Abstract
The expression of two cardiac myosin heavy chain (MyHC) isoforms in response to the thyroid status was studied in left ventricles (LVs) of Lewis rats. Major MyHC isoform in euthyroid and hyperthyroid LVs had a higher mobility on SDS-PAGE, whereas hypothyroid LVs predominantly contained a MyHC isoform with a lower mobility corresponding to that of the control soleus muscle. By comparing the MyHC profiles obtained under altered thyroid states together with the control soleus, we concluded that MyHCα was represented by the lower band with higher mobility and MyHCβ by the upper band. The identity of these two bands in SDS-PAGE gels was confirmed by western blot and mass spectrometry. Thus, in contrast to the literature data, we found that the MyHCα possessed a higher mobility rate than the MyHCβ isoform. Our data highlighted the importance of the careful identification of the MyHCα and MyHCβ isoforms analyzed by the SDS-PAGE.
1. Introduction
Cardiac muscle contractility and its efficiency depend on myosin heavy chain (MyHC) isoforms being present. Mammalian heart muscle cells express two MyHC gene products, Myh6 and Myh7, which correspond to the α and β isoforms respectively [1]. The molecular masses of the rat α and β isoforms are both about 223 kDa (Rat Gene Database: http://rgd.mcw.edu/), and their amino acid sequences are 93% identical [2]. However, the isoforms differ in their ATPase activity and in their effect on heart contractility. MyHCα represents a “fast myosin” with higher ATPase activity and faster contraction, whereas MyHCβ represents a “slow myosin” with lower ATPase activity and slower contraction [3–5]. In cardiac left ventricles (LVs), the α and β isoforms constitute three functional dimers marked V1, V2, and V3. V1 is the αα homodimer, while V3 is the ββ homodimer, and V2 is the αβ heterodimer. The ATPase activity of these three dimers corresponds to the α and β subunits involved [3, 6, 7]. The variations in the V-type ratio (V1 : V2 : V3) correlate with the heart rate of different species [7, 8]. Fast-contracting ventricles of mice and rats (heart rate > 300/min) have a high proportion of V1, while slow-contracting ventricles of humans and cows (heart rate about 70/min) contain a V3 majority. Rabbit and guinea pig ventricles, with an intermediate speed, consist predominantly of V3 and still possess some V1 and V2 dimers [9]. In vitro experiments showed the difference in the hydrolytic and kinetic characteristics of the two α and β isoforms, which could explain the difference in the economy of force development and the basis for cardiac adaptation mechanism [10, 11]. In general, the experimental evidence suggests that hearts expressing primarily MyHCα isoform have a significantly higher rate of muscle shortening, whereas the hearts expressing mostly MyHCβ possess the ability to generate force with higher efficiency [12].
The amount and changes in the ratio of V dimers have been determined using native gel electrophoresis under nondenaturating conditions, and it was found that the myosin dimers mobility decreases in order V1 > V2 > V3 [6–8, 13–16]. On the other hand, the experiments studying the mobility of individual α and β monomers by the sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) under denaturing conditions showed lower mobility of MyHCα compared to MyHCβ [17–24].
The relative proportion of α and β isoforms in cardiac LVs can be influenced by several physiological and pathophysiological factors like hemodynamic overload, diabetes, or thyroid hormones, and it also corresponds with heart fitness [12]. Thyroid hormones were found to be the most potent regulators of cardiac MyHC gene expression [25]. Increased levels of thyroid hormones stimulate MyHCα (V1) and restrain MyHCβ (V3) expression leading to the hyperthyroid phenotype, while lowered levels of thyroid hormones result in the inhibition of MyHCα (V1) and activation of MyHCβ (V3) expression resulting in the hypothyroid phenotype [26–32]. Alterations to the thyroid hormone levels, in both experimental animals and humans, contribute to various pathological changes including some of the most life-threatening cardiac events, like atrial and ventricular fibrillations or malignant ventricular arrhythmias [33–35]. Changes in the expression of cardiac MyHC isoforms are supposed to be the important aspect of a failing heart [36, 37]. Since many cardiovascular diseases are accompanied by the shift between cardiac α and β MyHC isoforms, the unequivocal determination of both isoforms separated by SDS-PAGE is extremely important, even for human pathology.
In this study, we present data on the MyHC isoforms mobility obtained by SDS-PAGE technique and unambiguous identification of α and β isoforms by mass spectrometry (MALDI TOF/TOF) [38] and western blot (WB) analyses.
2. Material and Methods
2.1. Animals
Male and female 4-week-old inbred Lewis strain rats obtained from an authorized laboratory of the rat-breeding unit of the Institute of Physiology, Academy of Sciences of the Czech Republic, v. v. i., Prague, Czech Republic (accreditation no. 1020/491/A/00) were used for the experiments. Male euthyroid 250 g Wistar rats, obtained from Velaz s.r.o., Prague, Czech Republic, were used for comparison to the experimental Lewis strain rats. The maintenance and handling of the experimental animals were in accordance with the EU Council Directive (86/609EEC) as well as with the local ethical committee guidelines of the Czech Ministry of Agriculture no. 1020/437/A/99. The investigation was approved by the Expert Committee of the Institute of Physiology, Academy of Sciences of the Czech Republic, v. v. i., Prague, Czech Republic.
2.2. Alteration of Thyroid Status
The hyperthyroid status (TH) was induced by intraperitoneal injections of 3,3′,5-triiodo-L-thyronine (sodium salt, T3, 150 μg/kg body weight) three times a week to the 4-week-old animals for 3 to 10 months. The hypothyroid (HY) status was induced with a 0.05% solution of methimazole (2-mercapto-1-methylimidazole) in drinking water given to the rats from the age of 4 weeks for 4 to 11 months. The euthyroid (EU) rats were age-matched littermates of the experimental animals. All the rats analyzed in this study were part of a larger group in which thyroid states were previously checked by the measurement of biochemical and anatomical parameters that are known to be affected by thyroid hormone level alterations [39, 40].
