Abstract
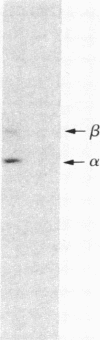
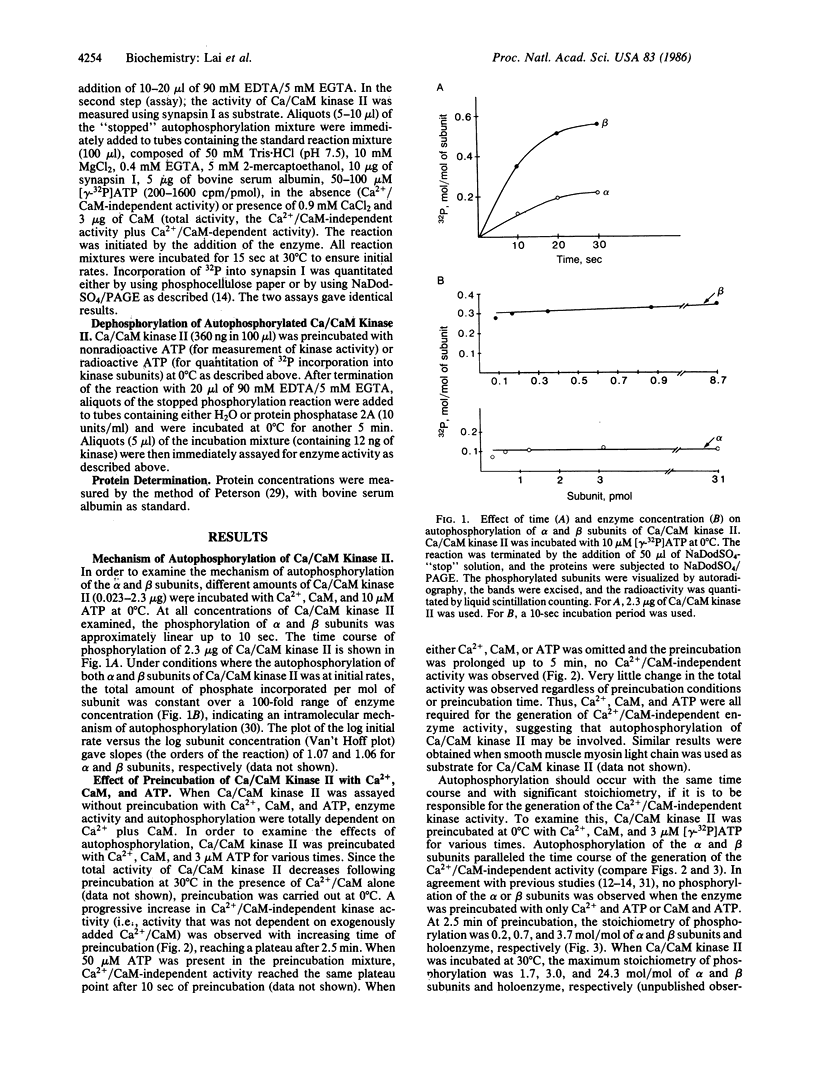
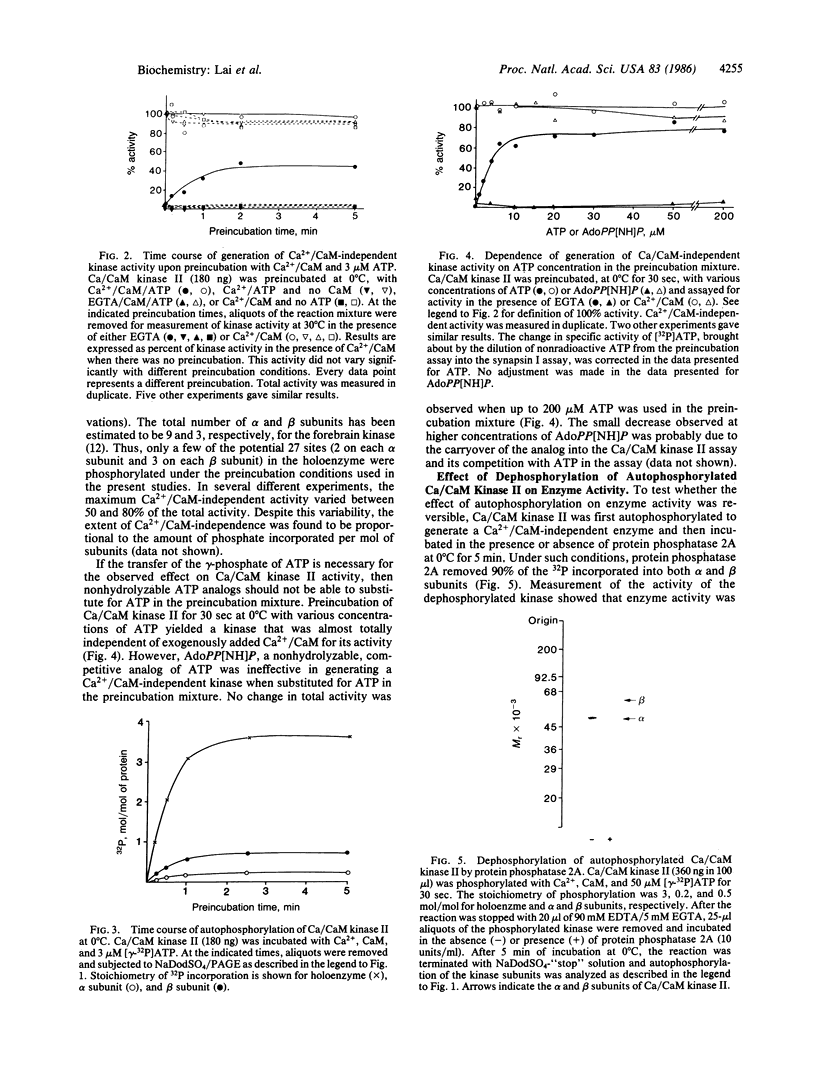
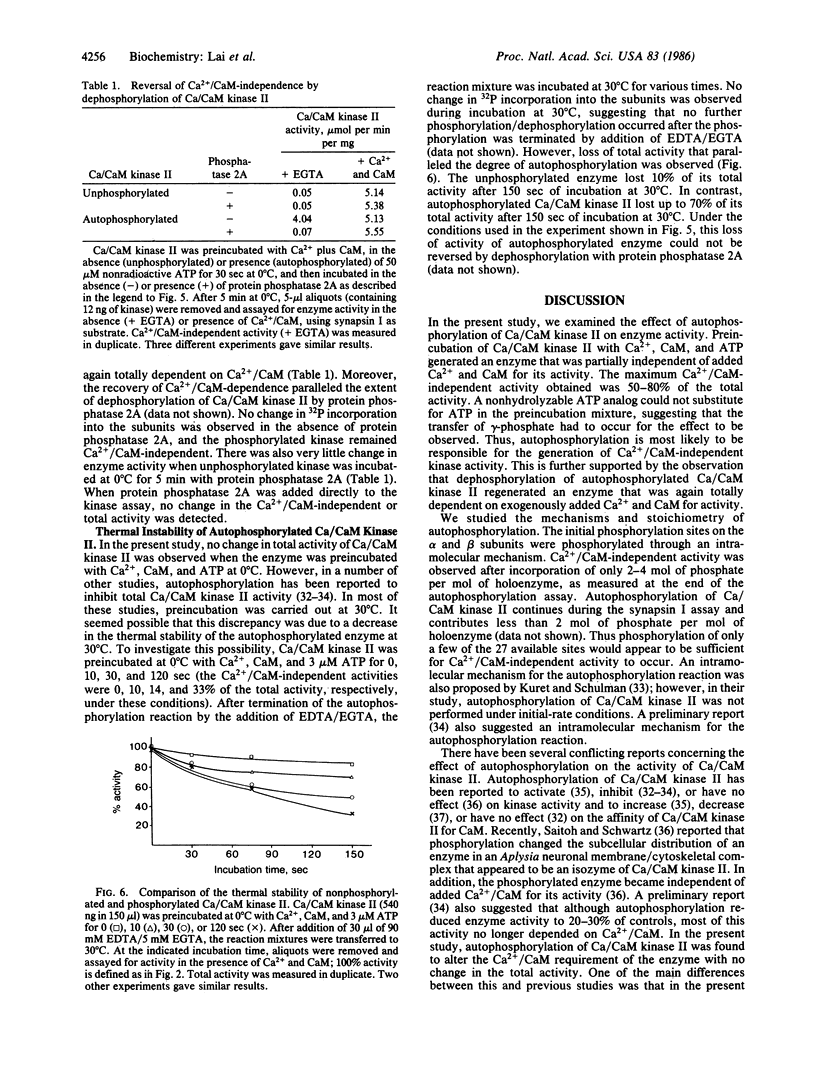
Ca2+/calmodulin-dependent protein kinase II contains two subunits, alpha (Mr 50,000) and beta (Mr 60,000/58,000), both of which undergo Ca2+/calmodulin-dependent autophosphorylation. In the present study, we have studied the mechanism of this autophosphorylation reaction and its effect on the activity of the enzyme. Both subunits are autophosphorylated through an intramolecular mechanism. Using synapsin I as substrate, Ca2+/calmodulin-dependent protein kinase II, in its unphosphorylated form, was totally dependent on Ca2+ and calmodulin for its activity. Preincubation of the enzyme with Ca2+, calmodulin, and ATP, under conditions where autophosphorylation of both subunits occurred, converted the enzyme to one that was only partially dependent on Ca2+ and calmodulin for its activity. No change in the total activity, measured in the presence of Ca2+ and calmodulin, was observed. The nonhydrolyzable ATP analog adenosine 5'-[beta, gamma-imido] triphosphate did not substitute for ATP in the preincubation. Moreover, dephosphorylation of autophosphorylated Ca2+/calmodulin-dependent protein kinase II with protein phosphatase 2A resulted in an enzyme that was again totally dependent on Ca2+ and calmodulin for its activity. We propose that autophosphorylation and dephosphorylation reversibly regulate the Ca2+ and calmodulin requirement of Ca2+/calmodulin-dependent protein kinase II.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad Z., DePaoli-Roach A. A., Roach P. J. Purification and characterization of a rabbit liver calmodulin-dependent protein kinase able to phosphorylate glycogen synthase. J Biol Chem. 1982 Jul 25;257(14):8348–8355. [PubMed] [Google Scholar]
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983 Oct 25;258(20):12735–12744. [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Fukunaga K., Yamamoto H., Matsui K., Higashi K., Miyamoto E. Purification and characterization of a Ca2+- and calmodulin-dependent protein kinase from rat brain. J Neurochem. 1982 Dec;39(6):1607–1617. doi: 10.1111/j.1471-4159.1982.tb07994.x. [DOI] [PubMed] [Google Scholar]
