Abstract
Oral immunization with a Salmonella vaccine vector expressing enterotoxigenic E. coli colonization factor antigen I (CFA/I) can protect against collagen-induced arthritis (CIA) by dampening IL-17 and IFN-γ via enhanced IL-4, IL-10, and TGF-β. To identify the responsible regulatory CD4+ T cells making the host refractory to CIA, Salmonella-CFA/I induced CD39+CD4+ T cells with enhanced apyrase activity relative to Salmonella vector-immunized mice. Adoptive transfer of vaccine-induced CD39+CD4+ T cells into CIA mice conferred complete protection, while CD39−CD4+ T cells did not. Subsequent analysis of vaccinated FoxP3-GFP mice revealed the CD39+ T cells were composed of FoxP3-GFP− and FoxP3-GFP+ subpopulations. Although each adoptively transferred Salmonella-CFA/I-induced FoxP3− and FoxP3+CD39+CD4+ T cells could protect against CIA, each subset was not as efficacious as total CD39+CD4+ T cells, suggesting their interdependence for optimal protection. Cytokine analysis revealed FoxP3− CD39+CD4+ T cells produced TGF-β, and FoxP3+CD39+CD4+ T cells produced IL-10, showing a segregation of function. Moreover, donor FoxP3-GFP− CD4+ T cells converted to FoxP3-GFP+ CD39+CD4+ T cells in the recipients, showing plasticity of these regulatory T cells. TGF-β was found to be essential for protection since in vivo TGF-β neutralization reversed activation of cAMP-response element-binding protein (CREB) and reduced the development of CD39+CD4+ T cells. Thus, CD39 apyrase-expressing CD4+ T cells stimulated by Salmonella-CFA/I are composed of TGF-β-producing FoxP3− CD39+CD4+ T cells and support the stimulation of IL-10-producing FoxP3+ CD39+CD4+ T cells.
Keywords: regulatory T cells, arthritis, CD39+, TGF-β, IL-10
Introduction
Regulatory CD4+ T cells have been studied extensively because of their ability to suppress autoimmunity. Controlled by FoxP3 transcription factor, natural regulatory CD25+CD4+ T (Treg) cells are generated in the thymus, suppressing the development of autoreactive T cells (1–4). More notably, a variety of inducible regulatory CD4+ T (iTreg) cells’ responses to soluble or infectious Ag exposure have been described (5, 6). These Treg cells mediate their suppression by inhibiting inflammatory cell proliferation and cytokine responses in an Ag-dependent or -independent fashion (7–10). Importantly, Ag-specific Treg cells show greater potency (7–10) and are particularly adept in suppressing autoimmunity (8, 9). Lacking CD25 expression, other regulatory T cell subsets (3, 11, 12) have been identified by their functional attribute of producing IL-10 (11, 12) or TGF-β (6). The former subset, commonly referred to as Tr1 cells, represents a diverse subset of regulatory T cells, which does not necessarily express CD25 or FoxP3 (2, 11, 12). Similarly, TGF-β-producing regulatory CD4+ T cells, also referred to as Th3 cells, require TCR stimulation, but feature bystander suppression in an Ag-independent manner (13). More recently, expression of CD39 and/or CD73 by regulatory FoxP3+ T cells has been shown (14–16). While we have shown that regulatory CD39+ T cells can be further fractionated, being CD25− or CD25+ (10, 17), the IL-35-induced CD25−CD39+ CD4+ T cell subset showed the greatest potency via IL-10 production, conferring complete protection against collagen-induced arthritis (CIA; 17).
CD39, an ectonuleoside triphosphate diphosphohydrolase, is expressed on the cell surface of lymphocytes and dampens proinflammatory cells by ultimately converting extracellular ATP to adenosine (14, 18). CD39 can be induced during apoptosis or after tissue damage when ATPs are released into the extracellular milieu (14, 16). Free extracellular ATP stimulates inflammatory responses by macrophages, particularly those that have been activated (19–21) via P2 purinergic receptors (20), promoting TNF-α release and stimulation of nitric oxide (20, 21). To dampen tissue destruction and inflammation, CD39 catalyzes extracellular ATP hydrolysis and, together with CD73, results in the generation of free adenosine, a known immunosuppressive agent (15). Adenosine receptor A2A is expressed by CD4+ T cells, and when adenosine agonists are added to CD25−CD4+ T cells, these can inhibit effector T cell proliferation (16). Additionally, Treg cells from CD39−/ − mice have been shown to lose their ability to suppress effector T cell proliferation (16). Hence, the expression of CD39 apyrase defines a subset of regulatory CD4+ T cells sufficiently potent to control inflammation.
CIA, a rodent model of human rheumatoid arthritis, is a CD4+ T cell-mediated inflammatory disease. In DBA/1 or C57BL/6 mice, CIA can be induced upon immunization with heterologous collagen type II (CII) (22, 23). While Th1 and Th17 cells are critical for establishing arthritis (24, 25), Th2 cells are associated with protection against disease (26). The regulatory cytokine, TGF-β, when given to mice after the induction of arthritis acts to protect against further CIA development (27). Interruption of endogenous TGF-β signaling, as in transgenic mice with truncated TGF-β type II receptor, develops a more severe disease mediated by enhanced Th1 cell involvement (28).
