Abstract
Previous efforts to improve the efficiency of cellular reprogramming for the generation of induced pluripotent stem cells (iPSCs) have focused mainly on transcription factors and small molecule combinations. Here, we report the results of our focus instead on the phenotype of the cells targeted for reprogramming. We find that adult mouse pancreatic tissue stem cells derived by the method of suppression of asymmetric cell kinetics (SACK) acquire increased potency simply by culture under conditions for the production and maintenance of pluripotent stem cells. Moreover, supplementation with the SACK agent xanthine, which promotes symmetric self-renewal, significantly increases the efficiency and degree of acquisition of pluripotency properties. In transplantation analyses, clonal reprogrammed pancreatic stem cells produce slow-growing tumors with tissue derivative of all three embryonic germ layers. This acquisition of pluripotency, without transduction with exogenous transcription factors, supports the concept that tissue stem cells are predisposed to cellular reprogramming, particularly when symmetrically self-renewing.
1. Introduction
Direct reprogramming of somatic cells into pluripotent stem cells (termed induced pluripotent stem cells (iPSCs)) has been achieved by inducing the expression of transcription factors associated with pluripotent cell states and cell transformation [1]. The first successful attempt used over-expression of four transcription factors, Oct4, Sox2, Klf4, and c-Myc, to convert somatic fibroblasts to iPSCs [2–4]. In subsequent studies, iPSCs were generated by overexpressing only the first three of these factors, and combinations using other transcription factors (e.g., Nanog, Lin28, Nr5a2, Esrrb) were also successful [5–8].
Progress in iPSC technology development has faced two main problems: (1) risk of cell transformation and mutation by transduced gene expression constructs and (2) poor efficiency. Transduction of somatic cells with synthetic mRNAs or proteins has addressed the gene transfer problem [9–11]. Other strategies have been the use of one or two of the transcription factors in combination with small molecules to achieve reprogramming [12–14]. On the other hand, the low efficiency of reprogramming continues to present a major challenge, especially with gene-free strategies. With the stochastic model for reprogramming now generally accepted [15], one approach to increasing reprogramming efficiency might be the use of cell populations whose active genetic networks inherently share similarities with those of pluripotent stem cells. Unipotent and multipotent tissue-specific stem cells may have this character.
Previous iPSC studies have focused mainly on transcription factor combinations necessary for the induction and maintenance of a pluripotent state. To the authors' knowledge, the possibility of tissue stem cells being involved as a predisposed population, though considered, previously has not been investigated specifically. In the closest example on the topic, cultures enriched for neural stem cells were shown to be more efficiently reprogrammed than routinely used fibroblast populations [16–18]. However, this property was attributed to the expression of complementing factors in these cells, instead of their tissue stem cell phenotype per se. Other specific investigations of the role of tissue stem cells may have been discouraged by the well-known difficulty of obtaining quantities of these cells sufficient for informative experimental analyses [19].
Here, we report the investigation of readily available adult mouse pancreatic stem cell strains for predisposition for pluripotency reprogramming. These clonal tissue stem cells were expanded by the method of suppression of asymmetric cell kinetics (SACK). The SACK method is based on the concept that tissue stem cells are difficult to expand ex vivo because of their inherent asymmetric self-renewal kinetics [19, 20]. In vivo, asymmetric self-renewal allows tissue stem cells to maintain their own number while continuously replenishing differentiated tissue cells. However, in vitro, the same process leads to the dilution and loss of tissue stem cells among their accumulating lineage-committed progeny cells. The SACK method uses specific guanine ribonucleotide precursors to shift tissue stem cells reversibly from asymmetric self-renewal kinetics to symmetric self-renewal kinetics, which promotes their exponential expansion in culture [21]. We found that, when simply transferred to culture conditions used for pluripotent stem cells, SACK-expanded pancreatic stem cells acquired properties of pluripotent stem cells. Moreover, inclusion of xanthine (Xn), the SACK agent used for their derivation, significantly increased their acquisition of properties characteristic of pluripotency. These findings suggest that targeting symmetrically self-renewing tissue stem cells could significantly increase the efficiency of pluripotency reprogramming when combined with other reprogramming strategies.
2. Materials and Methods
2.1. Cell Culture
Mouse pancreatic cell strain (mPanstra) cells; (see Section 3 for detailed description) were maintained in high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% dialyzed fetal bovine serum (DFBS; GIBCO, Invitrogen, Carlsbad, Calif, USA), 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 mM xanthine (Xn, Sigma Chemical Co., St. Louis, Mo, USA), at 37°C in a humidified incubator with 5% CO2. STO fibroblasts (American Type Culture Collection, Manassas, Va, USA) were maintained in the same media, except the DFBS which was replaced with fetal bovine serum (FBS; Sigma Chemical Co.), and no Xn was added. Mouse embryonic stem cells (mESCs) [22] were cultured, on STO feeder layers inactivated with mitomycin C (Roche Applied Science, Indianapolis, Ind, USA), in high-glucose DMEM supplemented with 20% heat-inactivated FBS (Sigma), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, nonessential amino acids (GIBCO), 50 μM β-mercaptoethanol, and 10 ng/mL leukemia inhibitory factor. Reprogrammed cells were cultured on mitotically inactivated STO feeder layers. The media were either the mPanstra maintenance medium, mESCs medium, or another mESCs medium in which the heat-inactivated serum was replaced with 15% KnockOut Serum Replacement (GIBCO). For each of these reprogramming conditions, one series was maintained without Xn and another with 1 mM Xn. For the initiation of reprogramming, cells were plated at a density of 1.5 × 105 cells per 10 cm diameter dish. Three plates were used for each condition. Two weeks later, cells from one plate were frozen, another plate was used for alkaline phosphatase staining, and isolated colonies were clonally expanded from the remaining plate.
2.2. Indirect In Situ Immunofluorescence (ISIF) Analyses
Cells were fixed for 20 minutes at room temperature with 4% formaldehyde in phosphate-buffered saline (PBS). They were permeabilized by incubating them with 2% bovine serum albumin (BSA), 0.2% dried milk, and 0.4% Triton X-100 in PBS for 10 minutes at room temperature. Cells were blocked for one hour at 4°C with a 3% dilution in PBS of the serum from the source-animal species of the secondary antibody. The rabbit polyclonal anti-Oct4 and mouse monoclonal anti-Klf4 antibodies (Stemgent, Cambridge, Mass, USA) were diluted at 1 : 200 in blocking solution and incubated overnight with the cells at 4°C. Incubation with 1 : 200 dilutions of secondary antibodies (goat polyclonal anti-rabbit-FITC, Santa Cruz Biotechnology Inc., Santa Cruz, Calif, USA; polyclonal rabbit anti-mouse-FITC, Dako, Glostrup, Denmark) was also performed overnight at 4°C. DAPI staining and mounting were performed using DAPI-containing Vectashield mounting medium (Vector Laboratories, Burlingame, Calif, USA).
