Summary
Sensation is an active process involving the selective sampling and central processing of external stimuli in space and time. Olfaction in particular depends strongly on active sensing due to the fact that - at least in mammals - inhalation of air into the nasal cavity is required for odor detection. This seemingly simple first step in odor sensation profoundly shapes nearly all aspects of olfactory system function, from the distribution of odorant receptors to the functional organization of central processing to the perception of odors. The dependence of olfaction on inhalation also allows for profound modulation of olfactory processing by changes in odor sampling strategies in coordination with attentional state and sensory demands. This review discusses the role of active sensing in shaping olfactory system function at multiple levels and draws parallels with other sensory modalities to highlight the importance of an active sensing perspective in understanding how sensory systems work in the behaving animal.
Introduction
Sensory systems gather and process information about the external world. For most modalities, sensation is an active operation in which the detection, representation and processing of sensory information is heavily modulated during behavior. Active sensing allows an animal to selectively sample regions in space and epochs in time, to regulate stimulus intensity and dynamics in order to optimize sensory processing, to extract features of interest from a complex stimulus and to protect sensory neurons from excessively strong or harmful stimuli. Classic examples of active sensation include finger movements during object manipulation, whisking in rodents and saccadic eye movements in vision. Each of these examples involves movement of the sense organs in order to optimally sample an area or object of interest. Active stimulus sampling can profoundly affect patterns of sensory neuron activation and, consequently, the postsynaptic processing of sensory inputs. In addition, active sensing involves the coordination of ‘bottom-up’ effects on sensory inputs with ‘top-down’ modulation of processing at multiple synaptic levels. Thus active sensation is a multi-level, system-wide process affecting sensory system function.
Olfaction, while not as extensively studied as other modalities, is in many respects an ideal model system for active sensing. First, for terrestrial vertebrates, olfactory sensation depends on stimulus acquisition by the animal; the inhalation of air into the nose is a necessary first step in olfaction. Second, mammals in particular have impressively complex behavioral repertoires for odorant sampling; this behavior - typically termed ‘sniffing’ - is precisely and strongly modulated as a function of task demands, behavioral state and stimulus context (Welker, 1964; Wesson et al., 2009; Youngentob et al., 1987). Finally, the olfactory system has in recent years matured into a highly tractable system in which its molecular, cellular and circuit-level organization can be examined, manipulated and integrated with behavioral experiments.
A central thesis of this review is that the active components of olfactory sensation are closely woven with fundamental processes of olfactory system function at levels ranging from receptor expression patterns, sensory neuron response properties, circuit dynamics in the olfactory bulb and cortex, and centrifugal systems. As a result, the reliance of olfaction on transient, active sampling of odors is manifest even in reduced experimental preparations that are far removed from an actively sampling animal. Thus considering olfaction as an active sense is not only essential to understanding how this system works in the behaving animal, it is a useful framework for understanding olfaction in many experimental contexts. A second point made here - and substantiated by examples from other sensory modalities - is that even descriptions of olfactory system function in the awake animal would benefit from considering sampling behavior as a primary factor in shaping how the brain represents and processes olfactory input. In general, considering sensory systems in the context of active sensing provides an important avenue for understanding key principles of sensory system function in the behaving animal.
Sniffing reflects behavioral strategies for olfactory sensing
In terrestrial vertebrates the olfactory epithelium is housed deep within the nasal cavity, such that inhalation of air is required for odorants to access olfactory receptor neurons (ORNs). Typically, this can only occur during the course of resting respiration or by the voluntary inhalation of air in the context of odor-guided behavior - i.e., sniffing. A sniff - like a whisk or a saccade - represents the basic unit of active odor sensing. Analogs of sniffing occur across the animal kingdom, with groups as diverse as crustaceans (Snow, 1973), fish (Nevitt, 1991), semi-aquatic mammals (Catania, 2006) and insects (Suzuki, 1975) showing active, intermittent odorant sampling. The persistence of sniffing behavior in different species and ecological settings together with its strong modulation during odor-guided behaviors suggests that intermittent sampling of odorant is fundamentally important to olfaction (Dethier, 1987).
Sniffing - while highly dynamic from cycle to cycle is precisely controlled during behavior (Figure 1). For example, when sampling odorant from a port in an odor discrimination task, rats show a brief bout of 6 – 10 Hz sniffing precisely timed to just precede odorant delivery and a slightly higher-frequency sniff bout (9 – 12 Hz) just prior to receiving a reward; each of these bouts is repeated with a temporal jitter of only a few hundred msec across hundreds of trials (Kepecs et al., 2007; Wesson et al., 2009). Humans also show stereotyped and task-dependent sniffing patterns and also can rapidly modulate sniffing in response to sensory input (Johnson et al., 2003; Laing, 1983).
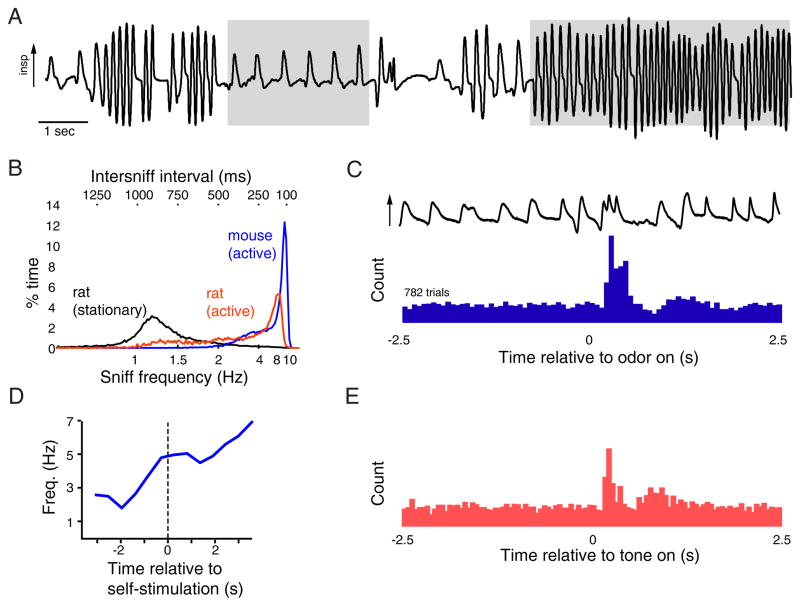
Figure 1. Sniffing as a dynamic, precisely-controlled and context-dependent behavior.
A. Sample of respiratory signals (measured as intranasal pressure) taken from an awake, freely-moving rat. Arrow indicates direction of inspiration. Shaded areas demarcate basal respiration (left) and a bout of sustained exploratory sniffing (right). Numerous other sniffing patterns involving changes in frequency, amplitude and duration of individual ‘sniffs’ - as well as pauses in breathing - can be seen within this ~ 20 second span.
B. Percent time spent sniffing at a given frequency for stationary rats, active rats and active mice. While sniffing behavior is highly variable and dynamic, active rodents spend the majority of time sniffing at frequencies above 4 Hz.
C. Sniff timing can be precisely controlled. Histogram shows inhalations measured in a head-fixed rat performing a two-odor discrimination task; trace at top shows intranasal pressure from a single trial. This rat displays a brief bout of high-frequency sniffing occurring just after odorant onset, which is repeated reliably over hundreds of trials. Modified with permission from (Wesson et al., 2009).
D. Animals modulate sniffing independent of any olfactory context. Plot shows increase in respiratory frequency evoked by self-administered electrical stimulation of the lateral hypothalamus in an unrestrained rat. Sniff frequency increases just prior to the rat beginning self-stimulation and persists throughout the stimulation period. Modified with permission from (Clarke, 1971).
E. Histogram of inhalations relative to time of tone presentation; same paradigm as in (C), but measured in a rat performing an auditory discrimination task. This rat displays a brief bout of high-frequency sniffing just after tone onset. Modified with permission from (Wesson et al., 2009).
Sniffing patterns thus reflect a particular strategy for olfactory sampling, chosen for a particular task and context. Sniffing strategies can also be individual-specific: both rodents and humans show individual differences in sniffing behavior when sampling odorants (Laing, 1983; Wesson et al., 2009). A compelling example of context-specific sampling strategies occurs in bird-hunting dogs: when tracking the scent of prey on the ground, dogs sniff at up to 4 – 6 Hz but when tracking the same scent in the air, the animal will raise its head and run forward, forcing a continuous stream of air into the nose for up to 40 sec (Steen et al., 1996). The presumed advantage of the latter strategy is to enable continuous odorant sampling while moving at high speed and to decouple sampling from respiration during a time of heavy load on the respiratory system.
Sniffing patterns - like saccadic eye movements in visual scene analysis and repeated whisking during somatosensory object identification - likely reflect strategies for optimally extracting and processing sensory information (Laing, 1983). How, then, does sniffing affect the detection, representation and processing of odor information by the nervous system? This question remains largely unanswered but crucial to understanding the role of active sensing in olfactory system function.