In our previous studies, we have demonstrated that an attenuated Salmonella vaccine vector expressing enterotoxigenic Escherichia coli colonization factor Ag I (CFA/I), Salmonella-CFA/I, promotes bystander suppression of experimental autoimmune encephalomyelitis by the means of Th2-type cytokine stimulation (29), as well as by the induction of regulatory Foxp3+CD25+CD4+ T cells (5). When this same vaccine is given prophylactically to DBA/1 mice, it prevents CIA development, suppressing both Th1 and Th17 cell responses to CII and stimulating the induction of IL-4-producing CD4+ T cells (10). Upon adoptive transfer of Salmonella-CFA/I-generated CD25− or CD25+CD4+ T cells, both were equally potent against CIA, but remained suboptimal in conferring protection. However, complete protection was conferred upon adoptive transfer of total CD4+ T cells, and this protective effect was abated upon TGF-β neutralization. Subsequently, it was found that an increased frequency of CD39+CD4+ T cells accompanied clinical protection conferred by Salmonella-CFA/I (10).
In the work described here, the regulatory CD39+CD4+ T cells stimulated subsequent immunization of C57BL/6 mice with Salmonella-CFA/I were further characterized. Adoptive transfer of Salmonella-CFA/I-induced CD39+CD4+, not CD39−CD4+, T cells protected mice against CIA. The enhanced expression of CD39 by CD4+ T cells correlated with accelerated ATP hydrolysis by Salmonella-CFA/I-induced CD4+ T cells. Considering the recently reported mechanism for transcriptional regulation of CD39 via cAMP-response element (CRE)-like motif (30), Salmonella-CFA/I-induced CD4+T cells featured enhanced activation of cAMP-response element-binding protein (CREB) when compared to Salmonella vector-induced CD4+ T cells. Since it was previously shown neutralization of TGF-β abates Salmonella-CFA/I’s protection (10), we sought to determine the source of TGF-β production. Interestingly, TGF-β production associated with FoxP3−CD25−CD39+ CD4+ T cells and the numbers of these T cells increased when compared to those induced by the Salmonella vector. Since it has been shown TGF-β induces phosphorylation of CREB (31), which in turn results in enhanced CD39 expression (30), our data strongly suggested that Salmonella-CFA/I stimulates the generation of regulatory CD39+ CD4+ T cells that confer protection against CIA via the production of TGF-β.
Materials and Methods
Mice
C57BL/6 8-wk-old males (Charles River Laboratories) and a breeding colony of FoxP3-GFP-transgenic mice (Jackson Laboratory) were maintained at Montana State University Animal Resources Center. Mice were kept in individual ventilated cages under high-efficiency particulate absorbing-filtered barrier conditions. All experimental procedures were concordant with institutional policies for animal health and well-being.
Immunizations
Mice were orally vaccinated with 5×109 CFU ΔaroA Salmonella enterica serovar Typhimurium-CFA/I (Salmonella-CFA/I, strain H696) or its isogenic vaccine vector (strain H647 lacking the cfa/I operon), as described in previous studies (10, 29). A separate control group of mice received sterile PBS. Vaccinations were conducted on day 14 or day 21 post-CII challenge, as described in the text.
Induction and clinical evaluations of CIA
CIA induction in C57BL/6 mice was similar to that previously described (6). Briefly, 100 μg of chicken CII (Chondrex) emulsified in complete Freund’s adjuvant containing 4 mg/ml killed Mycobacterium tuberculosis (Chondrex) was injected s.c. in the tail (Chondrex protocol; 22). Generally, clinical manifestation of arthritis occurred about day 21 post-challenge, and by day 35, nearly 100% of animals developed disease. Mice were scored using a scale of 0–3 for each limb for a maximal total score of 12 (10): 0, no clinical signs; 1, mild redness of a paw or swelling of single digits; 2, significant swelling of ankle or wrist with erythema; 3, severe swelling and erythema of multiple joints.
Histopathology
Upon study termination, joint sections were fixed in 10% neutral buffered formalin and decalcified in 5% formic acid for 3–6 days, embedded in paraffin, cut at 8 μm sections, and stained with H&E or toluidine blue, as previously described (10). Each joint was scored: 0, no changes; 1, synovial hyperplasia and mild inflammatory infiltration; 2, pannus formation with cartilage degeneration; and 3, severe inflammatory infiltration with bone and cartilage destruction. Paws and knee joint sections were evaluated with a total maximum score of 18 possible per mouse. Cartilage degeneration was scored in toluidine blue stained sections using a scale of 0–3: 0, no changes in cartilage morphology; 1, minimal proteoglycan and chondrocytes loss in superficial zone; 2, chondrocytes and proteoglycan loss into middle zone above tidemark; 3, severe matrix and chondrocytes loss through tidemark.
Cytokine ELISA
Upon termination of studies, axillary, popliteal, inguinal, and iliac lymph nodes (LNs) from Salmonella-CFA/I-, Salmonella vector-, or PBS-treated C57BL/6 mice were isolated; CD4+ T cells were then sorted by magnetic beads using Dynal Mouse CD4 Negative Isolation kit (Invitrogen) (>95% purity). CD4+ T cells (106/ml) were restimulated with 50 μg/ml CII (T cell Proliferation Grade, Chondrex) in a complete medium [CM; RPMI 1640 containing 10% of FBS (Atlanta Biologicals), 50 μM 2-ME (Sigma-Aldrich), and the supplements (Invitrogen-Life Technologies): 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, and 0.1 mM nonessential amino acids], in the presence of syngenic irradiated (3000 rad) APCs for 4 days at 37°C, 5% CO2. IFN-γ, IL-4, IL-10, IL-17, TGF-β capture ELISAs were performed on collected cell culture supernatants, as previously described (10,17).
