Abstract
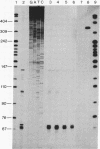
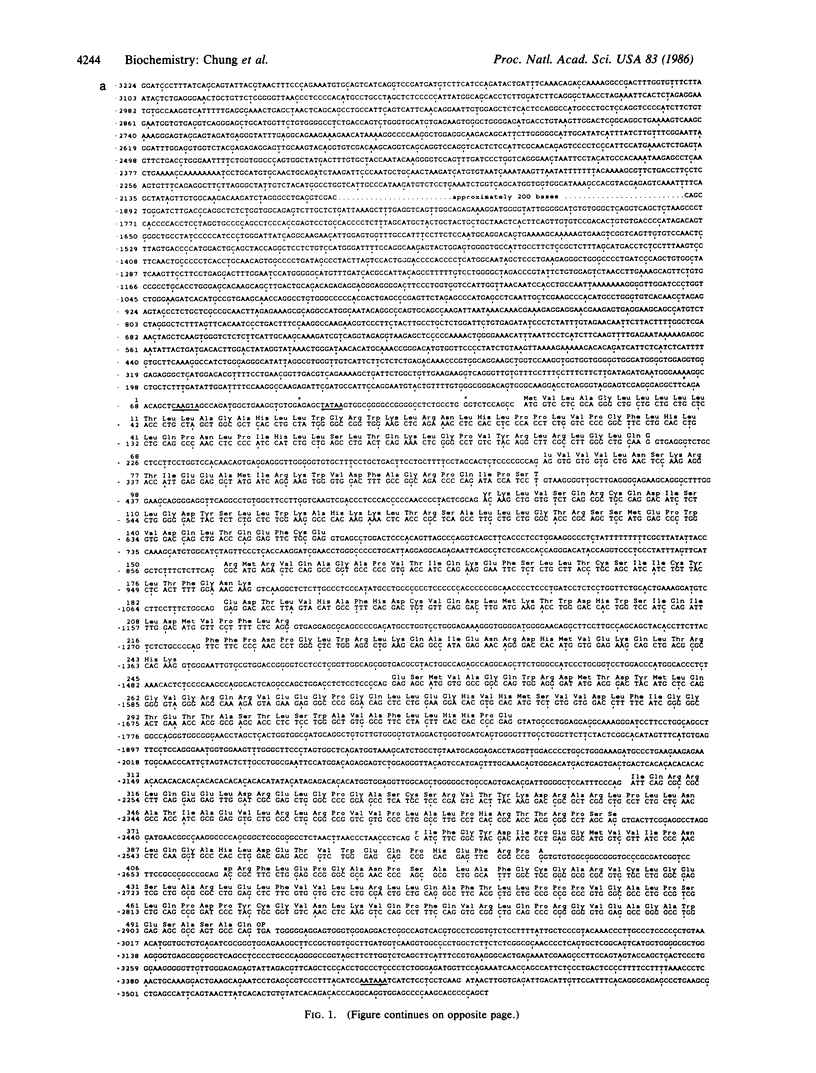
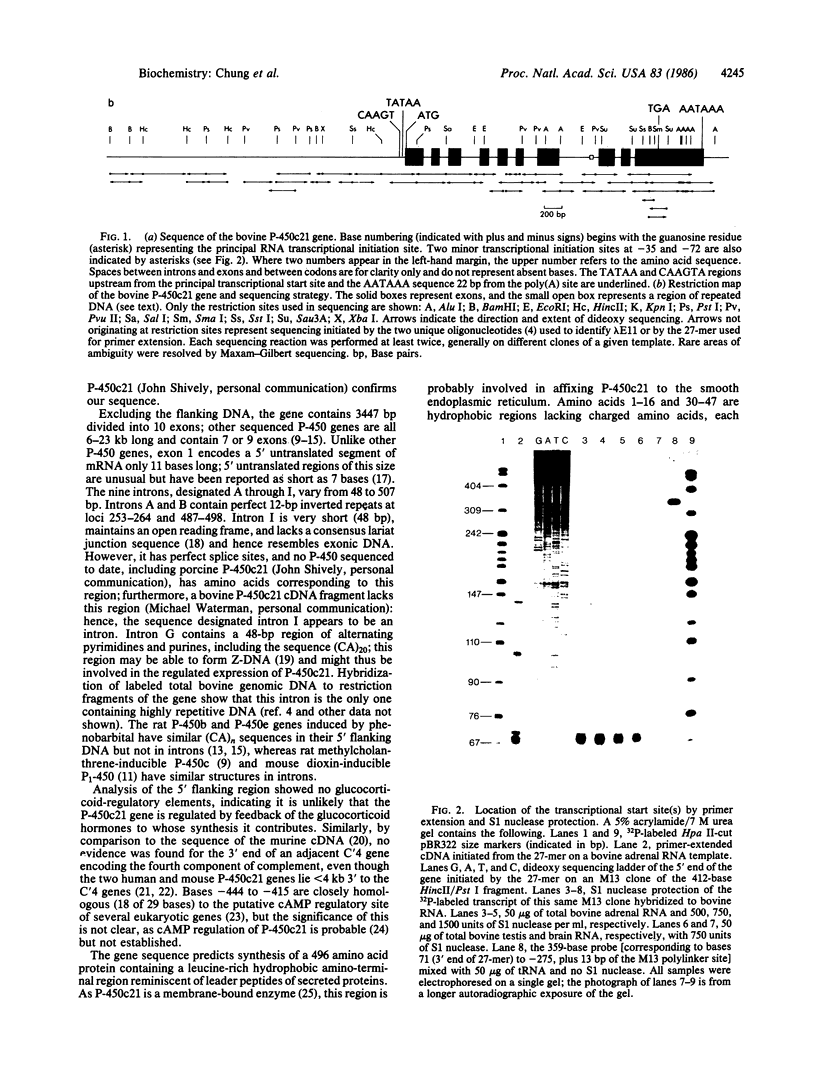
P-450c21, a cytochrome P-450 enzyme [steroid 21-monooxygenase (steroid 21-hydroxylase), EC 1.14.99.10], mediates the 21-hydroxylation of glucocorticoid and mineralocorticoid hormones in the adrenal gland. The complete sequence of a bovine P-450c21 gene shows it is 3447 base pairs long and contains 10 exons. The intron/exon organization and encoded amino acid sequence indicate that P-450c21 represents a unique family of genes in the P-450 gene superfamily. Primer extension and S1 nuclease protection experiments identified several cap sites for initiation of transcription; the principal cap site produces mRNA with a 5' untranslated region only 11 bases long. S1 nuclease protection experiments confirm that P-450c21 is actively expressed in the adrenal and the testis, an organ not known to secrete 21-hydroxylated steroids.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. A., Rose J. K. Structural requirements of a membrane-spanning domain for protein anchoring and cell surface transport. Cell. 1985 Jul;41(3):1007–1015. doi: 10.1016/s0092-8674(85)80081-7. [DOI] [PubMed] [Google Scholar]
- Amor M., Tosi M., Duponchel C., Steinmetz M., Meo T. Liver mRNA probes disclose two cytochrome P-450 genes duplicated in tandem with the complement C4 loci of the mouse H-2S region. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4453–4457. doi: 10.1073/pnas.82.13.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison M., Adesnik M. A cytochrome P-450 multigene family. Characterization of a gene activated by phenobarbital administration. J Biol Chem. 1983 Sep 25;258(18):11285–11295. [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggaram V., Simpson E. R., Waterman M. R. Induction of synthesis of bovine adrenocortical cytochromes P-450scc, P-45011 beta, P-450C21, and adrenodoxin by prostaglandins E2 and F2 alpha and cholera toxin. Arch Biochem Biophys. 1984 Jun;231(2):271–279. doi: 10.1016/0003-9861(84)90388-6. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- COOPER D. Y., LEVIN S., NARASIMHULU S., ROSENTHAL O. PHOTOCHEMICAL ACTION SPECTRUM OF THE TERMINAL OXIDASE OF MIXED FUNCTION OXIDASE SYSTEMS. Science. 1965 Jan 22;147(3656):400–402. doi: 10.1126/science.147.3656.400. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Porter R. R. Mapping of steroid 21-hydroxylase genes adjacent to complement component C4 genes in HLA, the major histocompatibility complex in man. Proc Natl Acad Sci U S A. 1985 Jan;82(2):521–525. doi: 10.1073/pnas.82.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey M. L., MacDonald P. C. Extraadrenal formation of a mineralocorticosteroid: deoxycorticosterone and deoxycorticosterone sulfate biosynthesis and metabolism. Endocr Rev. 1982 Fall;3(4):396–403. doi: 10.1210/edrv-3-4-396. [DOI] [PubMed] [Google Scholar]
- Chung B. C., Matteson K. J., Miller W. L. Cloning and characterization of the bovine gene for steroid 21-hydroxylase (P-450c21). DNA. 1985 Jun;4(3):211–219. doi: 10.1089/dna.1985.4.211. [DOI] [PubMed] [Google Scholar]
- Dieter H. H., Muller-Eberhard U., Johnson E. F. Rabbit hepatic progesterone 21-hydroxylase exhibits a bimodal distribution of activity. Science. 1982 Aug 20;217(4561):741–743. doi: 10.1126/science.6808664. [DOI] [PubMed] [Google Scholar]
- Finkelstein M., Shaefer J. M. Inborn errors of steroid biosynthesis. Physiol Rev. 1979 Apr;59(2):353–406. doi: 10.1152/physrev.1979.59.2.353. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Kimura S., Nebert D. W. Comparison of the flanking regions and introns of the mouse 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible cytochrome P1-450 and P3-450 genes. J Biol Chem. 1985 Apr 25;260(8):5040–5049. [PubMed] [Google Scholar]
- Jameson L., Chin W. W., Hollenberg A. N., Chang A. S., Habener J. F. The gene encoding the beta-subunit of rat luteinizing hormone. Analysis of gene structure and evolution of nucleotide sequence. J Biol Chem. 1984 Dec 25;259(24):15474–15480. [PubMed] [Google Scholar]
- Keller W. The RNA lariat: a new ring to the splicing of mRNA precursors. Cell. 1984 Dec;39(3 Pt 2):423–425. doi: 10.1016/0092-8674(84)90449-5. [DOI] [PubMed] [Google Scholar]
- Kimura S., Nebert D. W. Comparison of the mouse P(1)450 gene and flanking sequences from a MOPC 41 plasmacytoma and normal liver. DNA. 1985 Oct;4(5):365–375. doi: 10.1089/dna.1985.4.365. [DOI] [PubMed] [Google Scholar]
- Kominami S., Ochi H., Kobayashi Y., Takemori S. Studies on the steroid hydroxylation system in adrenal cortex microsomes. Purification and characterization of cytochrome P-450 specific for steroid C-21 hydroxylation. J Biol Chem. 1980 Apr 25;255(8):3386–3394. [PubMed] [Google Scholar]