2.3. Tissue Preparation
Cardiac tissue was obtained from adult EU, HY and TH inbred Lewis male and female rats. LVs from Wistar rats were used as a control of the MyHC band position in order to exclude strain differences. The animals were anesthetized with intraperitoneal injections of 1 mL (100 mg) of Narketan (Ketaminum ut hydrochloridum) per 1000 g of body weight, followed by 0.5 mL (10 mg) of the myorelaxant Xylapan (Xylazinum ut hydrochloridum) per 1000 g of body weight (Vetoquinol SA, France, and Vetoquinol Biowet, Poland, resp.). Whole hearts were quickly excised and placed in iced saline solution. LVs were readily isolated and immediately frozen in liquid nitrogen. Control soleus muscles (SOL) were excised immediately after the hearts and further treated with the same procedure described for LVs.
2.3.1. Homogenate Adjustment
The frozen LVs were pulverized in liquid nitrogen using porcelain mortar and homogenized 1 : 40 (w/v ratio) with homogenization buffer (5 M urea, 2 M thiourea, 0.4 M DL-dithiothreitol (DTT), 10 mM sodium pyrophosphate tetrabasic decahydrate) using a glass/glass homogenizer. The LV homogenates were aliquoted and stored at −80°C. Deep frozen tissue treatment and highly denaturating homogenization buffer protected proteins against eventual protease activity.
2.3.2. MyHC Extraction
The MyHC extraction was performed according to Agbulut et al. [41]. The LVs were pulverized in liquid nitrogen using a porcelain mortar. The powder was transferred into iced extraction buffer pH 6.5 containing 300 mM NaCl, 0.1 M NaH2PO4 monohydrate, 50 mM Na2HPO4 anhydrous, 10 mM sodium pyrophosphate, 1 mM MgCl2, 10 mM EDTA, and fresh 1.4 mM mercaptoethanol. MyHC were extracted for 1 hour on ice using four volumes of extraction buffer. The LV extracts were centrifuged at 12000 ×g for 10 minutes at 4°C and the supernatants were diluted 1 : 1 (v/v ratio) with conservation buffer pH 8.5 containing 40 mM sodium pyrophosphate and 50% glycerol, aliquoted and stored at −80°C.
2.4. MyHCs Separation
The SDS-PAGE method was performed on the Mini-PROTEAN Tetra Cell (BioRad) with gel thickness 0.75 mm according to a protocol used by Sant'Ana Pereira et al. [22] for gels running at constant current 13 mA (no pulse) for 7.5 hours. Separating and stacking gels were prepared on the same day as the electrophoresis was run. Separating gels (12% final concentration of acrylamide) contained 10% glycerol (v/v ratio), 12% acrylamide/bisacrylamide solution (the ratio of acrylamide/N,N′-methylenebisacrylamide was 200 : 1), 750 mM 2-amino-2-hydroxymethyl-propane-1,3-diol (TRIS) (pH 9.3), 0.1% sodium dodecyl sulphate (SDS) (w/v ratio), 0.03% ammonium persulphate (APS) (w/v ratio), and 0.14% tetramethylethylenediamine (TEMED). Stacking gels (3.7% final concentration of acrylamide) contained 10% glycerol, 3.7% acrylamide/N,N′-methylene-bisacrylamide (20 : 1 ratio), 125 mM TRIS (pH 6.8), 0.1% SDS, 0.03% APS, and 0.3% TEMED. Electrode buffer was composed of 0.05 M TRIS, 0.38 M glycine, and 0.346 mM SDS. Homogenates were diluted 1 : 1.5 (v/v ratio) with sample buffer (0.125 M TRIS pH 6.8, 0.5 mM EDTA pH 7, 5% SDS, 15 mM DTT, 20% glycerol, 0.1% Bromphenol Blue), LV extracts were diluted 1 : 1 (v/v ratio) with sample buffer, and SOL extracts were diluted 1 : 19 (v/v ratio) with sample buffer. Homogenates were loaded on the gels in the amount of 7 μL per sample (5.5 μg protein), LV extracts in the amount of 15 μL (1 μg protein) per sample, and SOL extracts of 4 μL (2 μg protein) per sample. As a control, we used SOL from hypothyroid animals (HY), containing basically 100% of the slow isoform identical with cardiac MyHCβ. The gels were directly used for WB analyses or stained by a mixture of Coomassie Brilliant Blue and Bismarck Brown R (CBB&BBR) [42] for mass spectrometry and stained by silver nitrate for densitometry quantification (Quantity One Software, BioRad) [43].
2.5. MALDI TOF/TOF Sample Preparation
Each single CBB&BBR stained protein band was cut out from a single lane of the gel, placed to a microtube, and covered with 100 μL 50 mM ammonium bicarbonate (ABC) buffer in 50% acetonitrile (ACN) with 50 mM DTT. All samples were subjected to sonication in an ultrasonic cleaning bath for 5 minutes. After 15 minutes the supernatant was discarded, and the gel was covered with 100 μL of 50 mM ABC/50% ACN with 50 mM iodoacetamide and sonicated for 5 minutes. After 25 minutes, the supernatant was discarded and exchanged for 100 μL 50 mM ABC/50% ACN with 50 mM DTT and sonicated for 5 minutes to remove any excess iodoacetamide. The supernatant was discarded, and the samples were sonicated for 5 minutes in 100 μL of HPLC-grade water. The water was discarded, and samples were sonicated for another 5 minutes in 100 μL of ACN. ACN was discarded, and the microtubes with the samples were left open for a couple of minutes to allow the rest of ACN to evaporate. Then, 5 ng of trypsin (Promega) in 10 μL of 50 mM ABC were added to the gel. The samples were incubated at 37°C overnight. Trifluoroacetic acid (TFA) and ACN were added to the final concentration of 1% TFA, 30% ACN. The samples were sonicated for 10 minutes, and a 0.5 μL drop was transferred onto MALDI targets and let to dry. The dried droplets were covered with a 0.5 μL drop of α-cyano-4-hydroxycinnamic acid solution (2 mg/mL in 80% ACN) and let to dry.