Addressing this question involves some important caveats, however. First, unlike in other sensory systems, active sampling in olfaction is confounded with an arguably more important function: respiration. In rodents, which are obligatory nose-breathers, odorants are unavoidably sampled with each inhalation (Verhagen et al., 2007) and sniffing strategies will be constrained within the limits required for proper respiratory function. For example, sniff frequency may increase in animals that are actively engaged with their environment due simply to increased respiratory demand. Autonomic or reflex-mediated effects on respiration might also be confused with active sniffing. Second, in the freely-moving animal, sniffing is expressed as part of a larger behavioral repertoire which may include head movements, whisking (in rodents), licking, and locomotion (Bramble and Carrier, 1983; Welker, 1964). The strong coupling between sniffing and other active sampling behaviors can confound interpretation of the role that sniffing plays in olfaction. Rodents increase respiration frequency prior to receiving a reward and when otherwise engaged in motivated behavior, independent of an olfactory context (Clarke, 1971; Kepecs et al., 2007; Wesson et al., 2008b) (Figure 1D). Rodents also increase respiration frequency (and initiate whisking) in response to unexpected stimuli of any modality (Macrides, 1975; Welker, 1964) and when inserting their nose into a port - even when performing non-olfactory tasks (Wesson et al., 2008b; Wesson et al., 2009) (Figure 1E). Finally, rodents and humans can make odor-guided decisions after only a single sample of odorant, which can occur via an inhalation that is indistinguishable from that of resting respiration (Verhagen et al., 2007). Thus, while in this review we use ‘sniffing’ to imply a voluntary inhalation (or repeated inhalations) in the context of odor-guided behavior, we include passive respiration as an effective means of olfactory sampling.
Sniffing controls the temporal structure of sensory input to the olfactory system
The most important function of sniffing is to control access of olfactory stimuli to the receptor neurons (ORNs) themselves. At least in awake rodents, ORNs are not activated when odorant is simply blown at the nose; the animal must inhale for odorant to reach the olfactory epithelium (Wesson et al., 2008a) (Figure 2A). Inhalation-driven ORN responses are transient, with each inhalation evoking a burst of ORN activity lasting only 100 – 200 ms (Carey et al., 2009; Chaput and Chalansonnet, 1997; Verhagen et al., 2007) (Figure 2B). Up to several thousand ORNs - each expressing the same odorant receptor - converge onto a single glomerulus in the OB (Mombaerts et al., 1996). An important aspect of inhalation-driven sensory activity is that the activation of the ORN population that converges onto one glomerulus is not instantaneous but instead develops over 40 – 150 msec (Carey et al., 2009). As a result, patterns of sensory input to OB glomeruli dynamically develop over the 50 – 200 milliseconds following an inhalation (Figure 2A).
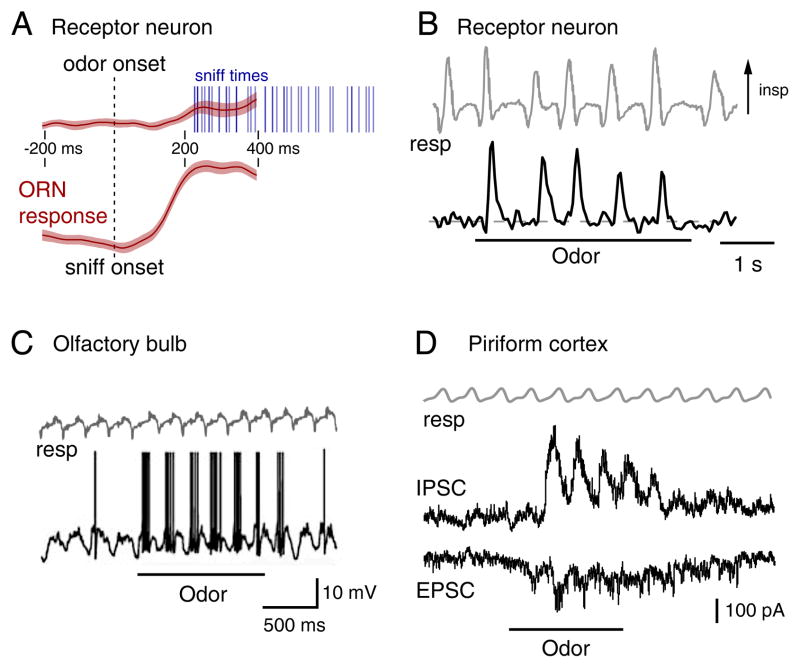
Figure 2. Inhalation controls the dynamics of odorant-evoked activity throughout the olfactory system.
A. Inhalation is required to activate sensory inputs to the olfactory system. Traces show activation of olfactory receptor neuron (ORN) inputs to the olfactory bulb (OB), averaged across multiple trials and aligned relative to odorant presentation (top) or relative to the time of the first sniff after odor presentation. For the latter, odorant is present before the dashed line. Responses occur only within a fixed time after inhalation. Shaded region around each trace indicates the variance across trials. Modified with permission from (Wesson et al., 2008a).
B. ORN input signals (bottom) and sniffing (top, gray) measured during odor presentation in an awake rat. Each sniff elicits a transient burst of ORN activity. Modified with permission from (Verhagen et al., 2007).
C. Current clamp recording from a mitral cell in an anesthetized, freely-breathing mouse. The cell shows oscillations in membrane potential synchronized with respiration (top, gray); odorant presentation elicits spike bursts after each inhalation. Modified with permission from (Schaefer et al., 2006).
D. Voltage clamp recording from a piriform cortex neuron in an anesthetized rat. Each inhalation (gray) in the presence of odor elicits transient excitatory as well as stronger inhibitory currents. Same time-scale as (B). Modified with permission from (Poo and Isaacson, 2009).
Inhalation-driven inputs drive the dynamics of postsynaptic olfactory processing
Temporal coupling between the dynamics of neural activity in the olfactory pathway and rhythmic odor sampling is the most distinctive feature of odorant-evoked activity in the CNS (Adrian, 1942; Buonviso et al., 2006; Macrides and Chorover, 1972). While long controversial, most recent evidence suggests that respiration-related rhythms in the CNS are dependent on the activation of ORNs during inhalation, as opposed to reflecting a central signal related to respiratory pattern-generation (Buonviso et al., 2006). In rodents, eliminating ORN activation or decoupling nasal airflow from respiration disrupts respiratory rhythms in the olfactory pathway in favor of nasal airflow rhythms (Grosmaitre et al., 2007; Sobel and Tank, 1993; Spors and Grinvald, 2002). Thus, olfactory network dynamics are primarily driven by the dynamics of inhalation-driven ORN input (Figures 2C, D). For example, the rise-time of odorant-evoked EPSPs in mitral/tufted (MT) cells - the principal OB output neuron - of anesthetized rats is approximately 100 msec, similar to that of the ORN response transients (Cang and Isaacson, 2003; Margrie and Schaefer, 2003). MT cells also show variation in temporal response patterns (e.g. - latency, rise-time and duration of an excitatory burst) that is unit- and odorant-specific (Bathellier et al., 2008; Macrides and Chorover, 1972) and varies over a range similar to that of ORNs (Carey and Wachowiak, 2011). Finally, pyramidal neurons in piriform cortex (PC) - a major target of OB output - also show strong inhalation-coupled dynamics in their spike output and in subthreshold synaptic inputs (Poo and Isaacson, 2009; Rennaker et al., 2007) (Figure 2D).
Given the temporal constraints on ORN responses imposed by respiration it seems likely that postsynaptic networks will be optimized for such input dynamics. Indeed, while the canonical view of the OB network has been that it shapes MT response properties in the spatial domain - e.g. relative to activity in other glomeruli and their associated MT cells (Johnson and Leon, 2007; Yokoi et al., 1995), recent data suggest that postsynaptic processing may primarily function to shape responses in the temporal domain relative to inhalation-driven bursts of input (Figure 3). Work supporting this view comes largely from experimental paradigms far removed from ‘active’ sensing. For example, in OB slice preparations, delivering patterned olfactory nerve stimulation at frequencies that mimic resting respiration amplifies MT responses to ORN input and leads to increased synchrony of MT firing and the emergence of gamma-frequency oscillations in MT cell membrane potential (Hayar et al., 2004b; Schoppa, 2006b).
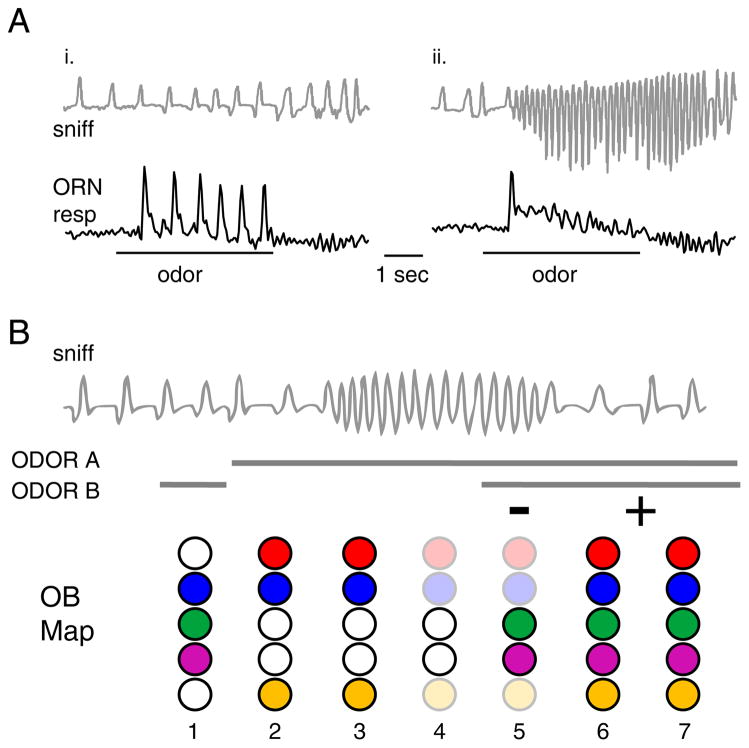
Figure 3. Dynamics of inhalation-driven olfactory inputs may set limits for the temporal integration of odor information.