- Goldenring J. R., Gonzalez B., McGuire J. S., Jr, DeLorenzo R. J. Purification and characterization of a calmodulin-dependent kinase from rat brain cytosol able to phosphorylate tubulin and microtubule-associated proteins. J Biol Chem. 1983 Oct 25;258(20):12632–12640. [PubMed] [Google Scholar]
- Grand R. J., Perry S. V., Weeks R. A. Troponin C-like proteins (calmodulins) from mammalian smooth muscle and other tissues. Biochem J. 1979 Feb 1;177(2):521–529. doi: 10.1042/bj1770521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., McGuinness T., Greengard P. A calcium/calmodulin-dependent protein kinase from mammalian brain that phosphorylates Synapsin I: partial purification and characterization. J Neurosci. 1983 Apr;3(4):818–831. doi: 10.1523/JNEUROSCI.03-04-00818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. M., Fitzgerald T. J., Carlson G. M. Characterization of initial autophosphorylation events in rabbit skeletal muscle phosphorylase kinase. J Biol Chem. 1983 Aug 25;258(16):9925–9930. [PubMed] [Google Scholar]
- Kuret J., Schulman H. Mechanism of autophosphorylation of the multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem. 1985 May 25;260(10):6427–6433. [PubMed] [Google Scholar]
- LeVine H., 3rd, Sahyoun N. E., Cuatrecasas P. Calmodulin binding to the cytoskeletal neuronal calmodulin-dependent protein kinase is regulated by autophosphorylation. Proc Natl Acad Sci U S A. 1985 Jan;82(2):287–291. doi: 10.1073/pnas.82.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., McGuinness T. L., Leonard C. S., Sugimori M., Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci U S A. 1985 May;82(9):3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness T. L., Lai Y., Greengard P. Ca2+/calmodulin-dependent protein kinase II. Isozymic forms from rat forebrain and cerebellum. J Biol Chem. 1985 Feb 10;260(3):1696–1704. [PubMed] [Google Scholar]