2.6. MALDI TOF/TOF Analysis
Peptide mapping was performed in multiple independent experiments and independent samples. Spectra were acquired in the range of 800–3500 m/z using a 4800 Plus MALDI TOF/TOF Analyzer (Applied Biosystems/MDS Sciex) equipped with a Nd : YAG laser (355 nm, firing rate 200 Hz). Peak lists from the MS spectra were generated by 4000 Series Explorer V 3.5.3 (Applied Biosystems/MDS Sciex) without smoothing, peaks with the local signal-to-noise ratio greater than 5 were picked automatically, deisotoped, and searched with local Mascot v. 2.1 (Matrix Science) against the nonredundant NCBI database of protein sequences as of 06/14/2010 (11186807 sequences; 3815639892 residues). The database search criteria were as follows—enzyme: trypsin, taxonomy: Rattus norvegicus (66703 sequences), fixed modification: carbamidomethylation, variable modification: methionine oxidation, peptide mass tolerance: 80 ppm, and one missed cleavage allowed. Only the hits that were scored by the Mascot software as significant (P < 0.0001) were accepted for further analyses.
2.6.1. Sequences Evaluation
Sequences for cardiac MyHC were obtained from GenBank databases: (MyHCα) P02563 and (MyHCβ) P02564. Sequences were aligned with ClustalW 1.83: http://www.ebi.ac.uk/clustalw/.
2.7. Western Blot and Immunodetection
After SDS-PAGE, gels were quickly washed in ultrapure H2O (mQ H2O) (Millipore-Q system) and equilibrated for 15 min in transfer buffer containing 25 mM TRIS, 192 mM glycine, and 80 mM urea. Subsequently, the proteins were electrotransferred onto nitrocellulose membranes (0.2 μm pore size, Protran BA 83, Whatman) at constant 100 V and 350 mA for 1 hour at 4°C using Mini Trans-Blot (BioRad). The membranes were quickly washed in mQ H2O, dried, wrapped in polypropylene film, and stored overnight at −20°C.
On the following day, the membranes were washed for 15 minutes in 0.05% Tween in Tris-buffered saline (TTBS) solution and blocked for 1 hour at room temperature (RT) in 5% nonfat dry milk in TTBS. To detect α and β isoforms, membranes were incubated with NB300-284 (1 : 1000, Novus Biologicals) or anti-Slow (1 : 1000, provided by BioTrend and Novocastra) antibodies that recognized both cardiac MyHC isoforms on WB membranes; for specific detection of MyHCα, we used the BA.G5 antibody (1 : 5000, a kind gift by Professor S. Schiaffino). Incubations with primary antibodies were performed for 1 hour at RT in the TTBS solution. TTBS washing (3 × 15 minutes) preceded and followed the membranes incubation with secondary anti-mouse IgG (Stabilized Goat Anti-Mouse IgG (H+L) – HRP Conjugated, no. 32430, Thermo Scientific Pierce) diluted 1 : 1000 under the same conditions as the primary antibodies. The membrane signals were detected by enhanced chemiluminescence (ECL) substrate (SuperSignal West Dura Extended Duration Substrate, no. 34076, Thermo Scientific Pierce) and visualized by the LAS-4000 system (Genetica, Fujifilm).
2.8. Statistical Analysis
Obtained data were analyzed by the GraphPad Prism 5 software. A one-way ANOVA and subsequent Student-Newman-Keuls test were used for comparison of differences in normally distributed variables between groups. Nonparametric Mann-Whitney's U-test and Kruskal-Wallis's test were used for a comparison of differences in nonnormally distributed variables between groups. All data are expressed as mean ± SD or as mean ± SEM. The differences were considered as statistically significant when P < 0.05.
3. Results and Discussion
3.1. Separation of Cardiac MyHC Isoforms
The SDS-PAGE of cardiac MyHC isoforms revealed that a major MyHC isoform in EU and TH hearts was that with a greater mobility in polyacrylamide gels, whereas HY LVs contained the predominantly MyHC isoform with a lower mobility corresponding to the mobility of slow-type MyHC1 isoform in control HY SOL (Figures 1(a) and 1(b)). The same results were obtained by homogenate as well as MyHC extract separations. Based on the MyHC profiles observed in LVs at different thyroid states and in the control SOL, we assumed that MyHCα was represented by the lower band with a higher mobility and MyHCβ by the upper band with a lower mobility. The densitometric quantification of the obtained bands supported our assumption, as the TH status was characterized by higher MyHCα expression, whereas the HY status significantly decreased MyHCα expression and increased MyHCβ expression when compared with EU and TH states (Table 1). Since the observed mobilities of α and β isoforms in polyacrylamide gels were quite opposite to those reported in the literature, we decided to recheck our results by WB immunodetection and MALDI TOF/TOF analyses.
Figure 1.

SDS-PAGE separation of cardiac MyHC isoforms from left ventricles of adult euthyroid (EU), hypothyroid (HY), and hyperthyroid (TH) Lewis rats. Control: soleus muscle (SOL) from the hypothyroid rat containing the MyHC1 isoform identical with cardiac MyHCβ. The gel was silver stained (a) or stained by CBB+BBR (b) before MALDI TOF/TOF analysis.
Table 1.