A. Responses of ORN inputs evoked by an inhalation of odorant to five different glomeruli, imaged in an awake rat. Traces are ‘sniff-triggered averages’ of presynaptic calcium signals (shaded areas indicate variance around the mean response). Vertical lines below trace indicate time to half-max for input to each glomerulus. Input to different glomeruli occurs with different latencies and rise-times, over a range of ~200 msec. Modified with permission from (Carey et al, 2009). The time-scale is the same in all panels (A – D).
B. Spike histogram showing inhalation-driven response across a population of 37 MT cells recorded during sniff ‘playback’ in an anesthetized rat; the rise-time of the population response is ~ 150 msec. Modified with permission from (Carey and Wachowiak, 2011).
C. Postsynaptic currents recorded from two neuron types in a piriform cortex slice preparation evoked by a train of stimuli (5 pulses at 20 Hz) delivered to the lateral olfactory tract, which contains the axons of MT cells. Recordings are from an inhibitory layer I interneuron (L1 INT, top trace) and a pyramidal neuron (PYR, lower trace). Blue dashed trace (bottom) shows the envelope of the in vivo MT cell population response evoked by inhalation from (B). MT inputs evoked over a time-course matching the inhalation-evoked response cause depression in the interneuron and facilitation in the pyramidal neuron. Modified with permission from (Stokes and Isaacson, 2010).
D. Schematic illustrating differences in odor discrimination times for mice performing a two-odor discrimination that is ‘easy’ (pair of dissimilar monomolecular odorants) or ‘difficult’ (pair of highly similar odor mixtures). Mice take 100 – 200 msec longer to perform the ‘difficult’ discrimination, similar to the amount of time needed for inhalation-driven odor response patterns to develop fully. Modified with permission from (Abraham et al., 2004).
Neurons in PC - the major cortical target of OB output neurons - also appear optimized to process information in a temporal domain organized around inhalation-driven bursts of input from MT cells. For example, MT cell axons from the OB provide direct but selective excitation to pyramidal neurons in PC while also driving more widespread feedforward inhibition via GABA-ergic local interneurons (Poo and Isaacson, 2009). For sparse and temporally unstructured MT cell inputs to PC, this strong feedforward inhibition creates an extremely short (5 – 10 ms) time window during which pyramidal neurons may integrate M/T inputs from the OB. However, if MT cell inputs to PC pyramidal neurons occur in sustained bursts - such as those as evoked by inhalation - this feedforward inhibition depresses while MT cell input to pyramidal neurons facilitates, allowing MT cell inputs to more effectively drive pyramidal neuron spiking (Stokes and Isaacson, 2010)(Figures 3B, C). This dynamic synaptic organization may act as a filter for OB inputs to PC that show strong inhalation-coupled temporal patterning.; this prediction could be tested in vivo by comparing PC neuron responses to inhalation-driven, odorant-evoked inputs with responses to brief electrical stimulation of MT axons.
Inhalation-driven neuronal dynamics and odor perception
There is strong behavioral evidence that a single, inhalation-driven ‘packet’ of activity can encode odor information sufficiently to support odor discrimination. Rodents (and humans) can discriminate two familiar odors after a single sniff and in as little as 150 – 250 msec (Abraham et al., 2004; Laing, 1986; Rinberg et al., 2006b; Uchida and Mainen, 2003). In fact, behavioral measurements of odor perception times in awake rats performed simultaneous with imaging of ORN inputs to the OB, indicate that a novel odor can be distinguished from a familiar one before the initial ORN response burst - as inferred from presynaptic calcium imaging - has even finished (Wesson et al., 2008a). In addition, rats performing an operant, two-choice odor discrimination task tend to make their choice after only a single sniff when that sniff evokes strong neural responses within an optimal time-window after inhalation but not when it evokes activity at later times (Cury and Uchida, 2010). Thus, the initial onset phase of the inhalation-evoked burst of ORN activity appears particularly important for olfactory processing and odor perception. Rodents require more time - an additional 100 – 200 msec - to discriminate highly similar odors (Abraham et al., 2004; Rinberg et al., 2006b)(Figure 3D). This additional time roughly matches the time-window over which patterns of ORN input and MT cell activity evolve after an inhalation (Figure 3A) (Cury and Uchida, 2010; Shusterman et al., 2011; Wesson et al., 2008a). Thus, the dynamics of inhalation-evoked ORN inputs to the OB may set an upper limit on the time-window for integration of odor information in the behaving animal (Schaefer and Margrie, 2007).
One longstanding - and still unresolved - question is whether the precise timing of odorant-evoked activity relative to the timing of inhalation plays a role in odor perception. Modeling and experimental data support the idea that spike timing relative to inhalation can robustly represent odor information (Chaput, 1986; Hopfield, 1995; Schaefer and Margrie, 2007; Shusterman et al., 2011). However, whether animals actually use a sniff-based temporal code remains unclear. Important evidence in support of such a coding strategy comes from a recent study using optogenetics in awake, head-fixed mice to activate the same ORN inputs at different times relative to inhalation or exhalation onset (Smear et al., 2011). Mice were able to perceive ORN inputs activated at different times after inhalation, with some mice able to discriminate input latency differences of as little as 10 msec; responses of individual OB units also showed sensitivity to the timing of ORN inputs relative to inhalation. Thus, the timing of sensory inputs relative to odorant sampling is, by itself, sufficient to mediate odor discrimination in the awake animal.
Actively shaping odor representations through sniffing
The fact that odor encoding and perception can occur after a single inhalation begs the question of why behaving animals modulate their sniffing behavior so profoundly when sampling odors. Here we discuss several hypotheses on how active control of sniff parameters shape the initial odor representations formed by ORNs; the following section discusses the consequences of changing sniffing patterns for the central processing of olfactory inputs.
One longstanding hypothesis is that animals actively shape ORN response patterns by modulating the rate of air flow over the olfactory epithelium and subsequently altering how odorant distributes across it (Adrian, 1950; Mozell, 1964). This idea - which we will call the sorption hypothesis - arises from the fact that the nasal cavity of most vertebrates – mammals in particular - is anatomically complex and forms a narrow space lined with epithelium and mucus onto which odorant molecules absorb as they flow through the cavity (Yang et al., 2007; Zhao et al., 2006). This arrangement causes a ‘chromatographic effect’ in which odorants are preferentially absorbed in different locations depending on their solubilities and their flow rate (Mozell and Jagodowicz, 1973; Yang et al., 2007). The topography of odorant receptor expression across the olfactory epithelium correlates with the areas of maximal sorption for the receptors’ respective ligands, suggesting that receptors are optimally localized to take advantage of the chromatographic effect (Schoenfeld and Cleland, 2006; Scott et al., 2000). Because the strength, duration and frequency of respiration can change dramatically during odor-guided behavior and because these parameters affect the rate and total volume of airflow into and out of the nasal cavity, sampling behavior has the potential to alter odorant sorption and, as a consequence, patterns of ORN activation (Mozell et al., 1987; Youngentob et al., 1987).
The sorption hypothesis makes specific predictions about how flow rate should shape activity in the intact animal, and applies to both rodent models and humans (Hahn et al., 1994; Mozell et al., 1987). The most directly testable is the following: at low flow rates, strongly-sorbed odorants - for example, polar compounds such as alcohols - will be largely removed from the air stream as they pass through the nasal cavity, resulting in fewer odorant molecules available to activate ORNs, particularly those positioned later in the path of airflow. Because less sorption occurs at higher flow rates, sniffs that elicit higher flows will bring more odorant molecules to ORNs and so evoke larger responses. In contrast, weakly-sorbed odorants - for example the terpene d-limonene, a principal component of orange odor - absorb slowly onto the epithelium and so tend to remain in the air stream as inhaled odorant passes through the nasal cavity. For these compounds, increasing flow rate will have little effect on odorant deposition. Thus, responses to a strongly-sorbed odorant should increase as flow rate increases, while responses to a weakly-sorbed odorant should remain constant or decrease (Hahn et al., 1994). Such effects have been measured at the level of the olfactory epithelium in reduced rodent preparations (Kent et al., 1996; Scott-Johnson et al., 2000) and, recently, in the olfactory bulb using artificial inhalation (Oka et al., 2009). The sorption hypothesis remains untested during natural odor sampling, however, with earlier studies relying primarily on steady-state flow rates, not the transient changes in airflow that occur during natural respiration and active sniffing. Thus, whether animals modulate sniff flow rate in order to actively modulate odorant response patterns remains unclear.
