analyzing or interpreting the data, preparing the article, or deciding to submit it for publication. All authors were independent from the funding sources.
Footnotes
Author contributions: CES, EL, RPW, KAF, and contributed to the conception and design of the study. KC, PC, FA, and HM acquired data for the study. KC performed the statistical analysis. CES, EL, RPW, KAF, ADP, and YY designed the model-based analysis. CES performed the model-based analysis. All authors participated in the interpretation of results. YYand CES drafted the article. All authors revised the article critically for important intellectual content. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have given final approval for publication. YY had full access to all the data in the study and had final responsibility for the decision to submit the article for publication.
Co-author information: CES, Project Coordinator (Massachusetts General Hospital, Division of General Medicine, 50 Staniford St, 9th floor, Boston MA 02114, USA); KC, PhD student (ATIP-Avenir Inserm U995, Faculté de medicine, 1 place de Verdun, 59045 Lille, France); PC, Research Nurse (Service Universitaire des Maladies Infectieuses et du Voyageur, Centre Hospitalier de Tourcoing, 135, rue du Preésident Coty, F 59208 Tourcoing, France); EL, Associate Professor of Biostatistics at Harvard Medical School (Massachusetts General Hospital, Division of General Medicine, 50 Staniford St, 9th floor, Boston MA 02114, USA); RPW, Associate Professor of Medicine at Harvard Medical School (Massachusetts General Hospital, Division of General Medicine, 50 Staniford St, 9th floor, Boston MA 02114, USA); BRS: Associate Professor and Chief of the Division of Health Policy (Department of Public Health, Weill Cornell Medical College, 402 E. 67th St. New York, NY 10065, USA); FA: Physician (Service Universitaire des Maladies Infectieuses et du Voyageur, Centre Hospitalier de Tourcoing, 135, rue du Président Coty, F 59208 Tourcoing, France); HM: Physician (Service Universitaire des Maladies Infectieuses et du Voyageur, Centre Hospitalier de Tourcoing, 135, rue du Président Coty, F 59208 Tourcoing, France); ADP, Professor (Yale School of Medicine, 60 College Street, Room 305, New Haven, CT 06510, USA); KAF, Director of the Program in HIV Epidemiology and Outcomes Research and Professor of Medicine at Harvard Medical School (Massachusetts General Hospital, Division of General Medicine, 50 Staniford St, 9th floor, Boston MA 02114, USA).
Conflicts of interest:
With the exception of Yazdan Yazdanpanah, none of the authors report any association that might pose a conflict of interest (e.g. pharmaceutical stock ownership, consultancy, advisory board membership, relevant patents, or research funding). Dr. Yazdanpanah has received travel grants, honoraria for presentation at workshops and consultancy honoraria from Bristol-Myers Squibb, Gilead, Merck, Pfizer, Roche and Tibotec, ViiV Healthcare.
Ethics Committee Approval: The medical ethics review committees of the contributing hospitals exempted this research from institutional review board approval, because all input data were obtained from secondary sources, we did not have access to any patient identifiers, and there was no direct contact with any human subjects.
References
- 1.Jaggy C, von Overbeck J, Ledergerber B, Schwarz C, Egger M, Rickenbach M, et al. Mortality in the Swiss HIV Cohort Study (SHCS) and the Swiss general population. Lancet. 2003;362:877–878. doi: 10.1016/S0140-6736(03)14307-3. [DOI] [PubMed] [Google Scholar]
- 2.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 3.Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300:51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 4.Prise en charge médicale des personnes infectées par le VIH: Rapport 2008. [Accessed September 30, 2008];Flammarion. 2008 at http://www.ladocumentationfrancaise.fr/rapports-publics/084000593/
- 5.Thompson MA, Aberg JA, Cahn P, Montaner JS, Rizzardini G, Telenti A, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 304:321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 6.Prise en charge médicale des personnes infectées par le VIH: Rapport 2010. [Accessed 9 February 2011];La documentation française. 2010 at http://www.sante-sports.gouv.fr/IMG/pdf/Rapport_2010_sur_la_prise_en_charge_medicale_des_personnes_infectees_par_le_VIH_sous_la_direction_du_Pr-_Patrick_Yeni.pdf.