Ratio of cardiac MyHCα/MyHCβ isoforms from left ventricles of adult euthyroid (EU), hyperthyroid (TH), and hypothyroid (HY) Lewis rats. MyHC isoforms were separated by SDS-PAGE, silver-stained, and quantified by densitometric evaluation (Quantity One Software, BioRad). The data are expressed as a mean ± SD, *significantly different from EU and TH, P < 0.05, number of animals = 5.
| Thyroid status | The ratio |
|---|---|
| EU | 3.84 ± 0.72 |
| TH | 4.39 ± 0.63 |
| HY | 0.09 ± 0.05* |
3.2. Identification of α and β MyHC Isoforms by WB Immunodetection and MALDI TOF/TOF Analyses
The WB analysis supported the conclusion based on results obtained by SDS-PAGE. The monoclonal BA.G5 antibody, shown to be specific for rat MyHCα [44], marked only the lower band with a higher mobility in EU and TH samples, while the HY samples and SOL control remained unstained with this antibody (Figure 2(b)). The NB300-284 antibody as well as anti-Slow antibody recognized both α and β isoforms. These antibodies marked the upper band with lower mobility in HY LVs and SOL samples and the lower band in the case of EU and TH rats (Figure 2(a)). The minor bands in all three thyroid states were under the detection limit of the WB method. These results confirm that cardiac LVs of EU and TH rats predominantly contain the MyHCα isoform showing higher mobility, whereas LVs of HY rats contain mainly the isoform MyHCβ with the lower mobility.
Figure 2.
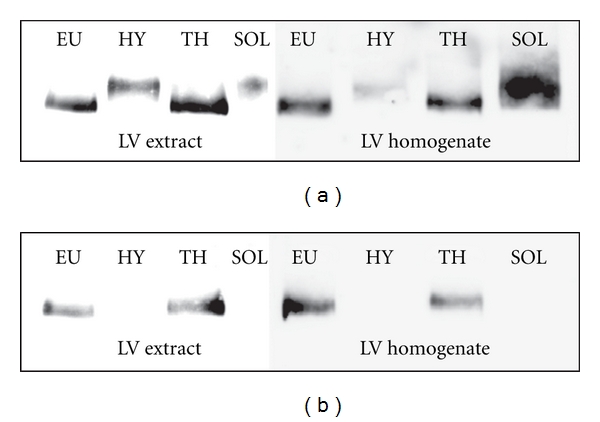
Western blot immunodetection of cardiac MyHC isoforms from left ventricle (LV) homogenates and extracts of adult euthyroid (EU), hypothyroid (HY), and hyperthyroid (TH) Lewis rats. The membranes were stained by NB300-284 antibody recognizing both MyHCα and MyHCβ isoforms (a) or by BA.G5 antibody, which was solely specific for cardiac MyHCα isoform (b).
The results of MALDI TOF/TOF analysis of EU, TH, HY, and SOL samples highly matched the sequences of MyHCα and MyHCβ and unambiguously identified both isoforms. The overall sequence coverage for trypsin digested samples was 52% for MyHCα and 58% for MyHCβ. Further experiments with Lys-C and Asp-N endoproteinases were used to increase the number of MyHCα isoform-specific (proteotypic) peptides and isoform-specific coverage which reached about 40% and 10% using Lys-C and Asp-N digestion, respectively. We highlighted the specific peptides resulting from different protease digestions in multiple sequence alignment (ClustalW 1.83) of MyHCα and MyHCβ (Figure 3). Analysis of HY samples digested by trypsin identified 27 peptides specific for MyHCβ (out of 61 in total). TH sample analysis using trypsin resulted in 14 peptides (out of 41 in total) and using Asp-N protease in 5 peptides specific for MyHCα (out of 13 in total). Analysis of EU samples cleaved by Asp-N endoproteinase identified 22 specific peptides (out of 51 in total) and cleaved by Lys-C endoproteinase identified 16 peptides specific for MyHCα (out of 37 in total). SOL tryptic digestion followed by mass spectrometric identification confirmed the presence of MyHCβ and obtained 27 specific peptides out of a total of 41 total peptides that were homologous with specific peptides of the HY sample. WB immunodetection as well as MALDI TOF/TOF analysis of Wistar EU rats confirmed the same identity of both cardiac MyHC isoforms.
Figure 3.
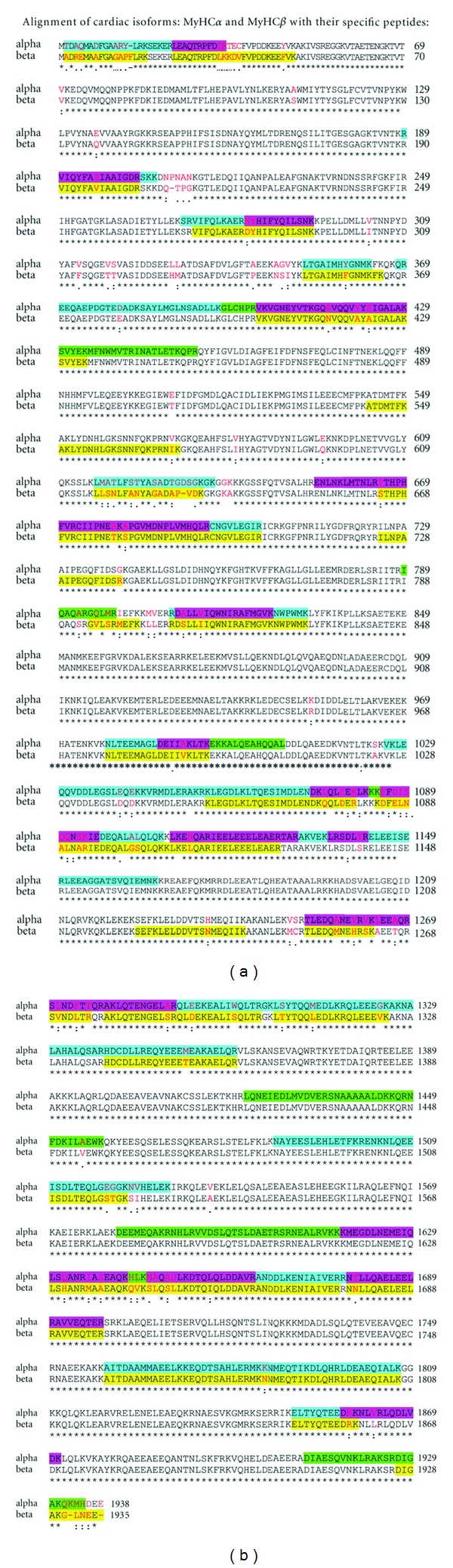
Specific peptides obtained by MALDI TOF/TOF (trypsin, Asp-N, and Lys-C proteases) and highlighted in multiple sequence alignment (ClustalW 1.83) of MyHCα and MyHCβ: yellow: HY MyHCβ, blue: EU MyHCα, green: TH MyHCα, pink: overlap of EU MyHCα and TH MyHCα. Red letters: specific amino acids for each MyHC isoform.