A second way in which sniffing behavior can alter ORN response patterns is through changes in sniff frequency. High-frequency (6 – 10 Hz) sniff bouts lasting up to several seconds are one of the most distinctive odor sampling strategies in mammals, particularly during exploratory behavior (Macrides, 1975; Welker, 1964). High-frequency sniffing shapes ORN responses in unexpected ways. An intuitive prediction is that increases in sniff frequency lead to increased ORN responses - and perhaps recruitment of activation of new ORN populations - due to an increased odorant influx. This prediction has been tested using presynaptic calcium imaging from ORN axon terminals in the OB of awake rats, which sampled the same odorant during low-frequency (1 – 2 Hz) respiration or during high-frequency (4 – 8 Hz) exploratory sniffing (Verhagen et al., 2007). Surprisingly, sampling an odorant at high-frequency only weakly enhanced the initial response to the odorant and did not recruit activation of new ORN populations. More importantly, sustained high-frequency sniffing of odorant led to a strong attenuation of ORN response magnitude (Figure 4A). Sniff frequency-dependent attenuation is rapidly reversible, with ORN response magnitudes recovering within one second after sniffing returns to below 4 Hz. A likely cellular mechanism mediating the frequency-dependent attenuation of ORN inputs to the OB is simple adaptation. At low respiration rates, ORNs can recover from adaptation in the interval between successive inhalations, but higher sniff frequencies allow less time for recovery between cycles (Reisert and Matthews, 2001).
Figure 4. Adaptive filtering of sensory inputs controlled by sniffing.
A. Rapid attenuation of receptor neuron activation during sustained high-frequency sniffing of an odorant. Top traces show sniffing in a head-fixed rat, lower traces show ORN responses (estimated from presynaptic calcium signals using temporal deconvolution). Responses are reliable across inhalations when sampled at low frequency (left), but attenuate rapidly during high-frequency sniffing of the same odorant. Modified with permission from (Verhagen et al., 2007).
B. Schematic illustrating effects of high-frequency sniffing on odor representations in a changing odor landscape. Top, representative sniffing pattern including a bout of high-frequency sniffing. Solid lines indicate preence of odorA, odor B or both A and B. Lower graphics (‘OB map’) represent patterns of activation across five glomeruli in the olfactory bulb at different times during the sniff bout. When sampled in isolation, A and B have overlapping representations (1 – 2). High-frequency sniffing of odor A causes an attenuation of the response map (3 – 4); when odor B is encountered against the background of odor A, only those glomeruli that differ from those activated by odor A are strongly activated, so that the representation resembles the difference between the two odor maps (5). A return to low-frequency sniffing removes the attenuation and the representation changes to resemble the sum of the two maps (6 – 7). Thus, changes in the odor landscape are represented in a subtractive or additive mode depending on sniff frequency.
What is the functional significance of peripheral frequency-dependent attenuation? The attenuation of ORN responses is specific to those glomeruli receiving odorant-evoked input, leaving other glomeruli free to respond to other odorants encountered during a sniff bout - for example, as an animal explores its environment. Thus, this attenuation constitutes an ‘adaptive filter’ of sensory input to the OB in which ORNs activated by odorants present at the beginning of exploratory sniffing (i.e. - ‘background’ odorants) are selectively suppressed in the representation of subsequently-sampled odorants (Verhagen et al., 2007). In contrast, during low-frequency sampling, odorants encountered against a background are represented as the sum of the background and ‘foreground’ response maps (Figure 4B). This filtering can enhance the contrast between odorants having overlapping molecular features (or mixtures with shared components). An equally important function of frequency-dependent attenuation may be to increase the salience of temporally dynamic or spatially localized odorants relative to broadly distributed background odorants. This effect is similar to that seen in active vision, in which repeated scanning of a complex visual scene induces adaptation to the scene statistics and increases the salience of novel stimuli appearing against this background (McDermott et al., 2010). Thus sniffing provides a bottom-up mechanism for the active modulation of odor salience.
Finally, odor representations may depend on whether odorants are sampled via inhalation of odorant through the nose - ‘orthonasal’ sampling - or via the oral cavity and through the nasopharynx - ‘retronasal’ sampling (Hummel, 2008). Retronasal odor sampling can occur during odorant exhalation or, as is more typically considered, after the release of odorant vapor from ingested liquids or solids; retronasally-sampled odorants are large contributors to flavor perception in humans (Murphy et al., 1977). Evidence from humans suggests that odors sampled orthonasally are perceived differently from those sampled retronasally, with retronasal odors perceived as less intense and originating from the oral cavity rather than externally (Murphy et al., 1977; Small et al., 2005). Ortho-versus retronasally sampled odors differentially activate brain areas involved in odor and flavor perception, suggesting that the route of odorant sampling can also impact central processing of odor information (Small et al., 2005). The specific role that retronasal olfaction plays in odor and flavor perception, including whether it is under active control during behavior, remains unclear, however. Retronasal odor sampling may also represent an important difference between human and rodent olfaction: in humans, both inhaled and exhaled air pass over the olfactory epithelium, while in rodents and other macrosmatic animals exhaled air largely bypasses the olfactory recess, severely limiting retronasal access of odorants to ORNs (Zhao et al., 2004; Craven et al., 2010).
Effect of sniffing patterns on central olfactory processing
Because the central circuits that process olfactory inputs are themselves dynamic, changes in the strength and temporal structure of ORN input during active sniffing should also change how these networks process olfactory information. For example, in the rodent somatosensory system, high-frequency inputs such as those that occur during active whisking lead to reduced responsiveness in cortical pyramidal neurons due to dynamic network properties such as short-term depression at the thalamocortical synapse and changes in the driving force of excitatory versus inhibitory inputs (Chung et al., 2002; Crochet et al., 2011). Frequency-dependent effects on olfactory network dynamics have primarily been studied in the OB (Figure 5), although olfactory processing in the PC likely also depends on sniff frequency.
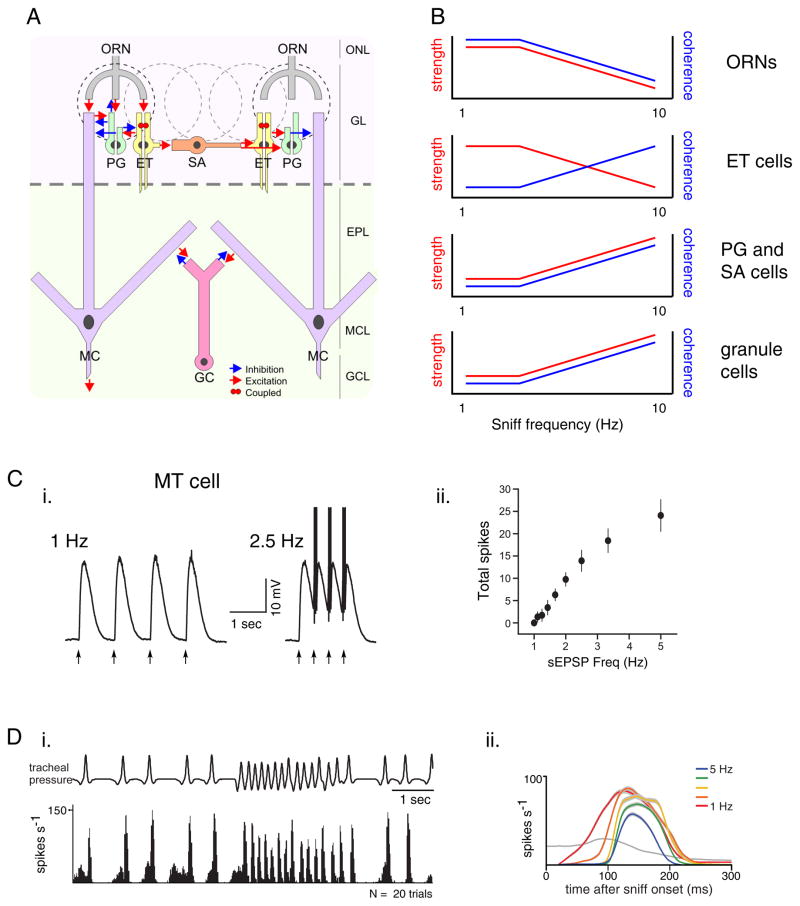
Figure 5. Potential effects of sniff frequency on odor processing in the olfactory bulb.
A. Principal cell types and canonical circuits of the olfactory bulb. Excitatory elements are ORN, olfactory receptor neurons (ORN), external tufted cells (ET), and mitral cells (MC). Inhibitory elements are periglomerular cells (PG), short axon cells (SA) and granule cells (GC). ET cells also provide excitatory input to MT cells (not shown). Schematic taken from (Wachowiak and Shipley, 2006).
B. Schematics illustrating predicted effects of increasing sniff frequency on the strength and coherence of activity in each cell type. Coherence reflects time-locking to sniffing and synchrony with other neurons of the same type. Experimental data supporting each prediction are cited in the text. The ultimate effect on MT cell output depends on emerging details of the OB circuitry.