- Matteson K. J., Chung B. C., Urdea M. S., Miller W. L. Study of cholesterol side-chain cleavage (20,22 desmolase) deficiency causing congenital lipoid adrenal hyperplasia using bovine-sequence P450scc oligodeoxyribonucleotide probes. Endocrinology. 1986 Apr;118(4):1296–1305. doi: 10.1210/endo-118-4-1296. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Coit D., Baxter J. D., Martial J. A. Cloning of bovine prolactin cDNA and evolutionary implications of its sequence. DNA. 1981;1(1):37–50. doi: 10.1089/dna.1.1981.1.37. [DOI] [PubMed] [Google Scholar]
- Mizukami Y., Sogawa K., Suwa Y., Muramatsu M., Fujii-Kuriyama Y. Gene structure of a phenobarbital-inducible cytochrome P-450 in rat liver. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3958–3962. doi: 10.1073/pnas.80.13.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K., Fujii-Kuriyama Y., Okada Y., Sogawa K., Hirose T., Inayama S., Omura T. Molecular cloning and nucleotide sequence of cDNA for mRNA of mitochondrial cytochrome P-450(SCC) of bovine adrenal cortex. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4647–4651. doi: 10.1073/pnas.81.15.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine Y., Reich E. Gene expression and cAMP. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4606–4610. doi: 10.1073/pnas.82.14.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K. L., Chaplin D. D., Wong M., Seidman J. G., Smith J. A., Schimmer B. P. Expression of murine 21-hydroxylase in mouse adrenal glands and in transfected Y1 adrenocortical tumor cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7860–7864. doi: 10.1073/pnas.82.23.7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W., Yamamoto T., Schneider W. J., Slaughter C. J., Brown M. S., Goldstein J. L. cDNA cloning of the bovine low density lipoprotein receptor: feedback regulation of a receptor mRNA. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7501–7505. doi: 10.1073/pnas.80.24.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepich D. S., Noonan D. J., Ogata R. T. Complete cDNA sequence of the fourth component of murine complement. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5895–5899. doi: 10.1073/pnas.82.17.