We have unambiguously shown that under our experimental conditions, the isoform with higher mobility in SDS-PAGE gels was the MyHCα, contrary to the prevailing literature data, considering MyHCβ as being the faster isoform.
3.3. Expression of Cardiac MyHC Isoforms under Altered Thyroid Status
The altered level of thyroid hormones is one of the most important pathophysiological factors leading to MyHC transitions and changes in heart contraction. It is generally accepted that the increased levels of thyroid hormones (hyperthyroidism) stimulate MyHCα expression, whereas the decreased levels of thyroid hormones (hypothyroidism) lead to the activation of MyHCβ expression [26–32]. It is obvious from our study that only the LVs of HY rats contain a significant amount of MyHCβ, while for the LVs of EU and TH rats the predominant isoform is the MyHCα.
To estimate the effect of thyroid status on the heart function or fitness of the rats, we measured the heart index (heart mass in mg/body mass in g, expressed as a mean ± SEM). In EU female and male rats, it was 3.23 ± 0.14 (n = 11) and 2.92 ± 0.06 (n = 13), respectively. The TH status led to cardiac hypertrophy, as the heart index significantly increased in female and male rats to 4.64 ± 0.23 (n = 11) and 3.78 ± 0.19 (n = 8), while the HY status resulted in cardiac atrophy, as the index significantly dropped to 2.9 ± 0.05 (n = 16) and 2.58 ± 0.11 (n = 10), respectively. Obviously, the application of thyroid hormones in our experiments corresponds to the physiological hypertrophy induced by chronic exercise and characterized by increased expression of MyHCα [45]. On the other hand, pathological hypertrophy, caused by pressure and volume overload in spontaneous hypertensive rats (SHR), leads to the shift from the MyHCα to the MyHCβ [46]. Thyroid hormone applications as well as physical training by swimming were able to prevent or repair contractile protein abnormalities in pathological hypertrophy in rat hearts [46, 47].
3.4. Electrophoretic Mobility of Cardiac MyHC Isoforms
Our experiments showed unexpected results relating to the mobility of MyHC isoforms. Contrary to the literature data [17–24], we observed higher mobility of the α and lower mobility of the β isoform in polyacrylamide gels. However, these results can be considered reliable, the identity of the MyHC isoforms was successfully verified by WB immunodetection and mass spectrometry (MALDI TOF/TOF) methods. Both analyses showed that MyHCα (no. 62029: 223.508.76 Da; 1939 AA; pI = 5.4500; charge = −28.5; Rat Gene Database) represented by a lower band, moves with a higher mobility in 12% polyacrylamide separating gels than MyHCβ (no. 62030: 222.899.28 Da; 1935 AA; pI = 5.4764; charge = −27; Rat Gene Database), represented by an upper band (Figures 1 and 2). Likewise, the control SOL sample analysis confirmed the position of MyHCβ as the upper band. Nevertheless, the reason for the different band position observed in our experiments remains to be elucidated.
3.4.1. SDS-PAGE Modifications
Factors affecting the electrophoretic mobility of cardiac isoforms appear to have a crucial importance for the observed mobility changes. We tested various concentrations of acrylamide and glycerol and various ratios of acrylamide and bisacrylamide, but all these changes affected only the quality of separation, not the position of the bands.
It is known that the separation and mobility of cardiac myosins by a native electrophoresis depend on several factors, such as the molecular mass, acidic and basic residues [48], and helix content and amino acid composition [49], and on the pH of the separating gel. Unlike the native gel electrophoresis, the SDS-PAGE method under denaturating conditions is supposed to separate cardiac MyHC isoforms only by their molecular mass. The cardiac α and β isoforms have nearly identical molecular masses (~223 kDa), so that their separation is rather difficult. Several recent works have attempted to improve the electrophoretic resolution of the cardiac MyHC isoforms. There were different concentrations of glycerol used: 5−45% [20], separating gels: 6% [24], 7% [21, 23], 8% [20], and 12% [22], and different acrylamide/bisacrylamide ratios: 37.5 : 1 [24], 50 : 1 [20, 21], and 200 : 1 [22]. All the mentioned papers, however, have reported an improvement of separation, but no change in the mobility of the isoforms.
For cardiac MyHC isoform separation, we used the method according to Sant'Ana Pereira et al. [22]: 12% separating gel, 200 : 1 acrylamide/bisacrylamide ratio, constant current 13 mA, temperature 10°C, and run time modified to 7.5 hours (7 hours originally). It is important to note that we used tissue processing according to Agbulut et al. [41] that differs significantly from the procedure used by Sant'Ana Pereira et al. [22] but in both cases the eventual activity of proteases was excluded. Furthermore, the same results were received with tissue homogenates and with MyHC extracts (cf. Figure 2).
The MyHC isoform mobility apparently depends on two forces acting against each other: the resistance and the electrical force. Thus, one of the possible explanations of the unexpected mobility observed might be the influence of the amount of bound SDS. MyHCα, which is slightly heavier, has 4 amino acids more than MyHCβ. Therefore, MyHCα should be covered by more SDS molecules and pulled through the separating gel by a higher electrical force, which is in favor of its higher mobility. The resistance, of the separating gel at given concentration of acrylamide and bisacrylamide, increases with the molecular weight of separated proteins. This factor would then favor the higher mobility of MyHCβ with a lower molecular weight. When these two factors were only taken into account, then the higher mobility of MyHCα could indicate that the electrical force have a greater impact on MyHC mobility than the resistance of the polyacrylamide gel.