C. Increasing input frequency leads to an increase in MT cell spike output in an OB slice preparation. (i) Voltage recordings from a MT cell showing responses evoked by four brief current injections simulating synaptic inputs (arrows) at 1 and 2.5 Hz. (ii) Plot of total spike output among MT cells as a function of input frequency. Precision of spike timing also increases (not shown). Modified with permission from (Balu et al., 2004).
D. Increasing sniff frequency shortens inhalation-evoked MT spike bursts in vivo. (i) Spike histogram of a MT cell during odorant sampling controlled by playback of sniffing recorded previously in an awake rat. This cell responds phasically to each sniff during both low-frequency respiration and a high-frequency sniff bout. Contrast with ORN responses in Figure 4A. (ii) Plots of inhalation-triggered spiking patterns for an MT cell evoked by inhalations repeated at 1 – 5 Hz. The cell shows a shortening of its spike burst, along with a modest decrease in peak firing rate and increase in response latency, all of which are consistent with increased inhibition. Modified with permission from (Carey and Wachowiak, 2011).
Predicted effects of sniff frequency on OB processing arise from experiments in anesthetized animals or slice preparations in which sniff frequency is mimicked with pulsed electrical stimulation or direct current injection (Balu et al., 2004; Hayar et al., 2004b; Margrie and Schaefer, 2003). These studies have led to predictions that increasing sniff frequency will have distinct, cell-type-specific effects on the strength of odorant-evoked activity and the coherence of activity across a population of neurons within the OB. For example, granule cells - GABA-ergic interneurons thought to mediate feedback- and lateral inhibition of MT cells - show increased synchrony and stronger inhibition onto MT cells at synaptic input frequencies corresponding to active sniffing (Young and Wilson, 1999; Schoppa, 2006a). In addition, MT cells themselves show increased spike output and temporal precision as input frequency increases into the range of active sniffing (Balu et al., 2004) (Figure 5C).
Another important element mediating sniff frequency-dependent changes in OB processing is the external tufted (ET) cell - an excitatory interneuron in the glomerular-layer. ET cells can drive direct feedforward excitation as well as indirect (disynaptic) feedforward inhibition of MT cells and are thus potent regulators of MT excitability (Hayar et al., 2004a; Najac et al., 2011). ET cells show spontaneous spike bursts but their bursts become increasingly entrained to rhythmic ORN inputs as input frequency increases (Hayar et al., 2004b), leading to an increase both in their excitation of MT cells and their activation of inhibitory periglomerular interneurons (PG cells) (Hayar et al., 2004a). In vivo, this effect is predicted to generate an increasingly sharp time-window over which MT cells integrate ORN inputs and may also increase the strength of lateral inhibition between glomeruli (Wachowiak and Shipley, 2006).
Overall, the consensus prediction from these circuit-level studies is that frequency-dependent effects within the OB network serve to enhance the inhalation-driven temporal patterning of ORN inputs and increase the reliability and temporal precision of MT cell firing relative to inhalation onset (Balu et al., 2004; Schaefer et al., 2006; Wachowiak and Shipley, 2006). MT cell recordings from anesthetized rats during artificial sniffing at different frequencies have found frequency-dependent changes in MT response dynamics consistent with this prediction (Bathellier et al., 2008; Carey and Wachowiak, 2011) (Figure 5D). Thus, the circuit organization and dynamics of central olfactory networks appear optimized to process sensory inputs organized by inhalation.
Integrating data across experimental paradigms in the context of active sensing
While data from slice experiments, anesthetized animals and computational studies all point to the fundamental importance of sniff-driven dynamics in shaping odor information processing, integrating these results with data from awake animals in which sampling behavior is truly ‘active’ (and highly variable) remains a major challenge. For example, no studies in awake animals have systematically explored the relationship between a particular parameter of sniffing behavior and circuit interactions in the OB or PC. In addition, slice experiments that mimic sniffing with electrical or optogenetic stimulation typically use synchronous activation of many neurons to mimic a sniff (Hayar et al., 2004b; Young and Wilson, 1999) rather than the slowly-rising, inhalation-driven packets of ORN input that develop over ~100 msec in vivo (Carey et al., 2009). Significant changes in synaptic transmission can develop during this time window - for example, synaptic depression and presynaptic inhibition of transmitter release from ORNs (Murphy et al., 2004; Wachowiak et al., 2005); these effects are not apparent following single shocks to the olfactory nerve. Extrapolating response properties from slice experiments or anesthetized animals to behaving animals is also complicated by differences in the spontaneous activity of MT cells, other interneurons, and centrifugal inputs to the OB and PC in awake versus anesthetized or slice preparations (Davison and Katz, 2007; Rinberg and Gelperin, 2006).
Nonetheless, many of the basic response properties of ORNs as well as MT and PC neurons are similar in anesthetized and awake animals. Inhalation-driven ORN responses show identical latencies and burst durations in awake and anesthetized rodents and similar degrees of frequency-dependent attenuation of response strength (Carey et al., 2009; Verhagen et al., 2007). Likewise, MT cells recorded from anesthetized and awake rodents show nearly identical response dynamics relative to inhalation in terms of their range of response latencies, duration and precision of spike timing (Carey and Wachowiak, 2011; Shusterman et al., 2011). Strategies of odor identity coding also appear similar in awake and anesthetized preparations, with MT cells showing roughly similar response specificities (Davison and Katz, 2007). Importantly, many of these similarities only become apparent when considered relative to inhalation or sniffing (Cury and Uchida, 2010; Shusterman et al., 2011); earlier studies that did not precisely monitor sniff timing noted significant differences in response features between anesthetized and awake animals (Rinberg et al., 2006a). Thus, while understanding olfactory system function in any realistic context will require work in behaving animals, anesthetized and slice preparations remain important tools by enabling systematic exploration of network dynamics and more direct probing of olfactory circuits. Considering these results from the perspective of active sensing and, specifically, sniff timing, appears key to integrating data across paradigms.
Attentional control and sensorimotor integration during active sensing
Thus far we have considered active sensing as a ‘bottom-up’ process in which the physical aspects of stimulus sampling shape sensory neuron activation and, subsequently, central processing. However, active sensing in any modality also involves ‘top-down’ mechanisms, which modulate sensory processing in coordination with stimulus sampling and other behavioral states. While ‘bottom-up’ processes are, as we have seen, amenable to a range of experimental approaches, investigating ‘top-down’ processes ultimately requires work in the awake animal, in which the systems modulating these processes are functioning normally. While the modulation of olfactory processing has been extensively studied - in particular in the rodent OB - much of this work has been performed in anesthetized animals and relatively little has been performed or interpreted in the context of active sensing, in which sensory processing is modulated in precise coordination with sampling behavior. Here we discuss potential pathways underlying the active modulation of olfactory processing, using parallels from other modalities - vision and somatosensation in particular - as instructive examples.
The modulation of sensory processing as a function of focal sampling in space or time has been termed ‘directed’ or ‘selective’ attention (Noudoost et al., 2010). For example, visual saccades involve directed attentional modulation of the responsiveness of visual neurons: responses of neurons with receptive fields in the region of spatial attention (e.g. - the target region of the saccade) show transient increases in sensitivity, while neurons with receptive fields in other regions show decreases in sensitivity (Winkowski and Knudsen, 2007; Noudoost et al., 2010). Similarly, cortical somatosensory neurons change their responsiveness to mechanosensory stimuli in the transition from passive to active touch mediated by reaching (in primates) or whisking (in rodents) (Hentschke et al., 2006; Nelson et al., 1991).
Like saccades and active touch, sniffing can provide an unambiguous and temporally precise behavioral readout of directed attention (Kepecs et al., 2007; Wesson et al., 2008a). In humans, anticipation of sniffing and attention to an olfactory task modulates activity in primary olfactory cortical areas (Zelano et al., 2005). Beyond these initial observations, however, attentional modulation of olfactory processing related to active sniffing remains largely unexplored. One prediction is that individual ‘active’ sniffs or high-frequency sniff bouts modulate odorant-evoked responses. A critical feature of directed attention in other modalities is that attentional modulation is transient and precisely timed to coincide with (and briefly precede) active sampling (Han et al., 2009). Tests for sniff-related modulation are less straightforward than for vision or touch because - at least in the awake mammal - inhalation is required to elicit odorant-evoked responses, precluding odorant presentation at different times relative to a sniff. Optogenetic approaches in which light is used to reliably activate sensory inputs independent of sniff timing (Smear et al., 2011) provide a promising solution to this problem.
What are the neural pathways underlying attentional modulation during active sensing?. In the heavily-studied visual system, multiple cortical as well as thalamic areas have been implicated in directed attention (Noudoost et al., 2010). One major source of attentional control is the frontal eye field - the premotor area controlling eye movements. In nonhuman primates, microstimulation of frontal eye field neurons enhances the responsiveness of visual cortex neurons with spatially overlapping receptive fields (Moore et al., 2003; Noudoost et al., 2010). In the somatosensory system, there are reciprocal connections between somatosensory neurons and the motor areas controlling active touch (Veinante and Deschênes, 2003). In addition, recent evidence has not only demonstrated monosynaptic connections between primary somatosensory and motor cortices corresponding to the same whisker (Ferezou et al., 2007) but direct control of whisker protraction by somatosensory cortex (Matyas et al., 2010). Thus, a tight coordination between the motor systems controlling stimulus sampling and the processing of incoming sensory signals mediated by this sampling is likely a fundamental component of top-down control in active sensing.