2.3. Alkaline Phosphatase Assay
Cells were fixed in 4% formaldehyde for two minutes at room temperature and rinsed with TBST (20 mM Tris-HCl, pH 7.4, 0.15 M NaCl, 0.05% Tween-20). They were then incubated, according to the manufacturer's protocol, in a mixture of Fast Red Violet, Naphthol AS-BI phosphate solution, and water from the Alkaline Phosphatase Detection Kit (Millipore, Billerica, Mass, USA). Before observation under the microscope, the cells were rinsed with TBST and covered with PBS. To estimate the alkaline phosphatase-positive (AP+) cell fraction, cells in colonies from representative 40X-magnification microscope fields were counted and evaluated as either AP+ or AP− based on staining intensity. The AP+ fraction was calculated by dividing the AP+ cell number by the total number of cells evaluated.
2.4. Microscopy
Immunofluorescence was viewed using an Imager. Z1 microscope (Zeiss, Gottingen, Germany). Live cells, alkaline phosphatase-tested colonies, and histological sections were viewed using an Observer. A1 microscope (Zeiss). Images were acquired using AxioVision software.
2.5. Immunoblotting
Cell lysates were prepared as previously described [16]. 100 μg of cell lysates from each cell strain was separated by SDS-PAGE (10% polyacrylamide). Proteins were blotted to a Hybond ECL membrane in Tris-glycine-methanol buffer (25 mM Tris-HCl, pH 7.5, 190 mM glycine, 20% methanol) for 2 hours at 120 volts on ice. The membrane was blocked with Tris-buffered saline (TBS) containing 10% dried milk and 0.1% Tween-20 at room temperature for one hour. Polyclonal rabbit anti-Pdx1 antibody (Millipore, Billerica, Mass, USA) was diluted 1 : 5000 in blocking solution and incubated overnight at 4°C with the membrane. Following four 15-minute washes in TBST, the membrane was incubated with ECL anti-rabbit-HRP (GE Healthcare, Waukesha, Wis, USA) diluted at 1 : 2000 in blocking solution. HRP-conjugated antibodies were detected using Amersham ECL Plus Western Blotting Detection System (GE Healthcare), followed by auto-photography.
2.6. Tumor Formation Assays
mPanstra-12 and mPanstra-25 cells, mESCs, and reprogrammed 12-KOSR-Xn-83, 12-KOSR-Xn-89, 25-KOSR-Xn-85, and 25-KOSR-Xn-86 cells were collected by trypsinization followed by three washes with PBS. Viable cell number was determined using a Vi-Cell XR Cell Viability Analyzer (Beckman Coulter, Brea, Calif, USA). For each cell type, two immunodeficient mice (3-month-old nude-Foxn1nu females; Harlan Laboratories) were injected subcutaneously in each hind limb (two injections per mouse, four injections per cell type) with 5 × 106 cells using a 26 G needle. Injected mice were evaluated twice a week for development of tumors. All animal procedures were approved by the BBRI Animal Care and Use Committee.
2.7. Histology
Tumors were dissected and fixed overnight in formalin. They were transferred to 70% ethanol and shipped to Mass Histology Service (Worcester, Mass, USA) for processing, embedding, sectioning (5 μm), and hematoxylin-eosin staining.
3. Background to the Study
Asymmetric self-renewal is a unique feature of tissue stem cells [19]. In adult tissues, tissue stem cells divide asymmetrically, resulting in the production of a new tissue stem cell and a lineage-committed progenitor, the latter having a limited proliferative potential. While this mechanism provides an equilibrium by maintaining a constant number of tissue stem cells in tissues, it limits the ex vivo expansion of tissue stem cells (Figure 1(a)). Asymmetric self-renewal by tissue stem cells in vitro leads to their dilution and eventual loss during serial passage. To overcome this asymmetric cell kinetics barrier, we developed the SACK method [20, 21]. SACK promotes tissue stem cells' expansion by reversibly converting asymmetric self-renewal kinetics to symmetric self-renewal kinetics. Symmetric cell kinetics promotes exponential expansion of tissue stem cells to the exclusion of production of lineage-committed progeny cells. Asymmetric cell kinetics requires a p53-dependent decrease in guanine ribonucleotide biosynthesis, specifically resulting from the repression of inosine-5′-monophosphate dehydrogenase II (IMPDH II) (Figure 1(b)) [23–25]. The SACK method uses specific exogenous guanine ribonucleotide salvage precursors and/or genes that circumvent the biochemical consequences of IMPDH II downregulation by acting either upstream or downstream of the reaction catalyzed by IMPDH II, which is rate limiting for guanine ribonucleotide biosynthesis.
Figure 1.
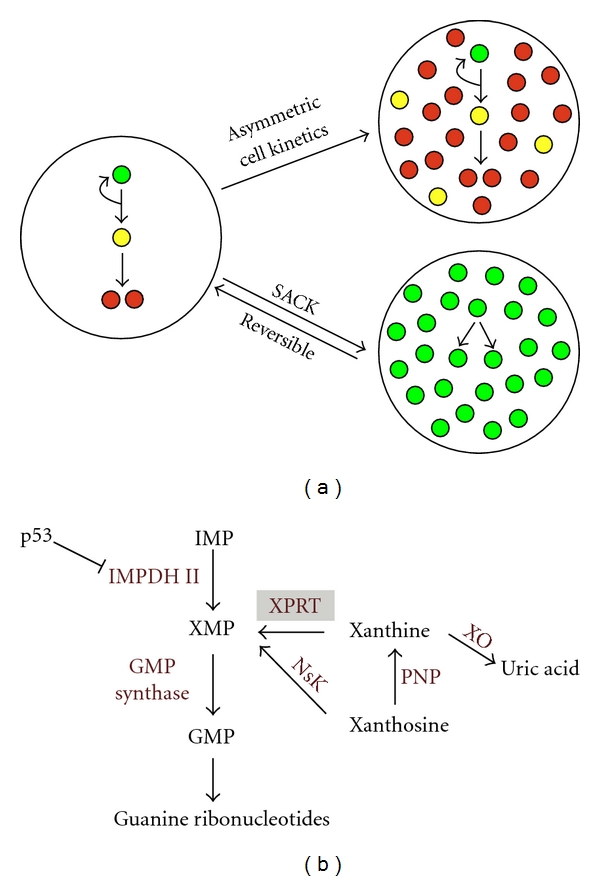
The SACK method for the expansion of tissue stem cells. (a) In vivo, tissue stem cells divide with asymmetric cell kinetics to produce progeny that eventually undergo a terminal cell cycle arrest and differentiate. Asymmetric cell kinetics maintains an equilibrium between renewal of differentiated tissue cells and preservation of constant tissue stem cell number. When tissue stem cells are cultured ex vivo, asymmetric cell kinetics results in their dilution and loss as a consequence of the accumulation of their differentiating progeny cells. The SACK method shifts tissue stem cells to symmetric cell kinetics that reversibly promotes their exponential expansion. Green circles, tissue stem cells; yellow circles, transient-amplifying lineage-committed cells; red circles, terminally arrested differentiated cells. (b) The guanine ribonucleotide biosynthesis pathway that controls tissue stem cells' self-renewal pattern. The rate-limiting step for cellular guanine ribonucleotide biosynthesis is the formation of xanthosine-5′-monophosphate (XMP) from inosine-5′-monophosphate (IMP), the reaction catalyzed by inosine-5′-monophosphate dehydrogenase II (IMPDH II). The asymmetric cell kinetics of tissue stem cells requires downregulation of IMPDH II expression by the tumor suppressor protein p53. Supplementation with exogenous guanine ribonucleotide precursors, like xanthosine, which expands guanine ribonucleotide pools, converts tissue stem cells to symmetric cell kinetics despite normal p53 expression. Cells expressing a xanthine phosphoribosyl transferase (XPRT) transgene can use the highly membrane-permeable precursor xanthine to achieve symmetric cell kinetics. GMP, guanosine monophosphate; NsK, nucleoside kinase; PNP, purine nucleoside phosphorylase; XO, xanthine oxidase.