- 7. [Accessed 9 February 2011];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2011 January 10; at http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 8.Ministére de la Santé et des Solidarités, Direction de l’Hospi-talisation et de l’Organisation des Soins. Circulaire n°DHOS/F1/MTAA/2006/376 relative aux conditions de facturation d’un GHS pour les prises en charge hospitaliéres en zone de surveillance de très courte durée ainsi que pour les prises en charge de moins d’une journée. 2006 August 31; [Google Scholar]
- 9.Levy AR, James D, Johnston KM, Hogg RS, Harrigan PR, Harri-gan BP, et al. The direct costs of HIV/AIDS care. Lancet Infect Dis. 2006;6:171–177. doi: 10.1016/S1473-3099(06)70413-3. [DOI] [PubMed] [Google Scholar]
- 10.Yazdanpanah Y, Goldie SJ, Losina E, Weinstein MC, Lebrun T, Paltiel AD, et al. Lifetime cost of HIV care in France during the era of highly active antiretroviral therapy. Antivir Ther. 2002;7:257–266. [PubMed] [Google Scholar]
- 11.Drummond M, O’Brien B, Stoddart G, Torrance G. Cost analysis. In: Drummond M, O’B B, Sotddart G, Torrance G, editors. Methods for the economic evaluation of a health care program. 2nd ed. Oxford: Oxford University Press; 1997. pp. 52–95. [Google Scholar]
- 12.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 13.Tolley K, Gyldmark M. The treatment and care costs of people with HIV infection or AIDS: development of a standardised cost framework for Europe. Health Policy. 1993;24:55–70. doi: 10.1016/0168-8510(93)90088-7. [DOI] [PubMed] [Google Scholar]
- 14.Yazdanpanah Y, Goldie SJ, Paltiel AD, Losina E, Coudeville L, Weinstein MC, et al. Prevention of human immunodeficiency virus-related opportunistic infections in France: a cost-effectiveness analysis. Clin Infect Dis. 2003;36:86–96. doi: 10.1086/344902. [DOI] [PubMed] [Google Scholar]
- 15.Schackman BR, Scott CA, Walensky RP, Losina E, Freedberg KA, Sax PE. The cost-effectiveness of HLA-B*5701 genetic screening to guide initial antiretroviral therapy for HIV. AIDS. 2008;22:2025–2033. doi: 10.1097/QAD.0b013e3283103ce6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedberg KA, Losina E, Weinstein MC, Paltiel AD, Cohen CJ, Seage GR, et al. The cost effectiveness of combination anti-retroviral therapy for HIV disease. N Engl J Med. 2001;344:824–831. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein MC, Goldie SJ, Losina E, Cohen CJ, Baxter JD, Zhang H, et al. Use of genotypic resistance testing to guide hiv therapy: clinical impact and cost-effectiveness. Ann Intern Med. 2001;134:440–450. doi: 10.7326/0003-4819-134-6-200103200-00008. [DOI] [PubMed] [Google Scholar]
- 18.Walensky RP, Wood R, Ciaranello AL, Paltiel AD, Lorenzana SB, Anglaret X, et al. Scaling up the 2010 World Health Organization HIV Treatment Guidelines in resource-limited settings: a model-based analysis. PLoS Med. 2010;7 doi: 10.1371/journal.pmed.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yazdanpanah Y, Chene G, Losina E, Goldie SJ, Merchadou LD, Alfandari S, et al. Incidence of primary opportunistic infections in two human immunodeficiency virus-infected French clinical cohorts. Int J Epidemiol. 2001;30:864–871. doi: 10.1093/ije/30.4.864. [DOI] [PubMed] [Google Scholar]
- 20.Manuel des groupes homogènes de malades. 10e`me version de la classification, version 10.10 de la fonction groupage. [Accessed 9 February 2011];Fascicule spécial n°2006/3 bis. 2006 March 1; at http://www.atih.sante.fr/?id=000250000EFF.
- 21.Echelle nationale de coûts par GHM, données 2004–2005. [Accessed 9 February 2011]; at http://www.atih.sante.fr/?id=000370000FFF.
- 22.Le programme de médicalisation des systèmes d’information en soins de courte durée: médecine, chirurgie et obstétrique L’algorithme de la classification des GHM. [Accessed 9 February 2011];2006 at http://www.atih.sante.fr/index.php?id=0002300005FF.