There is considerable evidence for coordination between olfactory sensory pathways and the motor systems controlling sniffing. First, in both humans and in rodents, olfactory stimuli can modulate sniffing behavior extremely quickly: humans show differences in the flow rate of inhalation that vary with odorant intensity within 200 msec after beginning an inhalation (Figure 6A) (Johnson et al., 2003); rats show an increase in sniff frequency in response to novel odorants in a similar time after inhalation and in as little as 50 – 100 msec after sensory input arrives at the OB (Figure 6B)(Wesson et al., 2008a). Motor signals related to sniffing also affect odor perception. For example, in human subjects in which odorant is injected into the bloodstream, sniffing can ‘gate’ odor perception (Mainland and Sobel, 2006). In addition, the degree of motor effort expended during a sniff affects perceived odor intensity (Hornung et al., 1997; Teghtsoonian and Teghtsoonian, 1984). Thus, motor information about sniffing is rapidly integrated with incoming sensory information and the motor component of sniffing appears to play an essential role in constructing an odor percept (Mainland and Sobel, 2006).
Figure 6. Sensorimotor integration during odor sensing.
A. Humans show rapid modulation of sniff magnitude as a function of odorant concentration. Blue and purple traces show mean intranasal flow rate for low and high concentrations of an odorant; black trace shows the p-value of the difference between the flow rates at each concentration. Sniff flow rates diverge and remain different at 160 msec after inhalation onset. Modified with permission from (Johnson et al., 2003).
B. Rats show similarly rapid changes in sniffing in response to a novel odorant. Red and blue plots show cumulative inhalation count over time during presentations of novel versus familiar (‘learned’) odorants. Black plot shows p-value of the difference between novel and learned trials. Sniff counts diverges significantly at 140 msec after the first inhalation. Modified with permission from (Wesson et al., 2008a).
C. Potential substrates for cortical control of sniffing behavior. Electrical stimulation of infralimbic cortex in an anesthetized rat elicits respiratory changes that closely resemble exploratory sniffing (compare with Figure 4A). Modified with permission from Aleksandrov et al. (2007).
D. Schematic illustrating the potential neural pathways involved in attentional modulation and sensorimotor integration during sniffing. Gray paths indicate the movement of odorant molecules through the nasal cavity; blue paths indicate sensory afferent pathways; red paths indicate centrifugal pathways targeting OB and PC. Dashed lines indicate more speculative connections. See text for references. IL, Infralimbic cortex; Ins, Insular cortex; LC, locus coeruleus; RN, Raphe nuclei; Cb, cerebellum; NTS, nucleus of the solitary tract. Brain template courtesy of A. Puche, Univ. of Maryland.
Potential pathways underlying sensorimotor integration during sniffing are outlined in Figure 6D. However, much of the neural circuitry mediating motor-related control of olfactory processing remains unclear, largely because the premotor control of sniffing is poorly understood. One important structure may be the cerebellum, which is activated during sniffing, may receive olfactory input from PC, and is involved in optimizing motor output for sensory acquisition in other modalities (Mainland and Sobel, 2006; Robinson, 1976; Sobel et al., 1998b). Sniffing is also likely under cortical control: several cortical areas, including the insular and infralimbic cortices, send projections to brainstem nuclei (such as the nucleus of the solitary tract) involved in respiratory pattern generation (Bianchi et al., 1995), and electrical stimulation of insular and infralimbic cortices alters respiration in anesthetized rats and can elicit increases in respiration frequency that mimic exploratory sniff bouts (Aleksandrov et al., 2007) (Figure 6C). Whether activation of these or other areas modulate processing in olfactory areas such as OB and PC remains untested.
Classical neuromodulatory pathways likely also play a role in directed attention. For example, cholinergic inputs to visual cortex alter visual responses via muscarinic acetylcholine receptors (Goard and Dan, 2009; Herrero et al., 2008), and dopaminergic signaling modulates the top-down control of visual responses by frontal eye field neurons (Noudoost and Moore, 2011). In the olfactory system, cholinergic, noradrenergic and serotonergic projections all target OB and PC (McLean and Shipley, 1992) and are capable of modulating olfactory responses (Chaudhury et al., 2009; Petzold et al., 2009; Shea et al., 2008) (Figure 6D). In addition, several olfactory cortical areas send strong centrifugal projections to the OB which are hypothesized to modulate odorant processing by affecting the strength of inhibition within the OB network (Strowbridge, 2009). Importantly, several of these areas are activated during sniffing in the absence of odorant, presumably by the airflow-driven, somatosensory component of a sniff (Adrian, 1942; Sobel et al., 1998a; Grosmaitre et al., 2007). Sniff-induced feedback from olfactory cortical areas to OB or PC might thus provide an alternate, ‘bottom-up’ mechanism by which a sniff can modulate olfactory processing.
Pursuing olfaction in the framework of active sensing
The active control of stimulus sampling and sensory information processing is fundamental to all sensory systems, and investigating sensory function from a perspective of active sensing us important not only for understanding sensation in the behaving animal, but also for integrating data obtained with different experimental approaches and at different levels of the nervous system. In the mammalian olfactory system, the act for odor inhalation through respiration or sniffing is a fundamental feature that is reflected in its anatomical, cellular and systems-level organization and thus can serve as a framework for unifying work at many levels. Considering olfaction as an active sense also serves to highlight areas ripe for future investigation.
For example, in the periphery, it seems important to gain a greater understanding of airflow patterns in the nasal cavity during the range of sniffing strategies expressed during behavior, analogous to the detailed descriptions of whisker movements described for the rodent somatosensory system (Ritt et al., 2008). Centrally, it is important to better understand how sniff-driven inputs shape the transformation of odor representations in the OB and its cortical targets - such questions have been addressed in other systems through replay of naturalistic stimuli (Goard and Dan, 2009) or recording from central neurons while carefully monitoring sampling behavior in the awake animal (Han et al., 2009; Nelson et al., 1991); to date only a handful of studies have used such approaches in the olfactory system (Cury and Uchida, 2010; Shusterman et al., 2011; Verhagen et al., 2007; Wesson et al., 2008a). Finally, a key to understanding how top-down pathways actively shape odor processing is understanding how and when these pathways are activated during odor sensing; this question has also been difficult to address in other modalities. While challenging, these and related questions outline a path towards achieving a more complete - and realistic - understanding of sensory system function during behavior.