In the presented study, we used SACK-expanded adult mouse pancreatic stem cell strains derived from transgenic mice expressing the xanthine phosphoribosyl transferase (XPRT) gene [26]. The XPRT gene is absent in mammalians. It catalyzes the conversion of Xn to xanthosine-5′-monophosphate (XMP), the product of the IMPDH II reaction with its substrate inosine-5′-monophosphate (IMP). Xn supplementation allowed expansion of pancreatic stem cells strains from adult XPRT-transgenic mice by promoting guanine ribonucleotide production downstream of the p53-regulation point. Properties of these cells will be reported in detail elsewhere. In brief, they were derived from isolated islets; their proliferation is optimal under Xn-supplementation conditions (i.e., more tissue stem cells are produced), and they have pancreatic endocrine cell differentiation potential. Although the cell strains are clonal, their cultures are composed of a mixture of tissue stem cells, lineage-committed pancreatic progenitor cells, and nonproliferating cells. We refer to these SACK-expanded, clonal, adult (i.e., eight-month old mice) mouse pancreatic stem cells as “mPanstra” strains. Although all mPanstra strains required Xn for their derivation, some are highly dependent on Xn to achieve their maximum proliferative rate (i.e., Xn-dependent, SACK-D[ependent] strains), whereas others maintain high rates of proliferation without Xn supplementation (i.e., Xn-independent, SACK-I[ndependent] strains). SACK-I strains are tumorigenic, but SACK-D strains are not. The SACK-D clones are nontumorigenic pancreatic stem cell strains (manuscript in preparation).
Tissue stem cells share more gene expression networks with pluripotent stem cells than do other somatic cells [27, 28]. We considered that these gene expression similarities, in combination with SACK-induced symmetric self-renewal, might predispose tissue stem cells for greater efficiency in conversion to a pluripotent phenotype. Here, we report evidence that adult mouse pancreatic stem cells derived by the SACK method can be converted to increased tissue potency without the use of exogenous transcription factors. Based on the criterion of formation of teratomas with tissues that originate from all three embryonic germ layers (i.e., endoderm, mesoderm, and ectoderm), the cells attain full pluripotency. We also show that the SACK agent Xn significantly increases the efficiency of SACK-D strains to convert to increased tissue potency. We propose that this ability reflects the common involvement of p53 gene regulation in induced pluripotent stem cell production [29–32] and tissue stem cell self-renewal kinetics [19, 20, 24, 25].
4. Results
4.1. Culture Conditions for Mouse Pluripotent Stem Cells Alter the Growth Kinetics of mPanstra Tissue Stem Cell Strains
Consistent with the concept of some shared gene expression networks between pluripotent stem cells and tissue stem cells, transcription factors used to induce formation of pluripotent stem cells are detected in mPanstra strains. Under Xn-supplementation conditions, two evaluated SACK-D strains, mPanstra-12 and mPanstra-25, show expression of nuclear Klf4 in a subset of cells (Figure 2(a)), while Oct4 expression can only be detected in the mPanstra-12 strain (Figure 2(b)). Although Klf4 detection was weaker, it was specific. STO fibroblast feeder cells in comparison to mESC cultures were negative for Klf4 fluorescence (Figure 2(a)). No Sox2 expression was detected.
Figure 2.
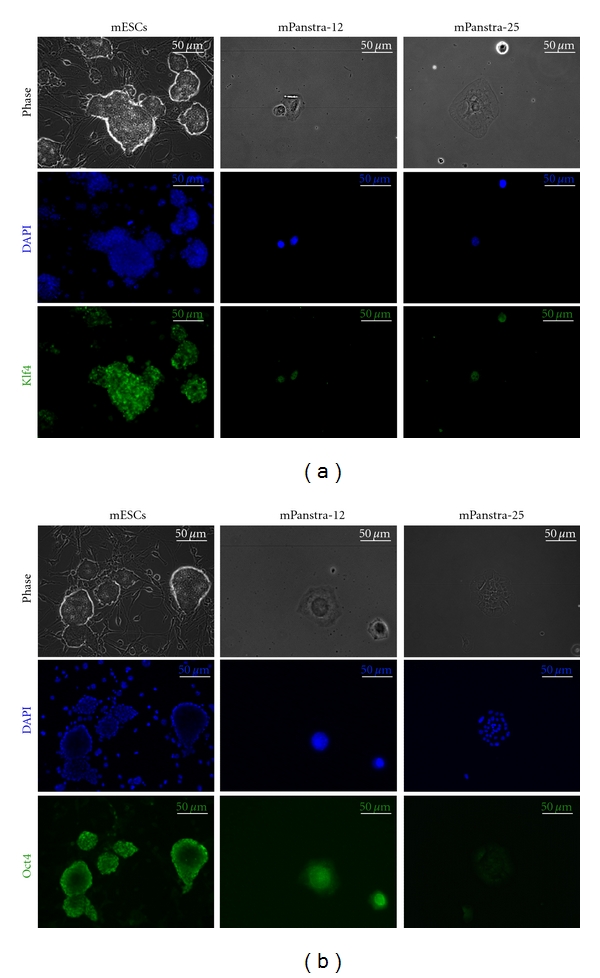
Expression of pluripotency factors in SACK-D mPanstra cells. (a) Nuclear expression of Klf4 was evaluated by indirect in situ immunofluorescence (ISIF) with Klf4-specific antibodies. (b) Nuclear expression of Oct4 was evaluated by ISIF with Oct4-specific antibodies. In each analysis, mouse embryonic stem cells (mESCs) were analyzed as a positive control. DAPI, nuclear DNA fluorescence.