Flow cytometry
For CD39+CD4+ T cells analysis, C57BL/6 or FoxP3-GFP transgenic mice were orally immunized with 5×109 CFU of Salmonella-CFA/I or Salmonella vector. Individual splenic, Peyer’s patch (PP), mesenteric LN (MLN), and head and neck LN (HNLN) cells were evaluated on days 7, 14, and 21 after immunization. Immunofluorescent staining was performed with fluorochrome-labeled mAbs: CD4, CD39, FoxP3 (eBioscience, San Diego, CA), and CD25 (BD Pharmingen). For surface TGF-β staining of CD4+ T cells, biotinylated anti-TGF-β mAbs (R&D Systems) paired with fluorochrome-conjugated streptavidin (BD Pharmingen) were used. For analysis of FoxP3 expression in recipients of Salmonella-CFA/I induced FoxP3− and FoxP3+ CD4+ T cells, spleens and LN cells were stained, as described, after following CIA course. For intracellular IL-10 detection, on day 14 post-immunization with Salmonella-CFA/I or Salmonella vector, splenic and MLN lymphocytes were stimulated in vitro with 25 ng/ml PMA and 1 μg/ml ionomycin and treated with 10 μg/ml brefeldin A for 6 hours. Following surface marker staining, cells were fixed with 2% paraformaldehyde, permeabilized with 0.2% saponin and stained with PE-labeled anti-IL-10 (BD Pharmingen). For analysis of phosphorylated CREB expression, on day 14 post-Salmonella immunization, lymphocytes from combined MLNs and HNLNs of individual mice were incubated for 15 min with 25 ng/ml PMA and 1 μg/ml ionomycin in CM, harvested, and washed with staining buffer (0.1% BSA in Ca2+/Mg2+ free PBS). After staining for cell surface expression of CD4, CD39, and CD25, lymphocytes were washed, fixed with 2% paraformaldehyde, and then permeabilized with ice-cold methanol at 4°C for 10 min. Following extensive washing, cells were then stained with PE-anti-CREB(pS133)/ATF-1 (pS63) mAb (BD™ Phosflow). Fluorescence was acquired on an LSR II flow cytometer (BD Biosciences) with BD FACSDiva software. All samples were analyzed using FlowJo software (Tree Star).
Evaluation of ectonucleoside triphosphate diphosphohydrolase activity
Mice were immunized, as described above. Quantification of ATP hydrolysis was performed using BioAssay Systems’ QuantiChrom™ ATPase/GTPase Assay Kit (BioAssay Systems). On day 14, post-immunization with Salmonella-CFA/I, Salmonella vector, or sterile PBS, dilutions of cell-sorted HNLN and MLN CD4+ T cells in 0.9% saline (Baxter Healthcare Corporation), buffered with 10 mM HEPES (Invitrogen Life Tech.), were incubated with 40 nmoles of ATP (Sigma-Aldrich) for 10 minutes at 37°C and 5% CO2 in 96-well cell culture plate per the kit manufacturer’s instructions. ATP was not added in negative control wells. PO43− concentrations were calculated from the standard curve after absorbance reading at 630 nm. Increases in PO43− concentrations as a result of extracellular ATP hydrolysis were determined as the difference between PO43− concentrations in reaction wells with added ATP from PO43− concentrations in corresponding control wells lacking exogenously added ATP. ATPase activity was calculated as fmoles of phosphate/sec per cell.
Adoptive transfer studies
Arthritis was induced in C57BL/6 mice on day 0. Simultaneously, as a source of donor cells, separate groups of C57BL/6 and FoxP3-GFP transgenic mice were immunized with either Salmonella-CFA/I or Salmonella-vector, and CD4+ T cell subsets were subsequently isolated from HNLNs and MLNs on day 14 post-immunization. To obtain CD39−CD4+ and CD39+CD4+ T cells, magnetic bead-sorted (CELLection Biotin Binder Kit; Invitrogen) total CD4+ T cells from Salmonella-CFA/I- or Salmonella vector-immunized donors were purified (purity >90% each). To obtain FoxP3-GFP− CD4+ and FoxP3-GFP+ CD4+ T cells, total CD4+ T cells were stained with PE-anti-CD4 mAb and cell-sorted using a FACSAria (purity >95% each). Each subset (1×106 donor cells) was subsequently adoptively transferred i.v. into to mice previously induced with CIA 2 wks earlier. To obtain FoxP3-GFP−CD39+CD4+ and FoxP3-GFP+CD39+CD4+ T cells, total CD4+ T cells stained with PE-anti-CD4 and Alexa Fluor 647-anti-CD39 and cell-sorted using a FACSAria (purity >95% each). Each subset (5×105 donor cells) was given i.v. to mice with CIA induced 2 weeks earlier. For each adoptive transfer experiment, development of disease was followed.
In vivo TGF-β neutralization
Mice were immunized with Salmonella-CFA/I or Salmonella vector, as described above. Beginning on the day of immunization, 250 μg of TGF-β-specific mAb (clone 1D11.16.8, Bio × Cell, West Lebanon, NH) was given i.p every other day for a total of four doses (total of 1 mg mAb/mouse).
Statistics
Non-parametric Mann-Whitney U test was used for statistical analysis of CIA clinical scores, histology scores, and cartilage destruction. Incidence of arthritis was statistically validated with Fisher’s exact probability test. Student’s t test and one-way ANOVA were used for analysis of cytokine ELISA results and flow cytometry data. Results were considered statistically significant if p-value was less than 0.05.