- 23.Nomenclature Générale des Actes Professionnels December. [Accessed 8 July 2009];2008 at http://www.ameli.fr/fileadmin/user_upload/documents/NGAP_dec2008.pdf.
- 24.Classification commune des actes médicaux. [Accessed 9 February 2011]; at http://www.ameli.fr/accueil-de-la-ccam/telechargement/historique/index.php.
- 25.Institut de Veille Sanitaire. Surveillance of HIV/AIDS infection in France, 2005. Bulletin Epidemiologique Hebdomadaire. 2006;48:371–378. [Google Scholar]
- 26.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 27.Prise en charge thérapeutique des personnes infectées par le VIH: Rapport 2004. [Accessed 9 February 2011];Flammarion. 2004 http://www.ladocumentationfrancaise.fr/rapports-publics/044000467/index.shtml.
- 28.Tourcoing Hospital Pharmacy. 2008. [Google Scholar]
- 29.Late diagnosis in the HAART era: proposed common definitions and associations with mortality. AIDS. 24:723–727. doi: 10.1097/QAD.0b013e328333fa0f. [DOI] [PubMed] [Google Scholar]
- 30.Cazein F, Pillonel J, Le Strat Y, Lot F, Pinget R, David D, et al. Surveillance de l’infection à VIH-sida en France, 2007. Bulletin Epidemiologique Hebdomadaire. 2008:434–443. [Google Scholar]
- 31.Verpillot E. La régulation du prix des médicaments et le marché des gériques (unpublished) Université de Franche-Comté, Ecole Doctorale Louis Pasteur. 2007 (Accessed at http://artur.univ-fcomte.fr/SJEPG/ECO/these/verpillot_protege.pdf)
- 32.Cooper DA, Steigbigel RT, Gatell JM, Rockstroh JK, Katlama C, Yeni P, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008;359:355–365. doi: 10.1056/NEJMoa0708978. [DOI] [PubMed] [Google Scholar]
- 33.Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, Markowitz M, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 34.Madruga JV, Cahn P, Grinsztejn B, Haubrich R, Lalezari J, Mills A, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-1: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:29–38. doi: 10.1016/S0140-6736(07)61047-2. [DOI] [PubMed] [Google Scholar]
- 35.Lazzarin A, Campbell T, Clotet B, Johnson M, Katlama C, Moll A, et al. Efficacy and safety of TMC125 (etravirine) in treatment-experienced HIV-1-infected patients in DUET-2: 24-week results from a randomised, double-blind, placebo-controlled trial. Lancet. 2007;370:39–48. doi: 10.1016/S0140-6736(07)61048-4. [DOI] [PubMed] [Google Scholar]
- 36.Nelson M, Arasteh K, Clotet B, Cooper DA, Henry K, Katlama C, et al. Durable efficacy of enfuvirtide over 48 weeks in heavily treatment-experienced HIV-1-infected patients in the T-20 versus optimized background regimen only 1 and 2 clinical trials. J Acquir Immune Defic Syndr. 2005;40:404–412. doi: 10.1097/01.qai.0000185314.56556.c3. [DOI] [PubMed] [Google Scholar]
- 37.Reynes J, Arasteh K, Clotet B, Cohen C, Cooper DA, Delfraissy JF, et al. TORO: ninety-six-week virologic and immunologic response and safety evaluation of enfuvirtide with an optimized background of antiretrovirals. AIDS Patient Care STDS. 2007;21:533–543. doi: 10.1089/apc.2006.0174. [DOI] [PubMed] [Google Scholar]
- 38.Yazdanpanah Y, Fagard C, Descamps D, Taburet AM, Colin C, Roquebert B, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009;49:1441–1449. doi: 10.1086/630210. [DOI] [PubMed] [Google Scholar]
- 39.Gulick RM, Lalezari J, Goodrich J, Clumeck N, DeJesus E, Horban A, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 41.Krentz HB, Gill MJ. Cost of medical care for HIV-infected patients within a regional population from 1997 to 2006. HIV Med. 2008;9:721–730. doi: 10.1111/j.1468-1293.2008.00613.x. [DOI] [PubMed] [Google Scholar]
- 42.Merito M, Bonaccorsi A, Pammolli F, Riccaboni M, Baio G, Arici C, et al. Economic evaluation of HIV treatments: The I.CO.N. A cohort study Health Policy. 2005;74:304–313. doi: 10.1016/j.healthpol.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JV, Berger DS, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374:796–806. doi: 10.1016/S0140-6736(09)60918-1. [DOI] [PubMed] [Google Scholar]
- 44.Prevention of cardiovascular disease: Guidelines for assessment and management of cardiovascular risk. [Accessed 9 February 2011]; at http://www.who.int/cardiovascular_diseases/guidelines/Full%20text.pdf.