Given their basal expression of genes associated with pluripotency, we investigated whether maintaining mPanstra cells under culture conditions for pluripotent stem cells would promote their conversion to a pluripotent state. Six related culture conditions were evaluated (see Figure 3(a)). All six conditions used gelatin-coated dishes covered with a monolayer of STO fibroblast feeder cells. In the first two conditions, evaluated mPanstra strains were cultured in their own maintenance medium, which is supplemented with dialyzed fetal bovine serum (DFBS), either without or with Xn supplementation (DFBS-Ctrl and DFBS-Xn, resp.). The third and fourth conditions employed culture medium commonly used for the growth of mouse embryonic stem cells (mESCs) that was supplemented with a high concentration of heat-inactivated, nondialyzed FBS and other factors required for the maintenance of pluripotency. This “hiFBS” condition was also evaluated either without or with Xn supplementation (hiFBS-Ctrl and hiFBS-Xn, resp.). The final two conditions were based on a more recently available defined condition used to maintain and grow pluripotent stem cells. Basically, heat-inactivated serum is substituted with KnockOut Serum Replacement (“KOSR”). Again, this medium was evaluated without or with Xn supplementation (KOSR-Ctrl and KOSR-Xn, resp.).
Figure 3.
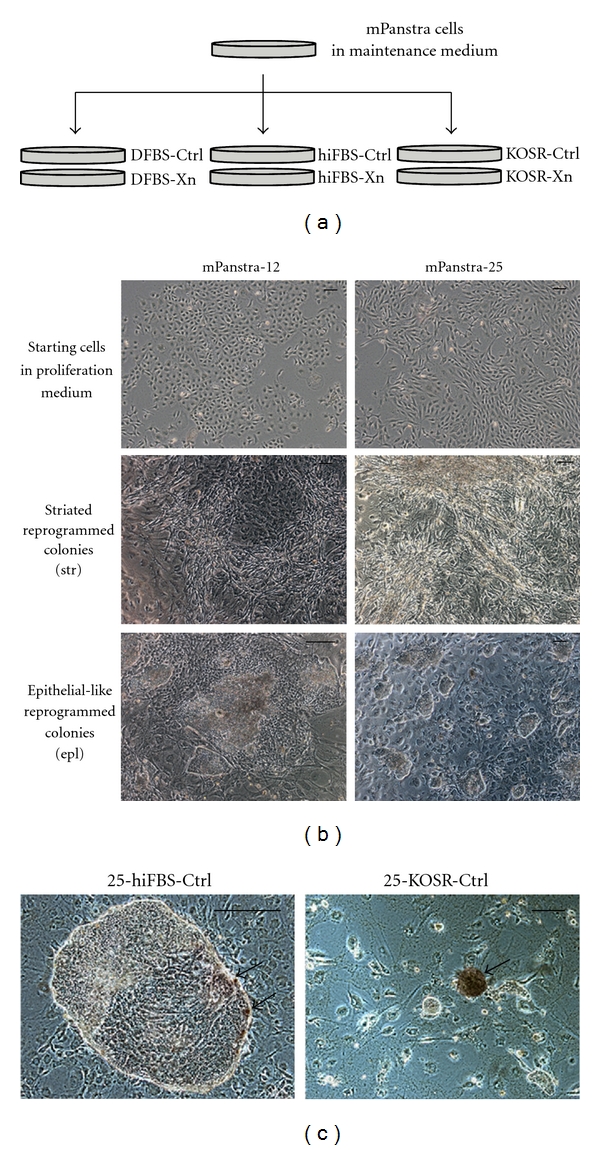
Derivation of reprogrammed colonies by culturing mPanstra cells under pluripotent stem cell culture conditions. (a) mPanstra cells grown in their maintenance medium were transferred to monolayers of STO feeder cells in medium supplemented with dialyzed fetal bovine serum (DFBS), heat-inactivated fetal bovine serum (hiFBS), or KnockOut Serum Replacement (KOSR). Each type of serum supplement was evaluated with (Xn) or without (Ctrl) Xn supplementation. Only SACK-D strains mPanstra-12 and mPanstra-25 formed colonies. (b) SACK-D mPanstra strains (top panel, under routine culture conditions) generate two types of colonies two weeks after transfer into reprogramming conditions: striated (str, middle panels) or epithelial-like (epl, lower panels). (c) A subset of cells from epithelial colonies express alkaline phosphatase (red color, indicated by arrows), a general pluripotency marker. Scale bars = 100 μm.
The most prominent effect noted upon transfer of the mPanstra strains to the six trial reprogramming conditions was a general inhibition of the cell proliferation of SACK-D strains. During the first week of growth, mPanstra cells plated in parallel at the same starting cell number in their routine maintenance conditions attained confluency. In contrast, only the SACK-I strains (mPanstra-11 and mPanstra-56) reached confluency in all reprogramming conditions. The SACK-D strains (mPanstra-12 and mPanstra-25) showed reduced cell proliferation in the reprogramming conditions, and even after two weeks, only cell colonies had formed.
4.2. Under Reprogramming Conditions, SACK-D mPanstra Stem Cell Strains Acquire Phenotypic Properties of Pluripotent Stem Cells
Two weeks after the transfer, two types of colonies were observed for the SACK-D strains in the reprogramming conditions: striated colonies and epithelial-like colonies (Figure 3(b)). The latter morphology resembled pluripotent stem cells. Some colonies developed subsets of cells with alkaline phosphatase activity (red cells in Figure 3(c)), a property of pluripotent stem cells [33].
For each SACK-D strain reprogramming condition, we picked 24 well-isolated colonies for clonal expansion. The proportion of striated versus epithelial colonies picked reflected their initial reprogramming fractions. Following expansion, each subclone was graded for morphology and alkaline phosphatase activity (Figure 4). All the subclones originating from the mPanstra-25 strain and derived in DFBS reprogramming conditions uniformly exhibited the striated morphology, and none of them showed detectable alkaline phosphatase activity (Table 1a). There was a significant shift toward the epithelial morphology in the subclones from the hiFBS conditions, with less than a third of the clones having the striated morphology. In the subclones showing alkaline phosphatase activity, the proportion of positive cells was estimated to vary from 5% to 50% (see example in Figure 4, top right panel). More dramatic was the change in the KOSR reprogramming conditions. Only the subclones with the epithelial morphology could be expanded, and the Xn-supplementation condition had more than twice the proportion of subclones showing alkaline phosphatase activity. Moreover, the proportion of alkaline phosphatase-positive cells was estimated to range from 5% to 60% with Xn supplementation, while never being higher than 20% in its absence.