Results
Intervention with Salmonella-CFA/I optimal 14 days post-CII challenge
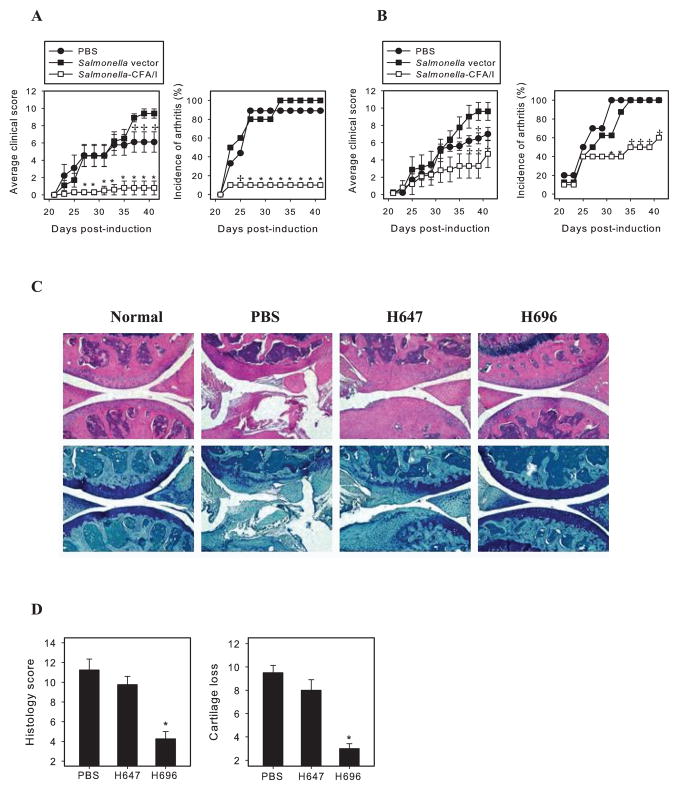
Previous work has shown that oral immunization with Salmonella-CFA/I seven days prior to CIA challenge could prevent subsequent development of disease (10). To determine the vaccine’s efficacy after CIA induction, groups of C57BL/6 mice were challenged with heterologous CII on day 0, and then on days 14 (Fig. 1A) or day 21 (Fig. 1B) mice were orally dosed with Salmonella-CFA/I (strain H696). As control, one group was orally dosed with its isogenic Salmonella vector (strain H647), and another group was given sterile PBS. Of the mice treated with Salmonella-CFA/I on 14 days post-CII challenge, only 10% of the mice developed disease, unlike the control groups with disease incidence of 90% – 100% (Fig. 1A). The average clinical score was also remarkably reduced in the Salmonella-CFA/I-treated group (Fig. 1A). In contrast, only partial protection of the mice treated with Salmonella-CFA/I on day 21 post-CII challenge was observed (Fig. 1B). Although disease incidence was reduced by this late intervention, 60% of the treated mice did develop CIA and, only after day 35, did the average clinical score stay significantly less than in control groups. Notably, immunization with Salmonella vector at either time point increased disease severity of arthritis when compared with the PBS group.
FIGURE 1.
Oral immunization with Salmonella-CFA/I after CII challenge reverses development of CIA. CIA was induced in C57BL/6 mice (10 per group) on day 0 with s.c. injection of 100 μg chick CII emulsified in complete Freund’s adjuvant. A, On day 14, or B, day 21, post-CII challenge, mice were orally dosed with 5×109 CFUs of Salmonella-CFA/I (H696) or Salmonella vector (H647). Control mice received sterile PBS. Average clinical score per group reflects severity of the disease; incidence of arthritis is percent of mice within a group with clinical symptoms. *p < 0.005 as compared with PBS or H647 group; +p<0.05 as compared with H647 group. One of 3 experiments with similar results is depicted. C, H&E - (top panels) and toluidine blue-stained (bottom panels) knee sections upon termination of study. D, Histology score (0–3 per section) and cartilage loss (0–3 per section) were graded for each limb and knee sections. Total maximum scores of 18 possible per mouse. *p < 0.05 as compared with PBS and H647 groups.
Histopathological analysis of joint sections was performed at study termination. Joints from mice treated with Salmonella-CFA/I on day 14 post-CII challenge showed only mild edema and synovial hyperplasia (Fig. 1C). Toluidine blue stained sections demonstrated minimal cartilage changes when compared to normal joints. In contrast, median sections from both control groups showed bone and cartilage erosions with proteoglycan and chondrocytes loss (Fig. 1C). Average histology scores and estimated cartilage losses in the Salmonella-CFA/I group were significantly lower than in the PBS- or Salmonella vector-treated groups (Fig. 1D). Hence, protective intervention by Salmonella-CFA/I was best on day 14 post-CII challenge and suboptimal when intervention occurred on day 21.
