Abstract
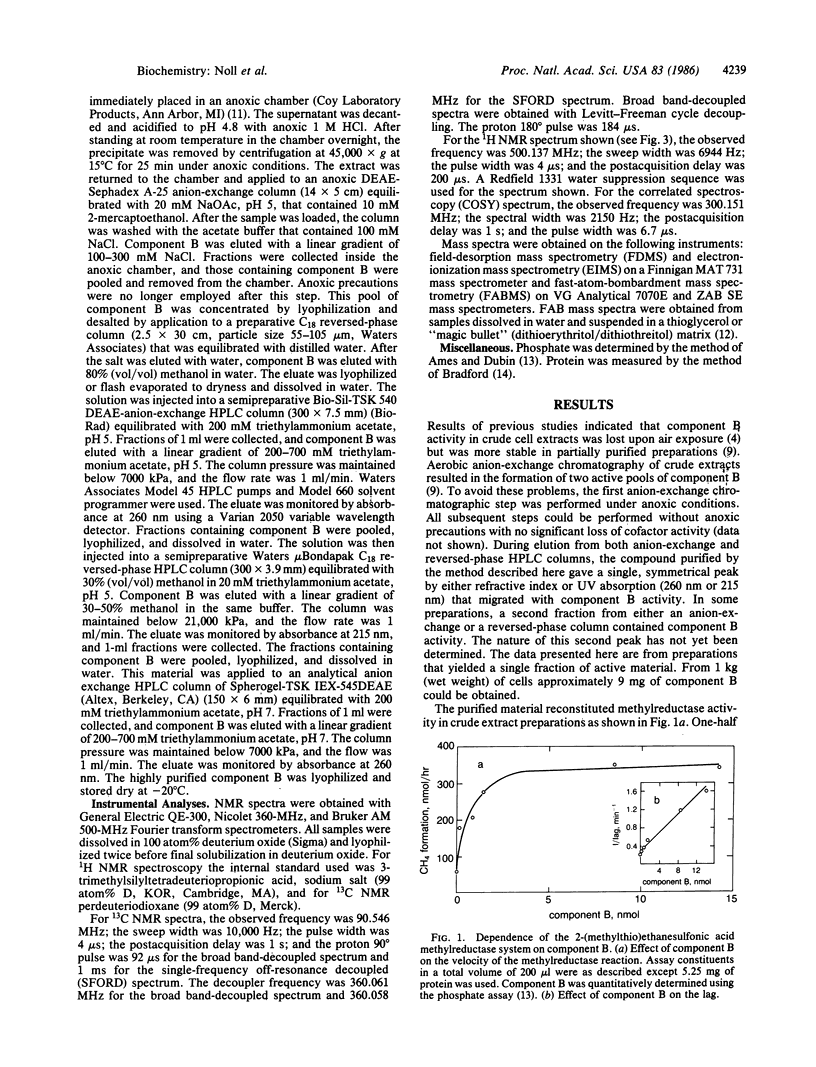
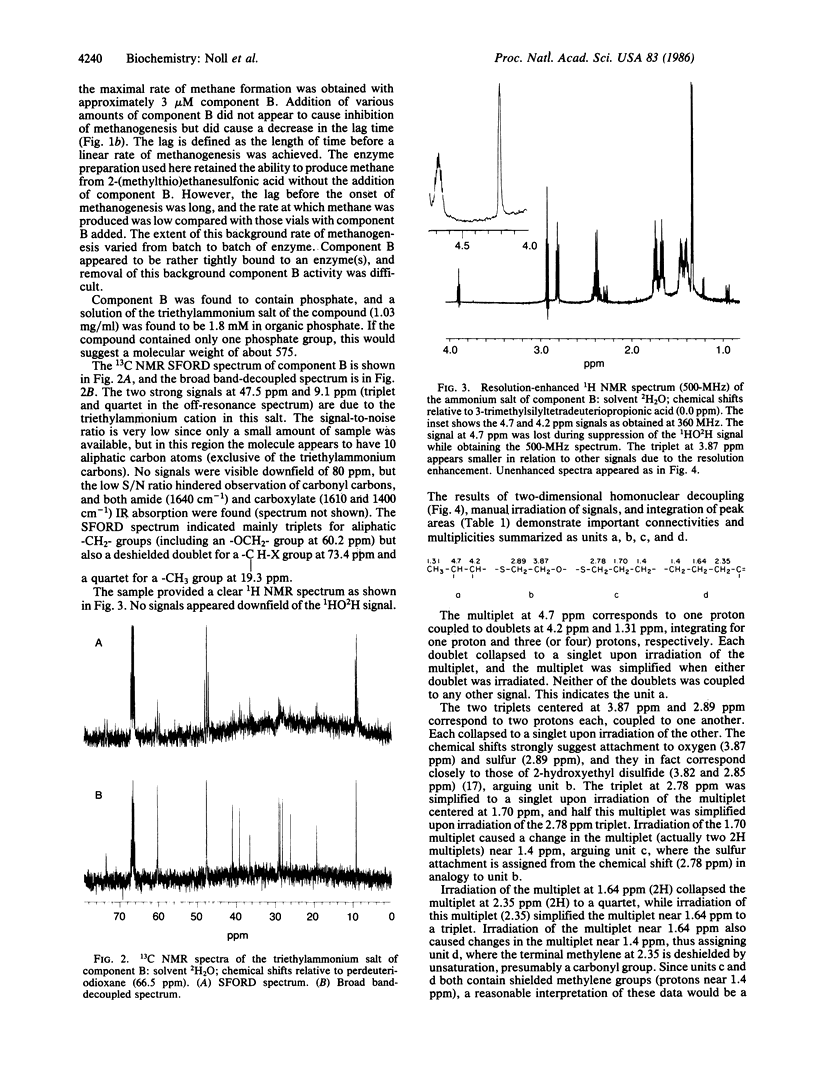
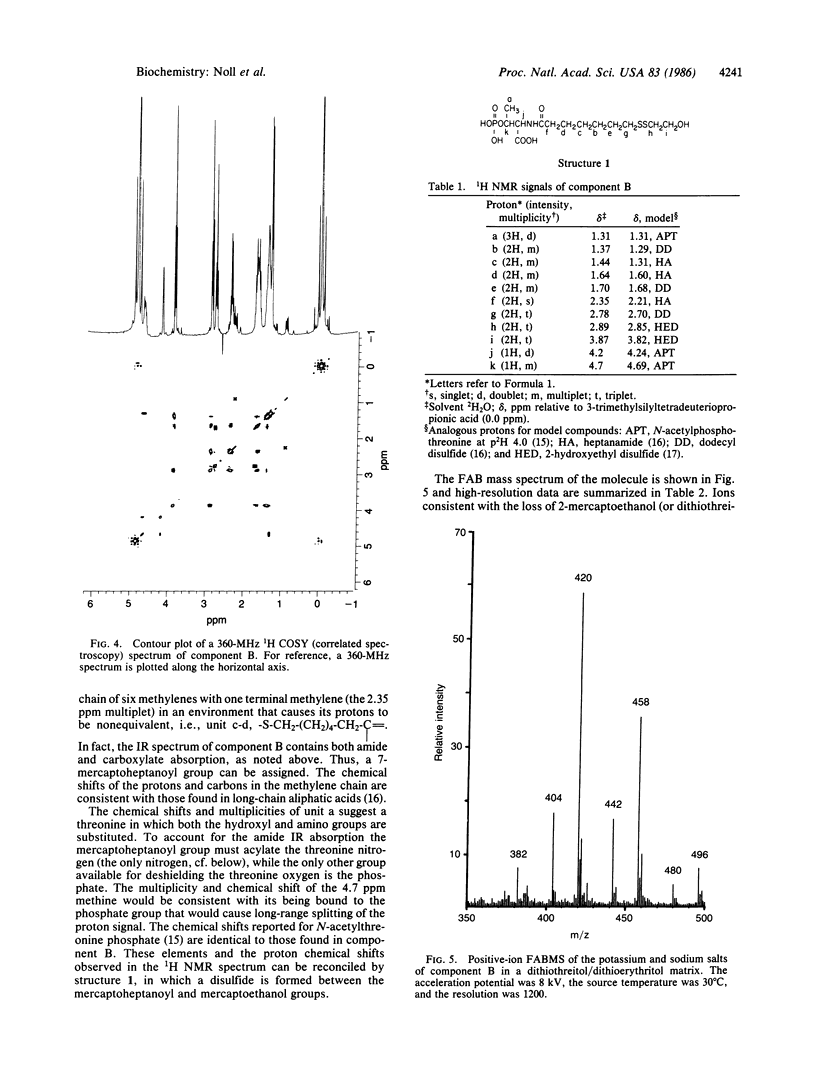
Component B, the heat-stable low-molecular-weight cofactor required for methane production by dialyzed cell-free extracts of Methanobacterium thermoautotrophicum, has been purified to homogeneity and its structure assigned. Results of low-resolution fast-atom-bombardment and field-desorption mass spectrometry indicated a molecular weight of 419, and high-resolution fast-atom-bombardment mass spectrometry agreed with the molecular formula C13H26NO8PS2. Evidence from fast-atom-bombardment and field-desorption mass spectrometry and 360-MHz 1H NMR in deuterium oxide argued that the compound was isolated as a mixed disulfide with 2-mercaptoethanol; so the proposed elemental formula of the free acid, free thiol would be C11H22NO7PS (molecular weight, 343). The proposed structure for an active form of the coenzyme is 7-mercaptoheptanoylthreonine phosphate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ellefson W. L., Whitman W. B., Wolfe R. S. Nickel-containing factor F430: chromophore of the methylreductase of Methanobacterium. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3707–3710. doi: 10.1073/pnas.79.12.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Tandon S. M., Wolfe R. S. A procedure for anaerobic column chromatography employing an anaerobic Freter-type chamber. Anal Biochem. 1980 Jan 15;101(2):327–331. doi: 10.1016/0003-2697(80)90195-5. [DOI] [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Methyl coenzyme M reductase from Methanobacterium thermoautotrophicum. Resolution and properties of the components. J Biol Chem. 1980 Mar 10;255(5):1891–1895. [PubMed] [Google Scholar]
- Gunsalus R. P., Wolfe R. S. Stimulation of CO2 reduction to methane by methylcoenzyme M in extracts Methanobacterium. Biochem Biophys Res Commun. 1977 Jun 6;76(3):790–795. doi: 10.1016/0006-291x(77)91570-4. [DOI] [PubMed] [Google Scholar]
- Leigh J. A., Wolfe R. S. Carbon dioxide reduction factor and methanopterin, two coenzymes required for CO2 reduction to methane by extracts of Methanobacterium. J Biol Chem. 1983 Jun 25;258(12):7536–7540. [PubMed] [Google Scholar]
- Nagle D. P., Jr, Wolfe R. S. Component A of the methyl coenzyme M methylreductase system of Methanobacterium: resolution into four components. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2151–2155. doi: 10.1073/pnas.80.8.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouvière P. E., Escalante-Semerena J. C., Wolfe R. S. Component A2 of the methylcoenzyme M methylreductase system from Methanobacterium thermoautotrophicum. J Bacteriol. 1985 Apr;162(1):61–66. doi: 10.1128/jb.162.1.61-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. D., Wolfe R. S. Structure and methylation of coenzyme M(HSCH2CH2SO3). J Biol Chem. 1974 Aug 10;249(15):4879–4885. [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Witten J. L., Schaffer M. H., O'Shea M., Cook J. C., Hemling M. E., Rinehart K. L., Jr Structures of two cockroach neuropeptides assigned by fast atom bombardment mass spectrometry. Biochem Biophys Res Commun. 1984 Oct 30;124(2):350–358. doi: 10.1016/0006-291x(84)91560-2. [DOI] [PubMed] [Google Scholar]