Figure 4.
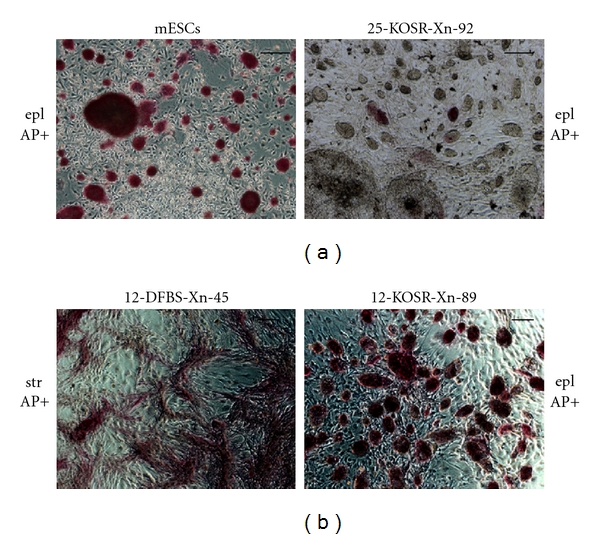
Evaluation of the morphology and alkaline phosphatase activity of expanded reprogrammed colonies of SACK-D strains mPanstra-12 and mPanstra-25. Shown are examples of phase micrographs of colonies developed from reprogrammed cell clones and stained for alkaline phosphatase activity. The estimated alkaline-phosphatase-positive (AP+) cell fractions for colonies with epithelial (epl) or striated (str) morphology were 100% for mESCs, 10% for 25-KOSR-Xn-92 (SACK-D strain #—derivation medium—xanthine supplementation—subclone #), 50% for 12-DFBS-Xn-45, and 90% for 12-KOSR-Xn-89. Scale bars = 100 μm.
Table 1.
(a) Phenotypes of individual reprogrammed subclones from the mPanstra-25 cell strain following expansion in respective reprogramming conditions.
| Condition | Phenotype | |||
| str, AP− | str, AP+ | epl, AP− | epl, AP+ | |
| DFBS-Ctrl | 23/23 (100%) | 0/23 (0%) | 0/23 (0%) | 0/23 (0%) |
| 0.06 | 0.00 | 0.00 | 0.00 | |
| DFBS-Xn | 22/22 (100%) | 0/22 (0%) | 0/22 (0%) | 0/22 (0%) |
| 0.06 | 0.00 | 0.00 | 0.00 | |
| hiFBS-Ctrl | 4/20 (20%) | 1/20 (5%) | 9/20 (45%) | 6/20 (30%) |
| 0.01 | 0.00 | 0.02 | 0.02 | |
| hiFBS-Xn | 5/19 (26%) | 1/19 (5%) | 10/19 (53%) | 3/19 (16%) |
| 0.01 | 0.00 | 0.03 | 0.01 | |
| KOSR-Ctrl | 0/16 (0%) | 0/16 (0%) | 14/16 (87.5%) | 2/16 (12.5%) |
| 0.00 | 0.00 | 0.04 | 0.01 | |
| KOSR-Xn | 0/20 (0%) | 0/20 (0%) | 14/20 (70%) | 6/20 (30%) |
| 0.00 | 0.00 | 0.04 | 0.02 | |
(b) Phenotypes of individual reprogrammed subclones from the mPanstra-12 cell strain following expansion in respective reprogramming conditions.
| Condition | Phenotype | |||
| str, AP− | str, AP+ | epl, AP− | epl, AP+ | |
| DFBS-Ctrl | 8/21 (38%) | 5/21 (24%) | 8/21 (38%) | 0/21 (0%) |
| 0.05 | 0.03 | 0.05 | 0.00 | |
| DFBS-Xn | 12/23 (52%) | 4/23 (17%) | 7/23 (31%) | 0/23 (0%) |
| 0.08 | 0.03 | 0.05 | 0.00 | |
| hiFBS-Ctrl | 18/24 (75%) | 0/24 (0%) | 6/24 (25%) | 0/24 (0%) |
| 0.12 | 0.00 | 0.04 | 0.00 | |
| hiFBS-Xn | 11/19 (58%) | 0/19 (0%) | 5/19 (26%) | 3/19 (16%) |
| 0.07 | 0.00 | 0.03 | 0.02 | |
| KOSR-Ctrl | 4/14 (29%) | 3/14 (21%) | 1/14 (7%) | 6/14 (43%) |
| 0.03 | 0.02 | 0.01 | 0.04 | |
| KOSR-Xn | 1/17 (6%) | 1/17 (6%) | 5/17 (29%) | 10/17 (59%) |
| 0.01 | 0.01 | 0.03 | 0.07 | |
AP−: alkaline phosphatase negative; AP+: alkaline phosphatase positive; epl: epithelial-like; str: striated
The number below each colony phenotype fraction corresponds to the estimated colony formation efficiency as a percentage of the number of cells used to initiate reprogramming cultures (see Figure 3(a)).
In the case of the mPanstra-12 subclones, about a third of these that were derived in the DFBS reprogramming conditions had the epithelial morphology. However, none of these epithelial subclones had detectable alkaline phosphatase activity, even though some of the striated subclones showed activity (Table 1b). In the hiFBS reprogramming conditions, there was no obvious shift from striated to epithelial morphology, but three of the eight epithelial subclones obtained showed alkaline phosphatase activity under conditions for Xn supplementation. For the KOSR reprogramming conditions, we observed a significant shift toward the epithelial morphology with Xn (15 out of 17 expanded clones; Table 1b), with two-thirds of them showing alkaline phosphatase activity. Overall, in KOSR medium, Xn increased the rate of epithelial colony formation by mPanstra-12 subclones 1.8-fold (P = 0.044; two-tailed Fisher's exact test).
The finding that colonies with an epithelial morphology readily developed in DFBS reprogramming conditions only from the mPanstra-12 strain suggested that mPanstra-12 might be a developmentally less committed stem cell strain than the mPanstra-25 strain. In earlier studies, we have found that the SACK method could expand distinct stem cell types from the same tissue [21]. Consistent with this hypothesis, mPanstra-12 cells do not express the early pancreatic marker Pdx1 [34] at detectable protein levels (Figure 5), suggesting that mPanstra-12 is less committed toward a specific differentiation pathway (e.g., pancreatic). Thereby, it could be more susceptible to conversion to increased tissue potency.
Figure 5.