- McGuinness T. L., Lai Y., Greengard P., Woodgett J. R., Cohen P. A multifunctional calmodulin-dependent protein kinase. Similarities between skeletal muscle glycogen synthase kinase and a brain synapsin I kinase. FEBS Lett. 1983 Nov 14;163(2):329–334. doi: 10.1016/0014-5793(83)80846-1. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J Biol Chem. 1985 Jul 25;260(15):9039–9046. [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986 Mar 28;44(6):861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Greengard P. Protein kinases in the brain. Annu Rev Biochem. 1985;54:931–976. doi: 10.1146/annurev.bi.54.070185.004435. [DOI] [PubMed] [Google Scholar]
- Payne M. E., Schworer C. M., Soderling T. R. Purification and characterization of rabbit liver calmodulin-dependent glycogen synthase kinase. J Biol Chem. 1983 Feb 25;258(4):2376–2382. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Resink T. J., Hemmings B. A., Tung H. Y., Cohen P. Characterisation of a reconstituted Mg-ATP-dependent protein phosphatase. Eur J Biochem. 1983 Jun 15;133(2):455–461. doi: 10.1111/j.1432-1033.1983.tb07485.x. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Schwartz J. H. Phosphorylation-dependent subcellular translocation of a Ca2+/calmodulin-dependent protein kinase produces an autonomous enzyme in Aplysia neurons. J Cell Biol. 1985 Mar;100(3):835–842. doi: 10.1083/jcb.100.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H. Phosphorylation of microtubule-associated proteins by a Ca2+/calmodulin-dependent protein kinase. J Cell Biol. 1984 Jul;99(1 Pt 1):11–19. doi: 10.1083/jcb.99.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields S. M., Vernon P. J., Kelly P. T. Autophosphorylation of calmodulin-kinase II in synaptic junctions modulates endogenous kinase activity. J Neurochem. 1984 Dec;43(6):1599–1609. doi: 10.1111/j.1471-4159.1984.tb06084.x. [DOI] [PubMed] [Google Scholar]
- Todhunter J. A., Purich D. L. Autophosphorylation of cardiac 3',5'-cyclic AMP-stimulated protein kinase. Kinetic evidence for the regulatory subunit directly acting at the active site in the R2C2 complex. Biochim Biophys Acta. 1977 Nov 23;485(1):87–94. doi: 10.1016/0005-2744(77)90195-4. [DOI] [PubMed] [Google Scholar]
- Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J Biol Chem. 1977 Jul 25;252(14):5155–5163. [PubMed] [Google Scholar]
- Woodgett J. R., Cohen P., Yamauchi T., Fujisawa H. Comparison of calmodulin-dependent glycogen synthase kinase from skeletal muscle and calmodulin-dependent protein kinase-II from brain. FEBS Lett. 1984 May 7;170(1):49–54. doi: 10.1016/0014-5793(84)81366-6. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Davison M. T., Cohen P. The calmodulin-dependent glycogen synthase kinase from rabbit skeletal muscle. Purification, subunit structure and substrate specificity. Eur J Biochem. 1983 Nov 15;136(3):481–487. doi: 10.1111/j.1432-1033.1983.tb07766.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Fukunaga K., Tanaka E., Miyamoto E. Ca2+- and calmodulin-dependent phosphorylation of microtubule-associated protein 2 and tau factor, and inhibition of microtubule assembly. J Neurochem. 1983 Oct;41(4):1119–1125. doi: 10.1111/j.1471-4159.1983.tb09060.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Disassembly of microtubules by the action of calmodulin-dependent protein kinase (Kinase II) which occurs only in the brain tissues. Biochem Biophys Res Commun. 1983 Jan 14;110(1):287–291. doi: 10.1016/0006-291x(83)91293-7. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Purification and characterization of the brain calmodulin-dependent protein kinase (kinase II), which is involved in the activation of tryptophan 5-monooxygenase. Eur J Biochem. 1983 Apr 15;132(1):15–21. doi: 10.1111/j.1432-1033.1983.tb07319.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Self-regulation of calmodulin-dependent protein kinase II and glycogen synthase kinase by autophosphorylation. Biochem Biophys Res Commun. 1985 May 31;129(1):213–219. doi: 10.1016/0006-291x(85)91424-x. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Tyrosine 3-monoxygenase is phosphorylated by Ca2+-, calmodulin-dependent protein kinase, followed by activation by activator protein. Biochem Biophys Res Commun. 1981 May 29;100(2):807–813. doi: 10.1016/s0006-291x(81)80246-x. [DOI] [PubMed] [Google Scholar]