3.4.2. Evaluation of Possible Mural Variability
A striking transmural variation of ATPase activity and MyHC isoform distribution was observed in rabbit [50] and rat [51] hearts. For our experiments, however, we have used longitudinal stripes containing epicardial, midwall, and endocardial portions in order to minimize any possible transmural variability.
3.4.3. Possible Strain Differences
To the best of our knowledge, this study was the first analysis of cardiac MyHCs performed on inbred Lewis strain rats. These animals have naturally higher levels of serum thyroxine compared with other routinely used rat strains [52]. Furthermore, in Lewis rats the fiber type compositions of skeletal muscles are very unique. When compared with other rat strains (Wistar, Sprague-Dawley, Fisher 344, WBN/Kob, Lister Hooded, and SHR rats), their soleus muscles were the slowest and their extensor digitorum longus muscles were the fastest [53]. However, we also analyzed cardiac MyHCs by the same methods in EU Wistar rats, and the positions of the MyHCs bands were quite similar to those obtained for the Lewis strain rats. In addition, no differences in cardiac MyHCs mobility among several rat strains in SDS-PAGE gels (Sprague Dawley, Brown Norway, Copenhagen, SHR, and Wistar rats) were seen by Reiser et al. [54]. Strain differences can apparently affect the MyHC isoform ratio in the heart, but they obviously cannot explain the observed switch of the band position of both cardiac isoforms.
4. Conclusions
The data we obtained by SDS-PAGE in the three thyroid states showed that only the left ventricles of HY rats contained a significant amount of MyHCβ, while the left ventricles of EU and TH rats were almost devoid of this isoform, expressing MyHCα as the major isoform. However, the higher mobility of MyHCα compared with that of MyHCβ was contrary to the literature data. Therefore, the identity of both bands was confirmed by western blot immunodetection using specific antibodies (BA.G5 and NB300-284) and by MALDI TOF/TOF that showed the specific peptides resulting from different protease digestions in multiple sequence alignment (ClustalW 1.83) of MyHCα and MyHCβ isoforms. The reasons for the observed unexpected mobility of the isoforms remain still unclear. The general conclusion from our results is that the order of migration of the two cardiac MyHC isoforms must always be verified and not just assumed.
Acknowledgments
The authors very grateful to Professor Stefano Schiaffino for BA.G5 antibody and Dr. Alberto C. Rossi for his help during experiments performed in Padua and for valuable comments during the preparation of the paper. They also thank to Professor Vaclav Pelouch for his kind advice during the experiments and to Bc. Eva Zikmundova for her excellent technical assistance. The study was supported by the Ministry of Education of the Czech Republic (Grant no. MSM0021620858), the Grant Agency of Academy of Sciences of the Czech Republic (Grant no. IAAX01110901), the Grant Agency of the Czech Republic (Grant no. 304/08/0256), and by the Research project AV0Z 50110509.
References
- 1.Mahdavi V, Periasamy M, Nadal-Ginard B. Molecular characterization of two myosin heavy chain genes expressed in the adult heart. Nature. 1982;297(5868):659–664. doi: 10.1038/297659a0. [DOI] [PubMed] [Google Scholar]
- 2.McNally EM, Kraft R, Bravo-Zehnder M, Taylor DA, Leinwand LA. Full-length rat alpha and beta cardiac myosin heavy chain sequences. Comparisons suggest a molecular basis for functional differences. Journal of Molecular Biology. 1989;210(3):665–671. doi: 10.1016/0022-2836(89)90141-1. [DOI] [PubMed] [Google Scholar]
- 3.Pope B, Hoh JF, Weeds A. The ATPase activities of rat cardiac myosin isoenzymes. FEBS Letters. 1980;118(2):205–208. doi: 10.1016/0014-5793(80)80219-5. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz K, Lecarpentier Y, Martin JL. Myosin isoenzymic distribution correlates with speed of myocardial contraction. Journal of Molecular and Cellular Cardiology. 1981;13(12):1071–1075. doi: 10.1016/0022-2828(81)90297-2. [DOI] [PubMed] [Google Scholar]
- 5.Ebrecht G, Rupp H, Jacob R. Alterations of mechanical parameters in chemically skinned preparations of rat myocardium as a function of isoenzyme pattern of myosin. Basic Research in Cardiology. 1982;77(2):220–234. doi: 10.1007/BF01908175. [DOI] [PubMed] [Google Scholar]
- 6.Hoh JFY, McGrath PA, Hale PT. Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. Journal of Molecular and Cellular Cardiology. 1978;10(11):1053–1076. doi: 10.1016/0022-2828(78)90401-7. [DOI] [PubMed] [Google Scholar]
- 7.Lompre AM, Mercadier JJ, Wisnewsky C. Species- and age-dependent changes in the relative amounts of cardiac myosin isoenzymes in mammals. Developmental Biology. 1981;84(2):286–290. doi: 10.1016/0012-1606(81)90396-1. [DOI] [PubMed] [Google Scholar]
- 8.Clark WA, Chizzonite RA, Everett AW. Species correlations between cardiac isomyosins. A comparison of electrophoretic and immunological properties. The Journal of Biological Chemistry. 1982;257(10):5449–5454. [PubMed] [Google Scholar]