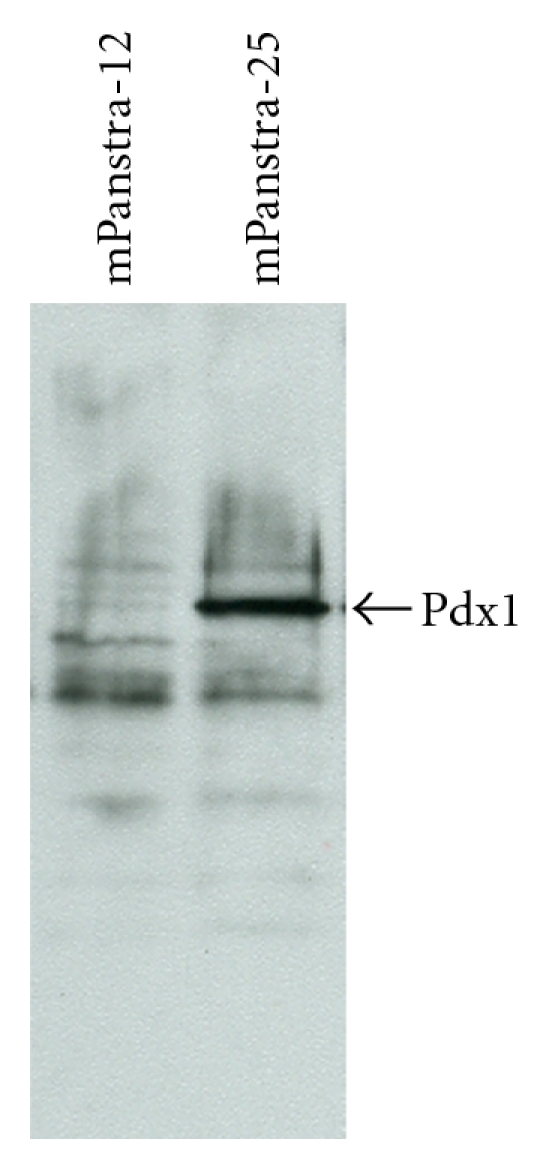
Pdx1 expression in SACK-D mPanstra pancreatic stem cell strains. Immunoblot analyses were performed with whole cell lysates (100 μg protein each) from the indicated mPanstra stem cell strains grown in their maintenance medium. The arrow indicates the 45 kDa Pdx1 protein detected with specific antibodies.
4.3. Reprogrammed Subclones of the SACK-D, Pdx1-Negative mPanstra-12 Strain Produce Teratomas Indicative of Pluripotency
To evaluate the developmental tissue potency of the subclones with epithelial morphology, we assayed them for their capacity to form teratomas following subcutaneous injections into immunodeficient mice. Mouse ESCs and iPSCs are characterized by their ability to form teratomas, that is, tumors that contain differentiated cells originating from the three embryonic germ layers.
For each SACK-D strain, we selected two subclones derived in the KOSR-Xn reprogramming conditions. For both SACK-D mPanstra strains, this was the condition leading to the highest fraction of subclones showing both the epithelial morphology and alkaline phosphatase activity. For each strain, one selected subclone had no detectable alkaline phosphatase activity (AP−; 12-KOSR-Xn-83 and 25-KOSR-Xn-86), whereas the other had the highest proportion of alkaline phosphatase-positive cells (AP+; 12-KOSR-Xn-89 (see Figure 4) and 25-KOSR-Xn-85; 90% and 60%, resp.).
Two mice were injected subcutaneously in their hind limbs with each of the four subclones, respectively (i.e., four injection sites for each subclone). In parallel, two mice were injected similarly with either mESCs or the parental SACK-D mPanstra cell strains. Four weeks after the injections, the mice receiving the mESCs showed tumors at all injection sites. The mESC-derived tumors had histological features of all three embryonic germ layers (Figure 6). Among the other cells injected, only the subclones derived from the mPanstra-12 strain formed small masses (at all injection sites, both AP− and AP+) four months after injection. The growth of these masses was slow, and two additional months were allowed before euthanizing the mice and analyzing the tumors (Figure 7), at which point the other injected mice were also euthanized and their tumor-free status confirmed. Histological analysis of the tumors derived from the reprogrammed mPanstra-12 cells (Figure 6) revealed the presence of tissues of mesodermal (e.g., cartilage (ca) and muscle (mu)), endodermal (e.g., digestive tract epithelium (de)), and ectodermal (e.g., nerves (ne)) origin, confirming acquisition of pluripotency.
Figure 6.
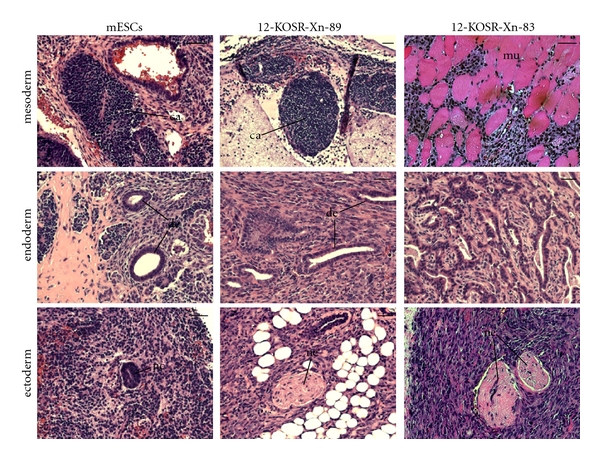
Reprogrammed cell clones derived from SACK-D pancreatic stem cell strain mPanstra-12 form teratomas in immunodeficient mice. Shown are micrographs of hematoxylin-and-eosin-stained tissue sections from subcutaneous tumors formed from mESCs or reprogrammed 12-KOSR-Xn-89 (AP+) and 12-KOSR-Xn-83 (AP−) cell clones. The corresponding embryonic germ layer of each histological example is indicated. ca, cartilage; mu, muscle; de, digestive tract epithelium; nc, neurectoderm; ne, nerve. Scale bars = 50 μm.
Figure 7.
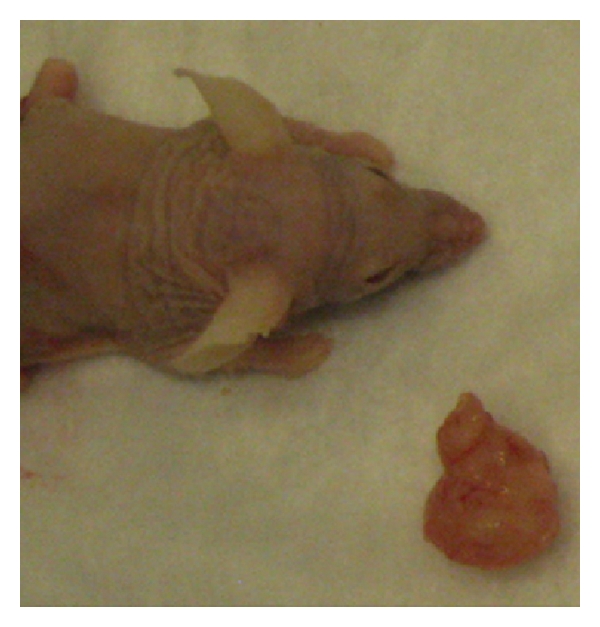
Example of a subcutaneous tumor derived from the 12-KOSR-Xn-89 reprogrammed cells. The photographed tumor (bottom right corner) was excised six months after subcutaneous injection of 5 × 106 cells. A recipient mouse is shown for scale.