Acknowledgments
I would like to thank the past and present members of the Wachowiak lab - in particular D. Wesson, J. Verhagen and R. Carey - for contributing to the views expressed here and for performing critical experiments described from our laboratory. I would also like to thank T. Bozza, D. Rinberg, M. Shipley, D. Katz, A. Yamaguchi, and K. Zhao for valuable discussions. The laboratory has been supported by the National Institutes of Health (NIDCD), Boston University and the University of Utah USTAR initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Adrian ED. Olfactory reactions in the brain of the hedgehog. J Physiol. 1942;100:459–473. doi: 10.1113/jphysiol.1942.sp003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED. Sensory discrimination with some recent evidence from the olfactory organ. Br Med Bull. 1950;6:330–331. doi: 10.1093/oxfordjournals.bmb.a073625. [DOI] [PubMed] [Google Scholar]
- Aleksandrov V, Invanova TG, Aleksandrov NP. Prefrontal control of respiration. J Physiol Pharmacol. 2007;58:17–23. [PubMed] [Google Scholar]
- Balu R, Larimer P, Strowbridge BW. Phasic stimuli evoke precisely timed spikes in intermittently discharging mitral cells. J Neurophysiol. 2004;92:743–753. doi: 10.1152/jn.00016.2004. [DOI] [PubMed] [Google Scholar]
- Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic Ensemble Odor Coding in the Mammalian Olfactory Bulb: Sensory Information at Different Timescales. Neuron. 2008;57:586–598. doi: 10.1016/j.neuron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Bramble DM, Carrier DR. Running and breathing in mammals. Science. 1983;219:251–256. doi: 10.1126/science.6849136. [DOI] [PubMed] [Google Scholar]
- Buonviso N, Amat C, Litaudon P. Respiratory modulation of olfactory neurons in the rodent brain. Chem Senses. 2006;31:145–154. doi: 10.1093/chemse/bjj010. [DOI] [PubMed] [Google Scholar]
- Cang J, Isaacson JS. In Vivo Whole-Cell Recording of Odor-Evoked Synaptic Transmission in the Rat Olfactory Bulb. J Neurosci. 2003;23:4108–4116. doi: 10.1523/JNEUROSCI.23-10-04108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Verhagen JV, Wesson DW, Pirez N, Wachowiak M. Temporal Structure of Receptor Neuron Input to the Olfactory Bulb Imaged in Behaving Rats. J Neurophysiol. 2009;101:1073–1088. doi: 10.1152/jn.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RM, Wachowiak M. Effect of sniffing on the temporal structure of mitral/tufted cell output from the olfactory bulb. J Neurosci. 2011;31:10615–10626. doi: 10.1523/JNEUROSCI.1805-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania KC. Olfaction: underwater ‘sniffing’ by semi-aquatic mammals. Nature. 2006;444:1024–1025. doi: 10.1038/4441024a. [DOI] [PubMed] [Google Scholar]
- Chaput MA. Respiratory-phase-related coding of olfactory information in the olfactory bulb of awake freely-breathing rabbits. Physiol Behav. 1986;36:319–324. doi: 10.1016/0031-9384(86)90023-5. [DOI] [PubMed] [Google Scholar]
- Chaput MA, Chalansonnet M. Recording the slow potentials evoked by odors in the olfactory mucosa of awake animals. Journal of Neuroscience Methods. 1997;75:193–198. doi: 10.1016/s0165-0270(97)00072-1. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Escanilla O, Linster C. Bulbar acetylcholine enhances neural and perceptual odor discrimination. J Neurosci. 2009;29:52–60. doi: 10.1523/JNEUROSCI.4036-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Li X, Nelson SB. Short-Term Depression at Thalamocortical Synapses Contributes to Rapid Adaptation of Cortical Sensory Responses In Vivo. Neuron. 2002;34:437–446. doi: 10.1016/s0896-6273(02)00659-1. [DOI] [PubMed] [Google Scholar]
- Clarke S. Sniffing and fixed-ratio behavior for sucrose and brain stimulation reward in the rat. Physiol Behav. 1971;7:695–699. doi: 10.1016/0031-9384(71)90133-8. [DOI] [PubMed] [Google Scholar]
- Craven BA, Paterson EG, Settles GS. The fluid dynamics of canine olfaction: unique nasal airflow patterns as an explanation of macrosmia. Journal of The Royal Society Interface. 2010;7:933–943. doi: 10.1098/rsif.2009.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, Poulet, James FA, Kremer Y, Petersen, Carl CH. Synaptic Mechanisms Underlying Sparse Coding of Active Touch. Neuron. 2011;69:1160–1175. doi: 10.1016/j.neuron.2011.02.022. [DOI] [PubMed] [Google Scholar]
- Cury KM, Uchida N. Robust odor coding via inhalation-coupled transient activity in the mammalian olfactory bulb. Neuron. 2010;68:570–585. doi: 10.1016/j.neuron.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Davison IG, Katz LC. Sparse and Selective Odor Coding by Mitral/Tufted Neurons in the Main Olfactory Bulb. J Neurosci. 2007;27:2091–2101. doi: 10.1523/JNEUROSCI.3779-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethier VG. Sniff, Flick, and Pulse: An Appreciation of Interruption. Proceedings of the American Philosophical Society. 1987;131:159–176. [Google Scholar]
- Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CC. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat Neurosci. 2007;10:348–354. doi: 10.1038/nn1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn I, Scherer PW, Mozell MM. A mass transport model of olfaction. J Theor Biol. 1994;167:115–128. doi: 10.1006/jtbi.1994.1057. [DOI] [PubMed] [Google Scholar]
- Han X, Xian SX, Moore T. Dynamic sensitivity of area V4 neurons during saccade preparation. Proceedings of the National Academy of Sciences. 2009;106:13046–13051. doi: 10.1073/pnas.0902412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: A major excitatory element that coordinates glomerular activity. J Neurosci. 2004a;24:6676–6685. doi: 10.1523/JNEUROSCI.1367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Shipley MT, Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci. 2004b;24:1190–1199. doi: 10.1523/JNEUROSCI.4714-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschke H, Haiss F, Schwarz C. Central signals rapidly switch tactile processing in rat barrel cortex during whisker movements. Cereb Cortex. 2006;16:1142–1156. doi: 10.1093/cercor/bhj056. [DOI] [PubMed] [Google Scholar]
- Herrero JL, Roberts MJ, Delicato LS, Gieselmann MA, Dayan P, Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JJ. Pattern recognition computation using action potential timing for stimulus representation. Nature. 1995;376:33–36. doi: 10.1038/376033a0. [DOI] [PubMed] [Google Scholar]
- Hornung DE, Chin C, Kurtz DB, Kent PF, Mozell MM. Effect of nasal dilators on perceived odor intensity. Chem Senses. 1997;22:177–180. doi: 10.1093/chemse/22.2.177. [DOI] [PubMed] [Google Scholar]
- Hummel T. Retronasal Perception of Odors. Chemistry & Biodiversity. 2008;5:853–861. doi: 10.1002/cbdv.200890100. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Chemotopic odorant coding in a mammalian olfactory system. J Comp Neurol. 2007;503:1–34. doi: 10.1002/cne.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BN, Mainland JD, Sobel N. Rapid olfactory processing implicates subcortical control of an olfactomotor system. J Neurophysiol. 2003;90:1084–1094. doi: 10.1152/jn.00115.2003. [DOI] [PubMed] [Google Scholar]
- Kent PF, Mozell MM, Murphy SJ, Hornung DE. The interaction of imposed and inherent olfactory mucosal activity patterns and their composite representation in a mammalian species using voltage-sensitive dyes. J Neurosci. 1996;16:345–353. doi: 10.1523/JNEUROSCI.16-01-00345.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. Rapid and precise control of sniffing during olfactory discrimination in rats. J Neurophysiol. 2007;98:205–213. doi: 10.1152/jn.00071.2007. [DOI] [PubMed] [Google Scholar]
- Laing DG. Natural sniffing gives optimum odour perception for humans. Perception. 1983;12:99–117. doi: 10.1068/p120099. [DOI] [PubMed] [Google Scholar]
- Laing DG. Identification of single dissimilar odors is achieved by humans with a single sniff. Physiol Behav. 1986;37:163–170. doi: 10.1016/0031-9384(86)90400-2. [DOI] [PubMed] [Google Scholar]
- Macrides F. Temporal relationships between hippocampal slow waves and exploratory sniffing in hamsters. Behav Biol. 1975;14:295–308. doi: 10.1016/s0091-6773(75)90419-8. [DOI] [PubMed] [Google Scholar]
- Macrides F, Chorover SL. Olfactory bulb units: activity correlated with inhalation cycles and odor quality. Science. 1972;175:84–87. doi: 10.1126/science.175.4017.84. [DOI] [PubMed] [Google Scholar]
- Mainland J, Sobel N. The sniff is part of the olfactory percept. Chem Senses. 2006;31:181–196. doi: 10.1093/chemse/bjj012. [DOI] [PubMed] [Google Scholar]
- Margrie TW, Schaefer AT. Theta oscillation coupled spike latencies yield computational vigour in a mammalian sensory system. J Physiol. 2003;546:363–374. doi: 10.1113/jphysiol.2002.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyas F, Sreenivasan V, Marbach F, Wacongne C, Barsy B, Mateo C, Aronoff R, Petersen CCH. Motor Control by Sensory Cortex. Science. 2010;330:1240–1243. doi: 10.1126/science.1195797. [DOI] [PubMed] [Google Scholar]
- McDermott KC, Malkoc G, Mulligan JB, Webster MA. Adaptation and visual salience. Journal of Vision. 2010:10. doi: 10.1167/10.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J, Shipley M. Neuroanatomical substrates of olfaction. In: Serby M, Chobor K, editors. Science of Olfaction. New York: Springer-Verlag; 1992. pp. 126–171. [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Mozell MM. Evidence for sorption as a mechanism of the olfactory analysis of vapours. Nature. 1964;203:1181–1182. doi: 10.1038/2031181a0. [DOI] [PubMed] [Google Scholar]
- Mozell MM, Jagodowicz M. Chromatographic separation of odorants by the nose: retention times measured across in vivo olfactory mucosa. Science. 1973;181:1247–1249. doi: 10.1126/science.181.4106.1247. [DOI] [PubMed] [Google Scholar]
- Mozell MM, Sheehe PR, Hornung DE, Kent PF, Youngentob SL, Murphy SJ. Imposed and inherent mucosal activity patterns. J Gen Physiol. 1987;90:625–650. doi: 10.1085/jgp.90.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C, Cain WS, Bartoshuk LM. Mutual action of taste and olfaction. Sens Processes. 1977;1:204–211. [PubMed] [Google Scholar]