Protection from CIA after immunization with Salmonella-CFA/I associated with suppressed Th1 and Th17 cells and enhanced regulatory responses
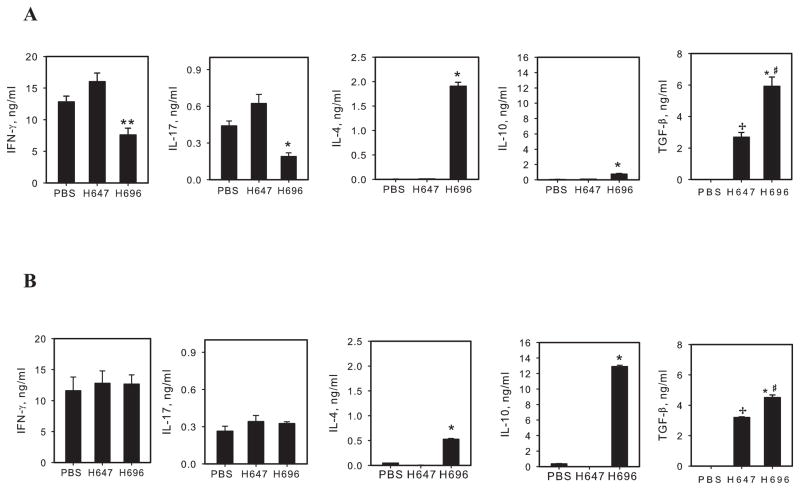
Previously, the Salmonella-CFA/I vaccine was shown to stimulate both Th2-type responses (9, 10, 29), as well as stimulate diverse regulatory T cell subsets (9, 10). To assess the types of cytokines induced, cytokine analysis was performed with purified CD4+ T cells restimulated with CII in the presence of naive APCs (Fig. 2). Examination of responses from CD4+ T cells obtained from mice treated 14 days post-CII challenge showed both IFN-γ and IL-17 were significantly suppressed by as much 2- and 3.3-fold, respectively, in the Salmonella-CFA/I-treated group (Fig. 2A). On the other hand, both IL-4 and IL-10 were potently stimulated with > 200-fold increases in IL-4 and with 6- to 7-fold increases in IL-10 when compared to control groups (Fig. 2A). TGF-β secretion was detected in both Salmonella vector- and Salmonella-CFA/I-treated groups, albeit 2-fold greater TGF-β production by the latter treatment group; none was produced by the PBS-dosed mice (Fig. 2A). In contrast, the observed suppression of IFN-γ and IL-17 was lost in mice treated with Salmonella-CFA/I on day 21 post-challenge (Fig. 2B), supporting the clinical findings. IL-4 was still potently induced, albeit the IL-4 response was reduced ~ 4-fold relative to day 14 treatment by Salmonella-CFA/I. Notably, IL-10 production in mice treated with Salmonella-CFA/I on day 21 post-induction essentially exceeded IL-10 level in day 14 treatment group (Fig. 2A, B), but this was insufficient to suppress the inflammatory responses. TGF-β was again induced in Salmonella vector and Salmonella-CFA/I day 21 treatment groups, though 30% decrease in TGF-β secretion resulted from Salmonella-CFA/I group relative to day 14 treatment (Fig. 2A, B). These results showed that intervention at day 14 post-challenge effectively suppressed the development of Th1 and Th17 cells.
FIGURE 2.
Collagen II-specific CD4+ T cell responses. Mice were immunized with CII, then treated with Salmonella-CFA/I (H696), Salmonella vector (H647), or PBS as described A, on day 14, or B, on day 21, post-CII challenge. Upon termination of the studies, CD4+ T cells were purified with magnetic beads (>95% purity) from axillary, popliteal, inguinal, and iliac lymph nodes (LNs). 106 cells/ml were restimulated with CII and syngenic irradiated APCs for 4 days. Mean cytokine concentrations from triplicate cultures ± SEM are shown. *p < 0.001; **p < 0.05 as compared with PBS and H647 groups; #p < 0.01 as compared with H647 group; +p < 0.001 as compared with PBS group.
Salmonella-CFA/I stimulates CD39+CD4+ T cells protective against CIA
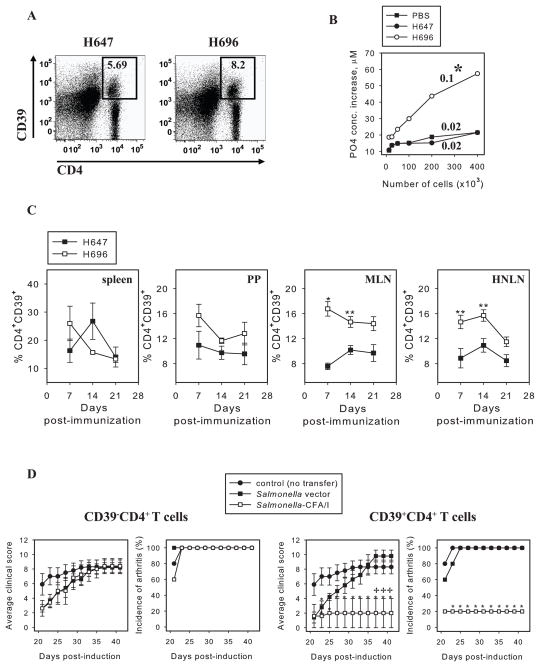
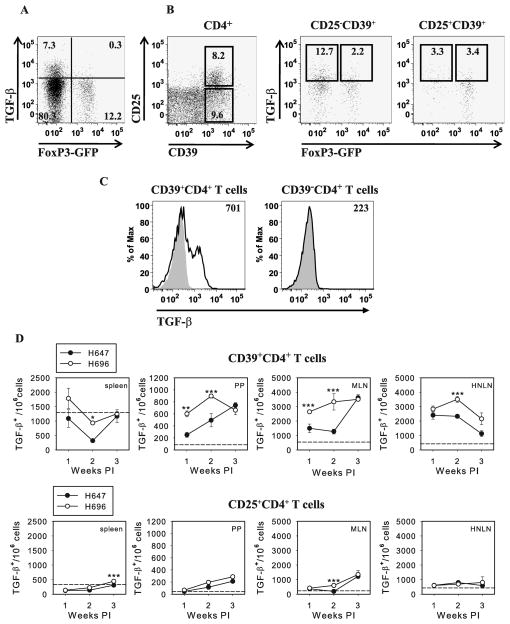
Previously, immunization of DBA/1 mice with Salmonella-CFA/I has been shown to stimulate the induction of CD25− and CD25+ FoxP3+CD4+ T cells, accompanied by enhanced expression of CD39 (10). Yet, adoptive transfer of total CD4+ T cells, not CD25+CD4+ T cells, protects against CIA (10), suggesting CD25 expression is not a correlative protective phenotype. In this study, increased frequency of CD39+CD4+ T cells was observed by C57BL/6 mice on day 14 after immunization with Salmonella-CFA/I (Fig. 3A). To assess whether this increased CD39 expression correlated to enhanced enzymatic activity, apyrase activity was measured (Fig. 3B). The rate of extracellular ATP hydrolysis by Salmonella-CFA/I-induced CD4+ T cells was significantly enhanced 5-fold in its apyrase activity (p = 0.026) when compared to CD4+ T cells from Salmonella vector- or PBS-immunized mice. Thus, a modest increase in CD39 expression corresponded to a robust apyrase activity enhanced by Salmonella-CFA/I extracellular ATP conversion into AMP by surface CD39.