5. Discussion
5.1. Pluripotency Reprogramming by Targeting Tissue Stem Cells
Ex vivo-expanded adult mouse pancreatic stem cell strains were evaluated for their ability to acquire phenotypic properties of pluripotent stem cells when transferred into culture conditions permissive for the derivation and maintenance of pluripotent stem cells. Because of the requirement that tissue stem cells maintain an undifferentiated phenotype with respect to the tissue lineages that they replenish, we considered that they might have gene expression networks that were more susceptible to pluripotent reprogramming by environmental factors found in pluripotent cell culture media. Subsequently, we found that the SACK-D mPanstra strains show expression of a subset of transcription factors (Oct4, Klf4) implicated in pluripotency state control mechanisms. This finding advanced further the possibility that these cells were, in fact, predisposed to pluripotency reprogramming.
Supporting this tissue stem cell target hypothesis, both of the evaluated SACK-D mPanstra stem cell strains showed evidence of reprogramming after exposure to pluripotent cell culture conditions. However, only strain mPanstra-12 achieved properties indicative of complete pluripotency conversion. Since the parental SACK-D strains were clonal, did not form tumors themselves, and were of endodermal origin, pluripotency was acquired after the transfer into reprogramming media. The particular differences noted between reprogrammed mPanstra-12 cells and reprogrammed mPanstra-25 cells may account for their difference in teratoma formation. The mPanstra-12 cells showed expression of two pluripotency factors (Klf4 and Oct4) in some cells, whereas only Klf4 was detected in mPanstra-25 cells. Also, when compared with mPanstra-25 cells, expression of the pancreatic determination factor Pdx1 was not detected in mPanstra-12 cells. These properties suggest that the mPanstra-12 cells were already less committed to the pancreatic differentiation pathway and that the gene expression networks necessary for reprogramming were more active, which could explain their increased potency.
Recent studies suggest that partial reprogramming of fibroblasts may provide attractive alternatives for producing cells that can replenish specific tissues [35, 36]. Such strategies highlight the well-recognized, but often understated, flaw in proposed pluripotency-based cell therapies, teratoma formation. The possibility of altering the tissue potency of tissue stem cells simply by transferring them into a defined culture environment underlies another approach to avoiding the inherent risks of pluripotency. In the future, culture environment might be used to instruct plentiful tissue stem cells (e.g., SACK-expanded) to switch their potency to a single tissue type of greater clinical need.
5.2. The Role of Guanine Ribonucleotide Precursors in Pluripotency Reprogramming
KOSR-supplemented medium was the most stringent reprogramming condition used. The presence of Xn in this reprogramming condition increased the frequency of subclones showing epithelial morphology and alkaline phosphatase activity. Xn is known to act on the parental stem cell strains by increasing the rate of symmetric self-renewal over asymmetric self-renewal, their default pattern [20]. Making the target cells adopt a symmetric self-renewal cell division pattern could advance them closer to a pluripotent stem cell phenotype, characterized by symmetric self-renewal (in the cases of mESCs and iPSCs). However, clearly more is at play than just acquisition of a symmetric self-renewal state. SACK-I strains which undergo constitutive symmetric self-renewal were unable to produce reprogrammed cells. The molecular mechanisms responsible for the emergence of SACK-I strains are not fully understood, but it seems most likely that they are pancreatic stem cells that acquired transforming cell mutations, prior to or after their culture. Because no stably propagated adult pancreatic cell strains emerged in the absence of XPRT transgenesis and Xn supplementation, it seems likely that the proposed mutations occur during or after SACK expansion. Although transformed clones like the SACK-I strains develop, the SACK method greatly limits their frequency, so that SACK-D stem cell strains can be obtained with a high efficiency.
Previous studies have established that supplemented purines like Xn regulate the self-renewing pattern of tissue-specific stem cells by increasing guanine ribonucleotide biosynthesis [21]. The upstream step in this pathway is catalyzed by IMPDH II, whose expression is controlled by the p53 tumor suppressor protein. Xn acts downstream of IMPDH II regulation by p53 and, thereby, overcomes the action of p53 on guanine ribonucleotide biosynthesis without altering the action of p53 on other vital cellular functions. Recently, several studies have demonstrated that loss of p53 function was a major factor responsible for more efficient derivation of iPSCs [29–32]. A large part of the effect of p53 loss on iPSC efficiency may be the increased symmetric self-renewal by tissue stem cells in treated cell preparations as a result of increased guanine ribonucleotide biosynthesis.
As noted earlier, among the many challenges to using iPSC-derived cells in transplantation medicine, carcinogenic transformation, beyond the gnomonic teratoma formation of pluripotent stem cells injected into mature tissues, is a major one. Thus, the recent major goal in iPSC research has been achieving complete reprogramming at high efficiency without resorting to gene transduction approaches. Many studies have shown that gene transduction by a variety of strategies can result in insertional mutagenesis that can lead to cellular transformation and neoplasia [3, 37]. Gene transduction was recently circumvented by transducing cells directly with mRNAs encoding reprogramming proteins or reprogramming proteins themselves [9–11]. Also, transduction with only one pluripotency factor in combination with cell culture supplementation with specific small molecules has led to successful reprogramming [12–14]. Adding Xn and/or other exogenous guanine ribonucleotide precursors (e.g., xanthosine, hypoxanthine [38]) to the reprogramming environment could provide an important element to increase iPSC production efficiency. Moreover, the use of starting cell populations containing tissue-specific stem cells undergoing symmetric self-renewal could provide even more efficient production of iPSCs.
Even with gene-free reprogramming, the long assumed inherent cellular transformation risk of pluripotency would still be present. Previously, this risk had been attributed to poor regulation of pluripotent cells by heterologous tissue environments [39, 40]. However, this view may change soon, because, recently, both human ESCs and human iPSCs were discovered to contain many mutations in well-known oncogenes and tumor-suppressor genes, indicating that their production is inherently carcinogenic [41, 42]. The reprogramming process of iPSCs is associated with deletions of tumor suppressor genes, followed by duplications of oncogenes during expansion in culture [42]. The slow tumor development that we observe after culture environment reprogramming of pancreatic tissue stem cells could lead to a safer alternative. Specific tissue stem cell types could prove more sensitive for tissue potency reprogramming and/or less prone to cellular transformation. Of course, recognizing such success will require assays that do not require tumor formation for evaluation. Looking for the ability of reprogrammed cells to replace injured tissues or expand normal tissues of a different embryonic germ layer than their origin is the ideal assay concept. Also, the development of better-defined culture conditions for directing reprogramming could increase the efficiency and decrease the time necessary for reprogramming.