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa K., Gotoh O., Kawajiri K., Fujii-Kuriyama Y. Distinct organization of methylcholanthrene- and phenobarbital-inducible cytochrome P-450 genes in the rat. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5066–5070. doi: 10.1073/pnas.81.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa K., Gotoh O., Kawajiri K., Harada T., Fujii-Kuriyama Y. Complete nucleotide sequence of a methylcholanthrene-inducible cytochrome P-450 (P-450d) gene in the rat. J Biol Chem. 1985 Apr 25;260(8):5026–5032. [PubMed] [Google Scholar]
- Suwa Y., Mizukami Y., Sogawa K., Fujii-Kuriyama Y. Gene structure of a major form of phenobarbital-inducible cytochrome P-450 in rat liver. J Biol Chem. 1985 Jul 5;260(13):7980–7984. [PubMed] [Google Scholar]
- Südhof T. C., Goldstein J. L., Brown M. S., Russell D. W. The LDL receptor gene: a mosaic of exons shared with different proteins. Science. 1985 May 17;228(4701):815–822. doi: 10.1126/science.2988123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey R. H., Okino S., Barnes H., Griffin K. J., Johnson E. F. Multiple gene-like sequences related to the rabbit hepatic progesterone 21-hydroxylase cytochrome P-450 1. J Biol Chem. 1985 Oct 25;260(24):13347–13354. [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- White P. C., Chaplin D. D., Weis J. H., Dupont B., New M. I., Seidman J. G. Two steroid 21-hydroxylase genes are located in the murine S region. 1984 Nov 29-Dec 5Nature. 312(5993):465–467. doi: 10.1038/312465a0. [DOI] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. Cloning and expression of cDNA encoding a bovine adrenal cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1986–1990. doi: 10.1073/pnas.81.7.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7505–7509. doi: 10.1073/pnas.81.23.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel C. A., Casey M. L., Worley R. J., Madden J. D., MacDonald P. C. Extraadrenal steroid 21-hydroxylase activity in a woman with congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1983 Jan;56(1):104–107. doi: 10.1210/jcem-56-1-104. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- de Bruijn M. H., Fey G. H. Human complement component C3: cDNA coding sequence and derived primary structure. Proc Natl Acad Sci U S A. 1985 Feb;82(3):708–712. doi: 10.1073/pnas.82.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]