FIGURE 3.
Salmonella-CFA/I stimulates CD39+CD4+ T cells protective against CIA. FoxP3-GFP Tg mice were immunized with Salmonella-CFA/I (H696) or Salmonella vector (H647), and on day 14 post-immunization, flow cytometry analysis was performed on stained lymphocytes of individual mice (5 mice per group). A, Representative FACS plots of CD39 expression by MLN CD4+ T cells out of total lymphocytes gated. B, Extracellular ATP hydrolysis by CD4+ T cells on day 14 post-immunization of mice with Salmonella-CFA/I, Salmonella vector, or PBS. ATP consumption was evaluated as an increase in PO43− concentrations relative to control reaction lacking exogenous ATP (base line hydrolysis). ATPase activity is calculated as fmoles of phosphate/sec per cell. Numbers above the curves represent ATPase activity. One of 4 experiments is depicted; p = 0.026. C, CD39 expression kinetics by CD4+ T cells in spleen, PPs, MLNs, and HNLNs of individual C57BL/6 mice (3/time point) after immunization with Salmonella-CFA/I or Salmonella vector. *p < 0.005; **p < 0.05. D, Adoptive transfer of Salmonella-CFA/I-induced CD39+, not CD39− CD4+, T cells protects against CIA. On day 0, arthritis was induced in recipient mice, and donor mice were immunized with Salmonella-CFA/I or Salmonella vector. On day 14 post-immunization, CD39+CD4+ and CD39− CD4+ T cells were sorted from immunized C57BL/6 donors and adoptively transferred to CIA recipients (5 mice per group). *p<0.05 versus PBS-treated group; +p < 0.05 versus recipients of Salmonella vector-induced cells.
To learn when CD39 expression was optimally induced, a kinetic analysis of CD39 expression by splenic, PP, MLN, and HNLN CD4+ T cells was performed. C57BL/6 mice were orally immunized with Salmonella-CFA/I or Salmonella vector, and CD39 expression was assessed by flow cytometry on days 7, 14, and 21 post-immunization. Significantly greater (~2-fold) frequencies of CD39+CD4+ T cells were observed in MLNs and HNLNs of Salmonella-CFA/I-immunized mice on days 7 and 14 compared with vector-immunized animals (Fig. 3C). No significant differences in CD39 expression by splenic and PP CD4+ T cells were observed.
To test if Salmonella-CFA/I-stimulated CD39+CD4+ T cells were sufficiently potent to protect against CIA, HNLN and MLN CD39+CD4+ and CD39−CD4+ T cells were cell-sorted 14 days post-immunization from Salmonella-CFA/I- or Salmonella vector-immunized mice. These T cell subsets were adoptively transferred to groups of mice already induced with CIA 14 days earlier. CD39−CD4+ T cells from either Salmonella-CFA/I- or Salmonella vector-immunized mice were not protective against CIA (Fig. 3D-left panel). In contrast, CD39+CD4+ T cells from Salmonella-CFA/I-immunized mice suppressed the development of arthritis, but the same subset from Salmonella vector-immunized mice was ineffective against disease (Fig. 3D-right panel). Thus, the results of adoptive transfer show that Salmonella-CFA/I-stimulated CD39+CD4+ T cell subset is protective against CIA.
Salmonella-CFA/I-induced CD39+CD4+ T cells are functionally heterogenous
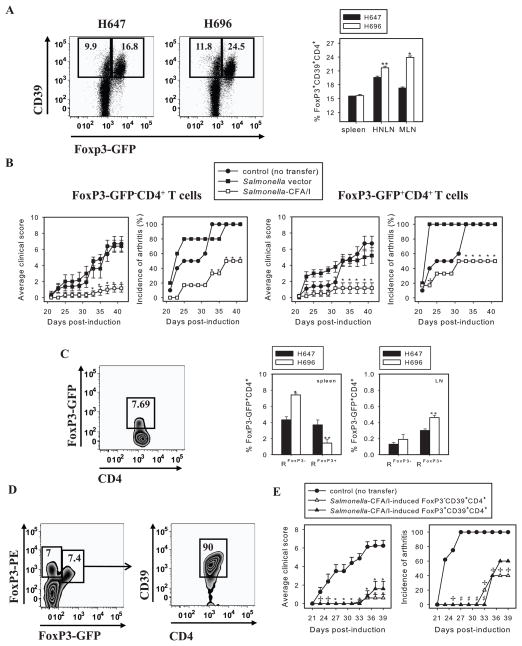
To determine whether CD39 expression by CD4+ T cells also correlated to FoxP3 expression, FoxP3-GFP transgenic mice were orally immunized with Salmonella CFA/I. Flow cytometry analysis on day 14 post-immunization revealed nearly 50% increased in FoxP3 expression by CD4+ T cells relative to Salmonella vector-immunized mice (Fig. 4A). The majority of FoxP3+CD4+ T cells did express CD39; however, not all CD39+CD4+ T cells in both immunization groups were FoxP3+ (Fig. 4A).