- 9.Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiological Reviews. 1986;66(3):710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- 10.Sugiura S, Kobayakawa N, Fujita H, et al. Comparison of unitary displacements and forces between 2 cardiac myosin isoforms by the optical trap technique: molecular basis for cardiac adaptation. Circulation Research. 1998;82(10):1029–1034. doi: 10.1161/01.res.82.10.1029. [DOI] [PubMed] [Google Scholar]
- 11.Vanburen P, Harris DE, Alpert NR, Warshaw DM. Cardiac V1 and V3 myosins differ in their hydrolytic and mechanical activities in vitro. Circulation Research. 1995;77(2):439–444. doi: 10.1161/01.res.77.2.439. [DOI] [PubMed] [Google Scholar]
- 12.Gupta MP. Factors controlling cardiac myosin-isoform shift during hypertrophy and heart failure. Journal of Molecular and Cellular Cardiology. 2007;43(4):388–403. doi: 10.1016/j.yjmcc.2007.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.d’Albis A, Pantaloni C, Bechet JJ. An electrophoretic study of native myosin isozymes and of their subunit content. European Journal of Biochemistry. 1979;99(2):261–272. doi: 10.1111/j.1432-1033.1979.tb13253.x. [DOI] [PubMed] [Google Scholar]
- 14.Lompre AM, Schwartz K, d’Albis A. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979;282(5734):105–107. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- 15.Mercadier JJ, Lompre AM, Wisnewsky C. Myosin isoenzymic changes in several models of rat cardiac hypertrophy. Circulation Research. 1981;49(2):525–532. doi: 10.1161/01.res.49.2.525. [DOI] [PubMed] [Google Scholar]
- 16.Rupp H, Maisch B. Separation of large mammalian ventricular myosin differing in ATPase activity. Canadian Journal of Physiology and Pharmacology. 2007;85(3-4):326–331. doi: 10.1139/y07-032. [DOI] [PubMed] [Google Scholar]
- 17.Esser KA, Boluyt MO, White TP. Separation of cardiac myosin heavy chains by gradient SDS-PAGE. American Journal of Physiology. 1988;255(3):H659–H663. doi: 10.1152/ajpheart.1988.255.3.H659. [DOI] [PubMed] [Google Scholar]
- 18.Caforio ALP, Grazzini M, Mann JM, et al. Identification of α- and β-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation. 1992;85(5):1734–1742. doi: 10.1161/01.cir.85.5.1734. [DOI] [PubMed] [Google Scholar]
- 19.Sweitzer NK, Moss RL. Determinants of loaded shortening velocity in single cardiac myocytes permeabilized with α-hemolysin. Circulation Research. 1993;73(6):1150–1162. doi: 10.1161/01.res.73.6.1150. [DOI] [PubMed] [Google Scholar]
- 20.Reiser PJ, Kline WO. Electrophoretic separation and quantitation of cardiac myosin heavy chain isoforms in eight mammalian species. American Journal of Physiology. 1998;274(3):H1048–H1053. doi: 10.1152/ajpheart.1998.274.3.H1048. [DOI] [PubMed] [Google Scholar]
- 21.Mansén A, Yu F, Forrest D, Larsson L, Vennström B. TRs have common and isoform-specific functions in regulation of the cardiac myosin heavy chain genes. Molecular Endocrinology. 2001;15(12):2106–2114. doi: 10.1210/mend.15.12.0735. [DOI] [PubMed] [Google Scholar]
- 22.Sant’Ana Pereira JAA, Greaser M, Moss RL. Pulse electrophoresis of muscle myosin heavy chains in sodium dodecyl sulfate-polyacrylamide gels. Analytical Biochemistry. 2001;291(2):229–236. doi: 10.1006/abio.2001.5018. [DOI] [PubMed] [Google Scholar]
- 23.Piao S, Yu F, Mihm MJ, et al. A simplified method for identification of human cardiac myosin heavy-chain isoforms. Biotechnology and Applied Biochemistry. 2003;37(1):27–30. doi: 10.1042/ba20020076. [DOI] [PubMed] [Google Scholar]
- 24.Warren CM, Greaser ML. Method for cardiac myosin heavy chain separation by sodium dodecyl sulfate gel electrophoresis. Analytical Biochemistry. 2003;320(1):149–151. doi: 10.1016/s0003-2697(03)00350-6. [DOI] [PubMed] [Google Scholar]
- 25.Izumo S, Nadal-Ginard B, Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- 26.Lompre AM, Nadal-Ginard B, Mahdavi V. Expression of the cardiac ventricular α- and β-myosin heavy chain genes is developmentally and hormonally regulated. The Journal of Biological Chemistry. 1984;259(10):6437–6446. [PubMed] [Google Scholar]
- 27.Morkin E, Flink IL, Goldman S. Biochemical and physiologic effects of thyroid hormone on cardiac performance. Progress in Cardiovascular Diseases. 1983;25(5):435–464. doi: 10.1016/0033-0620(83)90004-x. [DOI] [PubMed] [Google Scholar]
- 28.Morkin E. Control of cardiac myosin heavy chain gene expression. Microscopy Research and Technique. 2000;50(6):522–531. doi: 10.1002/1097-0029(20000915)50:6<522::AID-JEMT9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 29.Ojamaa K, Klein I. In vivo regulation of recombinant cardiac myosin heavy chain gene expression by thyroid hormone. Endocrinology. 1993;132(3):1002–1006. doi: 10.1210/endo.132.3.8440168. [DOI] [PubMed] [Google Scholar]
- 30.Fletcher AK, Weetman AP. Hypertension and hypothyroidism. Journal of Human Hypertension. 1998;12(2):79–82. doi: 10.1038/sj.jhh.1000574. [DOI] [PubMed] [Google Scholar]
- 31.Stevenson LW. Beta-blockers for stable heart failure. The New England Journal of Medicine. 2002;346(18):1346–1347. doi: 10.1056/NEJM200205023461802. [DOI] [PubMed] [Google Scholar]
- 32.Danzi S, Klein S, Klein I. Differential regulation of the myosin heavy chain genes α and β in rat atria and ventricles: role of antisense RNA. Thyroid. 2008;18(7):761–768. doi: 10.1089/thy.2008.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danzi S, Klein I. Thyroid hormone and the cardiovascular system. Minerva Endocrinologica. 2004;29(3):139–150. [PubMed] [Google Scholar]