- 45.Fleishman JA, Yehia BR, Moore RD, Gebo KA. The economic burden of late entry into medical care for patients with HIV infection. Med Care. 48:1071–1079. doi: 10.1097/MLR.0b013e3181f81c4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dépistage de l’infection par le VIH en France: Stratégies et dispositif de dépistage. [Accessed 9 February 2011];2009 October; at http://www.has-sante.fr/portail/upload/docs/application/pdf/2009-10/synthese_depistage_vih_volet_2_vfv_2009-10-21_16-48-3_460.pdf.
- 47.Yazdanpanah Y, Sloan C, Charlois-Ou C, Le Vu S, Semaille C, Costagliola D, et al. Routine HIV screening in France: clinical impact and cost-effectiveness. PloS One. 2010;5 doi: 10.1371/journal.pone.0013132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanoy E, Mary-Krause M, Tattevin P, Perbost I, Poizot-Martin I, Dupont C, et al. Frequency, determinants and consequences of delayed access to care for HIV infection in France. Antivir Ther. 2007;12:89–96. doi: 10.1177/135965350701200111. [DOI] [PubMed] [Google Scholar]
- 49.Cazein F, Pillonel J, Bousquet V, Imounga L, Le Vu S, Le Strat Y, et al. Caractéristiques des personnes diagnostiquées avec une infection à VIH ou un sida, France, 2008. Bulletin Epidemio-logique Hebdomadaire Web. 2009;2 [Google Scholar]
- 50.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 51.Tramarin A, Campostrini S, Postma MJ, Calleri G, Tolley K, Parise N, et al. A multicentre study of patient survival, dis- ability, quality of life and cost of care: among patients with AIDS in northern Italy. Pharmacoeconomics. 2004;22:43–53. doi: 10.2165/00019053-200422010-00004. [DOI] [PubMed] [Google Scholar]
- 52.Basuyau F, Josset V, Merle V, Czernichow P. Case fatality and health care costs in HIV-infected patients: evolution from 1992 to 2000 at Rouen University Hospital, France. Int J STD AIDS. 2004;15:679–684. doi: 10.1177/095646240401501009. [DOI] [PubMed] [Google Scholar]
- 53.Garattini L, Tediosi F, Di Cintio E, Yin D, Parazzini F. Resource utilization and hospital cost of HIV/AIDS care in Italy in the era of highly active antiretroviral therapy. AIDS Care. 2001;13:733–741. doi: 10.1080/09540120120076896. [DOI] [PubMed] [Google Scholar]
- 54.French Hospital Database on HIV (ANRS CO4 FHDH) [Accessed 9 February 2011]; at www.ccde.fr.
- 55.Lanoy E. Doctorat en SantéPublique: Épidémiologie et Sciences de l’information Biomédicale. Universite Pierre et Marie Curie - Paris VI; 2007. Prise en charge tardive des sujets séropositifs pour le virus de l’immunodéficience humaine (unpublished) [Google Scholar]
- 56.Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF emtricitabine, efavirenz vs zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 57.Johnson M, Grinsztejn B, Rodriguez C, Coco J, DeJesus E, Lazzarin A, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple vir-ological failures. AIDS. 2005;19:685–694. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 58.Grinsztejn B, Nguyen BY, Katlama C, Gatell JM, Lazzarin A, Vittecoq D, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369:1261–1269. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 59.Lalezari J, Goodrich J, DeJesus E, Lampiris H, Gulick R, Saag M, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3 study in the US and Canada [abstract 104bLB]. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 60.Cole S, Hernan M, Robins J, Anastos K, Chmiel J, Detels R, et al. Effect of Highly Active Antiretroviral Therapy on Time to AcquiredImmunodeficiency SyndromeorDeath using Marginal Structural Models. Am J Epidemiol. 2003;158:687–694. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]