6. Conclusion
We demonstrated that SACK-derived adult pancreatic tissue stem cells could acquire properties of pluripotent stem cells simply by culture under conditions used for production and routine maintenance of the latter. Strain mPanstra-12, the least developmentally committed SACK-D stem cell strain, showed the greatest potency. It was the only one of the two evaluated SACK-D strains that achieved teratoma formation. Xn supplementation significantly increased the efficiency and degree of potency. The combination of pancreatic tissue stem cells and Xn induction of symmetric self-renewal may reflect the same cellular effect as the loss of p53 function for increasing reprogramming efficiency. We predict that starting the reprogramming process with tissue stem-cell-enriched populations, and Xn supplementation (or other exogenous guanine ribonucleotide precursors) will independently and synergistically enhance pluripotent reprogramming into bona fide induced pluripotent stem cells while reducing the rate of emergence of clones with transforming cell mutations.
Acknowledgments
The authors thank the lab of C. E. Emerson, Jr. (Boston Biomedical Research Institute, Watertown, Mass, USA) for graciously providing mESCs, and they are grateful to Dr. S. Takayama (Boston Biomedical Research Institute, Watertown, Mass, USA) for expert histological consultation. This work was supported by a grant from the Charles E. Reed Faculty Initiatives Fund administered by the Massachusetts Institute of Technology (Cambridge, Mass, USA), NIH-NIEHS Grant no. 1R01ES011017, and NIH-NIGMS Director's Pioneer Award no. 5DP1OD000805.
References
- 1.Hanna JH, Saha K, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143(4):508–525. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 4.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 5.Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell. 2008;2(1):10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 7.Heng JC, Feng B, Han J, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6(2):167–174. doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Feng B, Jiang J, Kraus P, et al. Reprogramming of fibroblasts into induced pluripotent stem cells with orphan nuclear receptor Esrrb. Nature Cell Biology. 2009;11(2):197–203. doi: 10.1038/ncb1827. [DOI] [PubMed] [Google Scholar]
- 9.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, Wu S, Joo JY, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4(5):381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nature Biotechnology. 2008;26(11):1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 13.Feng B, Ng JH, Heng JC, Ng HH. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell. 2009;4(4):301–312. doi: 10.1016/j.stem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Ichida JK, Blanchard J, Lam K, et al. A small-molecule inhibitor of Tgf-β signaling replaces Sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5(5):491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460(7251):49–52. doi: 10.1038/nature08180. [DOI] [PubMed] [Google Scholar]
- 16.Kim JB, Zaehres H, Wu G, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454(7204):646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 17.Kim JB, Sebastiano V, Wu G, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136(3):411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 18.Kim JB, Greber B, Arazo-Bravo MJ, et al. Direct reprogramming of human neural stem cells by Oct4. Nature. 2009;461(7264):649–653. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 19.Sherley JL. Asymmetric cell kinetics genes: the key to expansion of adult stem cells in culture. Stem Cells. 2002;20(6):561–572. doi: 10.1634/stemcells.20-6-561. [DOI] [PubMed] [Google Scholar]
- 20.Paré JF, Sherley JL. Biological principles for ex vivo adult stem cell expansion. Current Topics in Developmental Biology. 2006;73:141–171. doi: 10.1016/S0070-2153(05)73005-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee HS, Crane GG, Merok JR, et al. Clonal expansion of adult rat hepatic stem cell lines by suppression of asymmetric cell kinetics (SACK) Biotechnology and Bioengineering. 2003;83(7):760–771. doi: 10.1002/bit.10727. [DOI] [PubMed] [Google Scholar]
- 22.Ai X, Do AT, Kusche-Gullberg M, Labosky PA, Emerson CP., Jr. SULF1 and SULF2 regulate heparan sulfate-mediated GDNF signaling for esophageal innervation. Development. 2007;134(18):3327–3338. doi: 10.1242/dev.007674. [DOI] [PubMed] [Google Scholar]
- 23.Sherley JL. Guanine nucleotide biosynthesis is regulated by the cellular p53 concentration. Journal of Biological Chemistry. 1991;266(36):24815–24828. [PubMed] [Google Scholar]
- 24.Sherley JL, Stadler PB, Johnson DR. Expression of the wild-type p53 antioncogene induces guanine nucleotide- dependent stem cell division kinetics. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(1):136–140. doi: 10.1073/pnas.92.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Bohn SA, Sherley JL. Inosine-5’-monophosphate dehydrogenase is a rate-determining factor for p53-dependent growth regulation. Molecular Biology of the Cell. 1998;9(1):15–28. doi: 10.1091/mbc.9.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jardim A, Bergeson SE, Shih S, et al. Xanthine phosphoribosyltransferase from Leishmania donovani. Molecular cloning, biochemical characterization, and genetic analysis. Journal of Biological Chemistry. 1999;274(48):34403–34410. doi: 10.1074/jbc.274.48.34403. [DOI] [PubMed] [Google Scholar]
- 27.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. Stemness: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298(5593):597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 28.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecutar signature. Science. 2002;298(5593):601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 29.Hong H, Takahashi K, Ichisaka T, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460(7259):1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawamura T, Suzuki J, Wang YV, et al. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460(7259):1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Utikal J, Polo JM, Stadtfeld M, et al. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460(7259):1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marión RM, Strati K, Li H, et al. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460(7259):1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pease S, Braghetta P, Gearing D, Grail D, Williams RL. Isolation of embryonic stem (ES) cells in media supplemented with recombinant leukemia inhibitory factor (LIF) Developmental Biology. 1990;141(2):344–352. doi: 10.1016/0012-1606(90)90390-5. [DOI] [PubMed] [Google Scholar]
- 34.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO Journal. 1993;12(11):4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabo E, Rampalli S, Risueño RM, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468(7323):521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 36.Efe JA, Hilcove S, Kim J, et al. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nature Cell Biology. 2011;13(3):215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- 37.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Sherley JL. Guanine nucleotide biosynthesis is regulated by the cellular p53 concentration. Journal of Biological Chemistry. 1991;266(36):24815–24828. [PubMed] [Google Scholar]
- 39.Baker DE, Harrison NJ, Maltby E, et al. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nature Biotechnology. 2007;25(2):207–215. doi: 10.1038/nbt1285. [DOI] [PubMed] [Google Scholar]
- 40.Maitra A, Arking DE, Shivapurkar N, et al. Genomic alterations in cultured human embryonic stem cells. Nature Genetics. 2005;37(10):1099–1103. doi: 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- 41.Mayshar Y, Ben-David U, Lavon N, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7(4):521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Laurent LC, Ulitsky I, Slavin I, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8(1):106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