- 34.Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocrine Reviews. 2005;26(5):704–728. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- 35.Tribulova N, Knezl V, Shainberg A, Seki S, Soukup T. Thyroid hormones and cardiac arrhythmias. Vascular Pharmacology. 2010;52(3-4):102–112. doi: 10.1016/j.vph.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circulation Research. 2000;86(4):386–390. doi: 10.1161/01.res.86.4.386. [DOI] [PubMed] [Google Scholar]
- 37.Reiser PJ, Portman MA, Ning XH, Moravec CS. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. American Journal of Physiology. 2001;280(4):H1814–H1820. doi: 10.1152/ajpheart.2001.280.4.H1814. [DOI] [PubMed] [Google Scholar]
- 38.Helmke SM, Yen CY, Cios KJ, et al. Simultaneous quantification of human cardiac alpha- and beta-myosin heavy chain proteins by MALDI-TOF mass spectrometry. Analytical Chemistry. 2004;76(6):1683–1689. doi: 10.1021/ac035144l. [DOI] [PubMed] [Google Scholar]
- 39.Soukup T, Zachařová G, Smerdu V, Jirmanová I. Body, heart, thyroid gland and skeletal muscle weight changes in rats with altered thyroid status. Physiological Research. 2001;50(6):619–626. [PubMed] [Google Scholar]
- 40.Rauchová H, Mráček T, Novák P, Vokurková M, Soukup T. Glycerol-3-phosphate dehydrogenase expression and oxygen consumption in liver mitochondria of female and male rats with chronic alteration of thyroid status. Hormone and Metabolic Research. 2011;43(1):43–47. doi: 10.1055/s-0030-1265220. [DOI] [PubMed] [Google Scholar]
- 41.Agbulut O, Li Z, Mouly V, Butler-Browne GS. Analysis of skeletal and cardiac muscle from desmin knock-out and normal mice by high resolution separation of myosin heavy-chain isoforms. Biology of the Cell. 1996;88(3):131–135. [PubMed] [Google Scholar]
- 42.Choi JK, Yoon SH, Hong HY, Choi DK, Yoo GS. A modified Coomassie blue staining of proteins in polyacrylamide gels with Bismark brown R. Analytical Biochemistry. 1996;236(1):82–84. doi: 10.1006/abio.1996.0134. [DOI] [PubMed] [Google Scholar]
- 43.Blum H, Beier H, Gross HJ. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:33–99. [Google Scholar]
- 44.Rudnicki MA, Jackowski G, Saggin L, McBurney MW. Actin and myosin expression during development of cardiac muscle from cultured embryonal carcinoma cells. Developmental Biology. 1990;138(2):348–358. doi: 10.1016/0012-1606(90)90202-t. [DOI] [PubMed] [Google Scholar]
- 45.Rupp H. Differential effect of physical exercise routines on ventricular myosin and peripheral catecholamine stores in normotensive and spontaneously hypertensive rats. Circulation Research. 1989;65(2):370–377. doi: 10.1161/01.res.65.2.370. [DOI] [PubMed] [Google Scholar]
- 46.Scheuer J, Malhotra A, Hirsch C. Physiologic cardiac hypertrophy corrects contractile protein abnormalities associated with pathologic hypertrophy in rats. The Journal of Clinical Investigation. 1982;70(6):1300–1305. doi: 10.1172/JCI110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang B, Ouyang J, Xia Z. Effects of triiodo-thyronine on angiotensin-induced cardiomyocyte hypertrophy: reversal of increased β-myosin heavy chain gene expression. Canadian Journal of Physiology and Pharmacology. 2006;84(8-9):935–941. doi: 10.1139/y06-043. [DOI] [PubMed] [Google Scholar]
- 48.Yazaki Y, Raben MS. Effect of the thyroid state on the enzymatic characteristics of cardiac myosin. A difference in behavior of rat and rabbit cardiac myosin. Circulation Research. 1975;36(1):208–215. doi: 10.1161/01.res.36.1.208. [DOI] [PubMed] [Google Scholar]
- 49.Thyrum PT, Kritcher EM, Luchi RJ. Effect of l-thyroxine on the primary structure of cardiac myosin. Biochimica et Biophysica Acta. 1970;197(2):335–336. doi: 10.1016/0005-2728(70)90048-4. [DOI] [PubMed] [Google Scholar]
- 50.Eisenberg BR, Edwards JA, Zak R. Transmural distribution of isomyosin in rabbit ventricle during maturation examined by immunofluorescence and staining for calcium-activated adenosine triphosphatase. Circulation Research. 1985;56(4):548–555. doi: 10.1161/01.res.56.4.548. [DOI] [PubMed] [Google Scholar]
- 51.Bugaisky LB, Anderson PG, Hall RS, Bishop SP. Differences in myosin isoform expression in the subepicardial and subendocardial myocardium during cardiac hypertrophy in the rat. Circulation Research. 1990;66(4):1127–1132. doi: 10.1161/01.res.66.4.1127. [DOI] [PubMed] [Google Scholar]
- 52.Esber HJ, Menninger FF, Bogden AE. Variation in serum hormone concentrations in different rat strains. Proceedings of the Society for Experimental Biology and Medicine. 1974;146(4):1050–1053. doi: 10.3181/00379727-146-38244. [DOI] [PubMed] [Google Scholar]
- 53.Novák P, Zacharová G, Soukup T. Individual, age and sex differences in fiber type composition of slow and fast muscles of adult lewis rats: comparison with other rat strains. Physiological Research. 2010;59(5):783–801. doi: 10.33549/physiolres.931827. [DOI] [PubMed] [Google Scholar]
- 54.Reiser PJ, Wick M, Pretzman CI. Electrophoretic variants of cardiac myosin heavy chain-α in Sprague Dawley rats. Electrophoresis. 2004;25(3):389–395. doi: 10.1002/elps.200305709. [DOI] [PubMed] [Google Scholar]