FIGURE 4.
Salmonella-CFA/I-induced CD39+CD4+ phenotype represents a heterogenous T cell subset. A, Representative FACS plots of gated CD4+ T cells from MLNs of Salmonella-CFA/I or Salmonella vector-immunized mice and percentages of FoxP3 expressing CD39+CD4+ T cells. FoxP3-GFP transgenic mice were immunized, and flow cytometry analysis of individual mice lymphocytes (5 mice/group) was performed as described in Figure 3; *p < 0.001; **p = 0.01 between groups. B, FoxP3+CD4+ and FoxP3− CD4+ T cells were sorted from FoxP3-GFP donors on day 14 post-immunization with Salmonella-CFA/I or Salmonella vector, then adoptively transferred to recipients with induced CIA 2 wks earlier (5 mice/group). Disease course was followed; *p < 0.05 versus PBS-treated group; +p < 0.01 versus Salmonella vector-induced CD4+ T cell recipients. C, Representative plot of gated splenic CD4+ T cells (left panel) and frequencies of FoxP3-GFP+CD4+ T cells in individual recipients of Salmonella-CFA/I-induced FoxP3− CD4+ (RFoxP3−) and FoxP3+CD4+ (RFoxP3+) T cells (right panel). Mean frequencies of spleen and combined axillary, popliteal, inguinal, and iliac LN FoxP3-GFP+CD4+ T cells ± SEM in recipients given Salmonella vector- or Salmonella-CFA/I -generated FoxP3− CD4+ or FoxP3+CD4+ T cells are shown; *p < 0.005 and **p < 0.05 versus Salmonella vector-derived cells. D, The converted FoxP3-GFP+CD4+ T cells express CD39. Examination of gated splenic CD4+ T cells from recipients given Salmonella-CFA/I-induced FoxP3-GFP− CD4+ T cells reveal positive recognition by PE-FoxP3 mAb and are mostly CD39+. In addition, a separate FoxP3+ CD4+ T cell subset, that is GFP-negative, is detected with the PE-FoxP3 mAb. E, Adoptive transfer of cell-sorted Salmonella-CFA/I-induced FoxP3− CD39+CD4+ and FoxP3+CD39+CD4+ T cells from Foxp3-GFP donors to CIA recipients (5–8 mice per group) on day 14 post-induction of the disease; +p < 0.05, *p < 0.005, #p < 0.001 versus PBS control group.
Thus, we queried the relative contribution of FoxP3-GFP− and FoxP3-GFP+ CD4+ T cells to protect against CIA. As with previous adoptive transfers, FoxP3-GFP male mice were orally immunized with either Salmonella vector or Salmonella-CFA/I, and after 14 days, CD4+ T cells were sorted for FoxP3-GFP− and FoxP3-GFP+ subsets and adoptively transferred into B6 males induced with CIA 2 wks earlier. Notably, Salmonella-CFA/I-stimulated FoxP3−CD4+ and FoxP3+CD4+ T cells each conferred ~50% protection against CIA, but importantly, clinical disease was significantly suppressed, bearing a clinical score of 1 versus 6 for control groups (Fig. 4B). Salmonella vector-induced FoxP3-GFP+CD4+ T cells provided partial protection only until day 30 post-challenge, but eventually all of the mice developed disease and were not significantly different from PBS control group (Fig. 4B-right panel). Upon termination of the study, flow cytometry analysis of recipients’ splenic and LN CD4+ T cells was performed. The spleens of recipients given FoxP3-GFP+CD4+ or FoxP3-GFP−CD4+ T cells from Salmonella vector-treated mice showed a similar frequency of FoxP3-GFP+ CD4+ T cells, ~ 4% of total CD4+ T cells. In contrast, significantly lower percentages of FoxP3-GFP+ CD4+ T cells were observed in recipient spleens of Salmonella-CFA/I-induced FoxP3-GFP+ CD4+ T cells relative to recipients of Salmonella vector -induced FoxP3-GFP+CD4+ T cells (Fig. 4C). Interestingly, recipients of FoxP3-GFP− CD4+T cells from either Salmonella-CFA/I- or Salmonella vector-immunized mice exhibited converted FoxP3-GFP+ CD4+ T cells predominantly in spleens, and a significantly higher frequency of the converted phenotype was observed in recipients of FoxP3−CD4+ T cells from Salmonella-CFA/I-immunized mice (Fig. 4C). Further analysis of converted FoxP3−GFP+ CD4+ cells showed 90% of them being CD39+ (Fig. 4D).