- Murphy GJ, Glickfeld LL, Balsen Z, Isaacson JS. Sensory neuron signaling to the brain: properties of transmitter release from olfactory nerve terminals. J Neurosci. 2004;24:3023–3030. doi: 10.1523/JNEUROSCI.5745-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najac M, De Saint Jan D, Reguero L, Grandes P, Charpak S. Monosynaptic and Polysynaptic Feed-Forward Inputs to Mitral Cells from Olfactory Sensory Neurons. The Journal of Neuroscience. 2011;31:8722–8729. doi: 10.1523/JNEUROSCI.0527-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Li B, Douglas VD. Sensory response enhancement and suppression of monkey primary somatosensory cortical neurons. Brain Research Bulletin. 1991;27:751–757. doi: 10.1016/0361-9230(91)90059-s. [DOI] [PubMed] [Google Scholar]
- Nevitt GA. Do fish sniff? A new mechanism of olfactory sampling in pleuronectid flounders. J Exp Biol. 1991;157:1–18. doi: 10.1242/jeb.157.1.1. [DOI] [PubMed] [Google Scholar]
- Noudoost B, Chang MH, Steinmetz NA, Moore T. Top-down control of visual attention. Current Opinion in Neurobiology. 2010;20:183–190. doi: 10.1016/j.conb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature. 2011;474:372–375. doi: 10.1038/nature09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y, Takai Y, Touhara K. Nasal Airflow Rate Affects the Sensitivity and Pattern of Glomerular Odorant Responses in the Mouse Olfactory Bulb. J Neurosci. 2009;29:12070–12078. doi: 10.1523/JNEUROSCI.1415-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci. 2009 doi: 10.1038/nn.2335. [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor Representations in Olfactory Cortex: “Sparse” Coding, Global Inhibition, and Oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Response properties of isolated mouse olfactory receptor cells. J Physiol. 2001;530:113–122. doi: 10.1111/j.1469-7793.2001.0113m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennaker RL, Chen CFF, Ruyle AM, Sloan AM, Wilson DA. Spatial and Temporal Distribution of Odorant-Evoked Activity in the Piriform Cortex. J Neurosci. 2007;27:1534–1542. doi: 10.1523/JNEUROSCI.4072-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Gelperin A. Olfactory neuronal dynamics in behaving animals. Semin Cell Dev Biol. 2006;17:454–461. doi: 10.1016/j.semcdb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006a;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron. 2006b;51:351–358. doi: 10.1016/j.neuron.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Ritt JT, Andermann ML, Moore CI. Embodied Information Processing: Vibrissa Mechanics and Texture Features Shape Micromotions in Actively Sensing Rats. Neuron. 2008;57:599–613. doi: 10.1016/j.neuron.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. Adaptive gain control of vestibuloocular reflex by the cerebellum. Journal of Neurophysiology. 1976;39:954–969. doi: 10.1152/jn.1976.39.5.954. [DOI] [PubMed] [Google Scholar]
- Schaefer AT, Angelo K, Spors H, Margrie TW. Neuronal Oscillations Enhance Stimulus Discrimination by Ensuring Action Potential Precision. PLoS Biology. 2006;4 doi: 10.1371/journal.pbio.0040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AT, Margrie TW. Spatiotemporal representations in the olfactory system. Trends Neurosci. 2007;30:92–100. doi: 10.1016/j.tins.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Cleland TA. Anatomical contributions to odorant sampling and representation in rodents: zoning in on sniffing behavior. Chem Senses. 2006;31:131–144. doi: 10.1093/chemse/bjj015. [DOI] [PubMed] [Google Scholar]
- Schoppa NE. AMPA/kainate receptors drive rapid output and precise synchrony in olfactory bulb granule cells. J Neurosci. 2006a;26:12996–13006. doi: 10.1523/JNEUROSCI.3503-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa NE. Synchronization of olfactory bulb mitral cells by precisely timed inhibitory inputs. Neuron. 2006b;49:271–283. doi: 10.1016/j.neuron.2005.11.038. [DOI] [PubMed] [Google Scholar]
- Scott-Johnson PE, Blakley D, Scott JW. Effects of air flow on rat electroolfactogram. Chem Senses. 2000;25(25):761–768. doi: 10.1093/chemse/25.6.761. [DOI] [PubMed] [Google Scholar]
- Scott JW, Brierley T, Schmidt FH. Chemical determinants of the rat electro-olfactogram. J Neurosci. 2000;20:4721–4731. doi: 10.1523/JNEUROSCI.20-12-04721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, Katz LC, Mooney R. Noradrenergic Induction of Odor-Specific Neural Habituation and Olfactory Memories. The Journal of Neuroscience. 2008;28:10711–10719. doi: 10.1523/JNEUROSCI.3853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman R, Smear MC, Koulakov AA, Rinberg D. Precise olfactory responses tile the sniff cycle. Nat Neurosci. 2011;14:1039–1044. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- Small DM, Gerber JC, Mak YE, Hummel T. Differential Neural Responses Evoked by Orthonasal versus Retronasal Odorant Perception in Humans. Neuron. 2005;47:593–605. doi: 10.1016/j.neuron.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Smear M, Shusterman R, O’Connor R, Bozza T, Rinberg D. Perception of sniff phase in mouse olfaction. Nature. 2011 doi: 10.1038/nature10521. in press. [DOI] [PubMed] [Google Scholar]
- Snow PJ. The Antennular Activities of the Hermit Crab, Pagurus Alaskensis (Benedict) J Exp Biol. 1973;58:745–765. doi: 10.1242/jeb.63.1.1. [DOI] [PubMed] [Google Scholar]
- Sobel EC, Tank DW. Timing of odor stimulation does not alter patterning of olfactory bulb unit activity in freely breathing rats. J Neurophysiol. 1993;69:1331–1337. doi: 10.1152/jn.1993.69.4.1331. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature. 1998a;392:282–286. doi: 10.1038/32654. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, Gabrieli JD, Sullivan EV. Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci. 1998b;18:8990–9001. doi: 10.1523/JNEUROSCI.18-21-08990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spors H, Grinvald A. Spatio-temporal dynamics of odor representations in the mammalian olfactory bulb. Neuron. 2002;34:301–315. doi: 10.1016/s0896-6273(02)00644-x. [DOI] [PubMed] [Google Scholar]
- Steen JB, Mohus I, Kvesetberg TK, Walloe L. Olfaction in bird dogs during hunting. Acta Physiol Scand. 1996;157:115–119. doi: 10.1046/j.1365-201X.1996.479227000.x. [DOI] [PubMed] [Google Scholar]
- Stokes CCA, Isaacson JS. From Dendrite to Soma: Dynamic Routing of Inhibition by Complementary Interneuron Microcircuits in Olfactory Cortex. Neuron. 2010;67:452–465. doi: 10.1016/j.neuron.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowbridge BW. Role of Cortical Feedback in Regulating Inhibitory Microcircuits. Annals of the New York Academy of Sciences. 2009;1170:270–274. doi: 10.1111/j.1749-6632.2009.04018.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Antennal movements induced by odour and central projection of the antennal neurones in the honey-bee. Journal of Insect Physiology. 1975;21:831–847. [Google Scholar]
- Teghtsoonian R, Teghtsoonian M. Testing a perceptual constancy model for odor strength: the effects of sniff pressure and resistance to sniffing. Perception. 1984;13:743–752. doi: 10.1068/p130743. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Veinante P, Deschênes M. Single-cell study of motor cortex projections to the barrel field in rats. The Journal of Comparative Neurology. 2003;464:98–103. doi: 10.1002/cne.10769. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci. 2007;10:631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, McGann JP, Heyward PM, Shao Z, Puche AC, Shipley MT. Inhibition of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J Neurophysiol. 2005;94:2700–2712. doi: 10.1152/jn.00286.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Semin Cell Dev Biol. 2006;17:411–423. doi: 10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing in the albino rat. Behavior. 1964;22:223–244. [Google Scholar]
- Wesson DW, Carey RM, Verhagen JV, Wachowiak M. Rapid Encoding and Perception of Novel Odors in the Rat. PLoS Biology. 2008a;6:e82. doi: 10.1371/journal.pbio.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Donahou TN, Johnson MO, Wachowiak M. Sniffing behavior of mice during performance in odor-guided tasks. Chemical Senses. 2008b;33:581–596. doi: 10.1093/chemse/bjn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Verhagen JV, Wachowiak M. Why Sniff Fast? The Relationship Between Sniff Frequency, Odor Discrimination, and Receptor Neuron Activation in the Rat. J Neurophysiol. 2009;101:1089–1102. doi: 10.1152/jn.90981.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GC, Scherer PW, Zhao K, Mozell MM. Numerical Modeling of Odorant Uptake in the Rat Nasal Cavity. Chem Senses. 2007;32:273–284. doi: 10.1093/chemse/bjl056. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci U S A. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TA, Wilson DA. Frequency-dependent modulation of inhibition in the rat olfactory bulb. Neuroscience Letters. 1999;276:65–67. doi: 10.1016/s0304-3940(99)00781-8. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Mozell MM, Sheehe PR, Hornung DE. A quantitative analysis of sniffing strategies in rats performing odor discrimination tasks. Physiol Behav. 1987;41:59–69. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N. Attentional modulation in human primary olfactory cortex. Nat Neurosci. 2005;8:114–120. doi: 10.1038/nn1368. [DOI] [PubMed] [Google Scholar]
- Zhao K, Dalton P, Yang GC, Scherer PW. Numerical modeling of turbulent and laminar airflow and odorant transport during sniffing in the human and rat nose. Chem Senses. 2006;31:107–118. doi: 10.1093/chemse/bjj008. [DOI] [PubMed] [Google Scholar]
- Zhao K, Scherer PW, Hajiloo SA, Dalton P. Effect of anatomy on human nasal air flow and odorant transport patterns: implications for olfaction. Chem Senses. 2004;29:365–379. doi: 10.1093/chemse/bjh033. [DOI] [PubMed] [Google Scholar]