To assess whether the protective phenotype was retained with FoxP3-GFP−CD39+CD4+ T cells, an additional cell sorting experiment was performed. On day 14 after immunization of FoxP3-GFP Tg mice with Salmonella-CFA/I, FoxP3-GFP− and FoxP3-GFP+ CD39+CD4+ T cells were sorted and each adoptively transferred into mice with CIA. Both recipient groups showed a similar onset of CIA delayed until day 33–35, whereas PBS control group’s first symptoms of arthritis appeared on day 23 (Fig. 4E). Upon termination of the study, 60% and 40% protection was conferred by FoxP3-GFP−CD39+CD4+ and FoxP3-GFP+CD39+CD4+ T cells, respectively (Fig. 4E). Both FoxP3− and FoxP3+ subsets of Salmonella-CFA/I-stimulated CD39+CD4+ T cells provided strong suppression of clinical disease (Fig. 4E). Maximum average clinical score of 0.6 was found in recipients of FoxP3-GFP−CD39+CD4+ T cells, maximum average clinical score of 1.6 was seen in recipients of FoxP3-GFP+CD39+CD4+ T cells versus average score of 6.25 in control group. Yet disease incidence was still apparent in both recipient groups given T cells from Salmonella-CFA/I-vaccinated mice. Collectively, these data showed the vaccine-induced protective CD39+CD4+ T cells are heterogenous, and Salmonella-CFA/I-induced FoxP3−CD39+CD4+ and FoxP3+CD39+CD4+ T cells are both required for protection against CIA.
Salmonella-CFA/I-induced FoxP3− CD39+CD4+ T cells are a TGF-β-producing subset
Past studies have shown the relevance of exogenous and endogenous TGF-β to dampen CIA (27, 28). Previously, it was reported treatment with an anti-TGF-β mAb abated the protective capacity by the adoptively transferred CD4+ T cells induced by Salmonella-CFA/I vaccine (10). In this current investigation, the role of TGF-β was further analyzed to determine which CD4+ T cell subset was induced by Salmonella-CFA/I and responsible for TGF-β production. FoxP3-GFP transgenic mice were immunized with Salmonella-CFA/I or Salmonella vector, and flow cytometry analysis was performed on day 14 post-immunization for surface TGF-β expression. Interestingly, TGF-β was mostly expressed by FoxP3−CD4+ T cells by Salmonella-CFA/I-immunized mice (Fig. 5, A and B). Amongst the gated CD39+CD4+ T cells, the frequency of TGF-β+ cells was nearly 4-fold less in the CD25+CD39+CD4+ T cell subset than in CD25−CD39+CD4+ T cells (Fig. 5B). Moreover, TGF-β-producing cells associated for the most part with CD39+CD4+ T cells and minimal to none with the CD39−CD4+ T cells (Fig. 5C). A kinetic analysis was performed to determine absolute numbers of TGF-β+CD39+CD4+ T cells, and on day 14 post-immunization with Salmonella-CFA/I, the TGF-β-producing cells were significantly enhanced compared to Salmonella vector-immunized mice in all tested tissues, spleens, PPs, MLNs, and HNLNs (Fig. 5D). Analyzing gated CD25+CD4+ T cells, we did not find any difference in TGF-β production between Salmonella-CFA/I- and Salmonella vector-immunized mice, except in spleens at 3 wks post-immunization and MLNs at 2 wks time point (Fig. 5D), but the number of TGF-β+ CD25+CD4+ T cells in MLN was ~6-fold less than TGF-β+CD39+CD4+ T cells (Fig. 5D).
FIGURE 5.
Salmonella-CFA/I-induced FoxP3− CD39+CD4+ T cells produce TGF-β. A and B, On day 14 after immunization of Foxp3-GFP mice (5/group) with Salmonella-CFA/I, lymphocytes were stained and analyzed for surface TGF-β expression. A representative plot using MLN CD4+ gated lymphocytes is depicted. C, CD39− CD4+ T cells do not make TGF-β. On day 14 post-immunization with Salmonella-CFA/I, MLN CD39+ and CD39− CD4 +T cells were evaluated for TGF-β expression. Plots are representative of five mice. D, Comparative kinetic analysis of TGF-β expression by CD39+CD4+ and CD25+CD4+ T cells of individual mice (3 mice/group/time point) after immunization with Salmonella-CFA/I (H696) or Salmonella vector (H647) is shown; *p < 0.001; **p < 0.01; ***p < 0.05 between groups. Dashed line indicates the average number of TGF-β+ cells in naive mice.
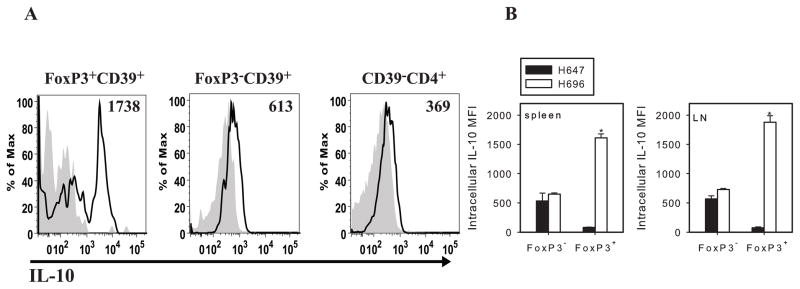
Analyzing IL-10 expression by CD4+ T cells on day 14 after immunization with Salmonella-CFA/I, FoxP3+CD39+CD4+ T cells showed greater intracellular IL-10 staining than the FoxP3− or CD39− T cells (Fig. 6A); IL-10-specific MFI was ~2.5-fold greater than FoxP3−CD39+CD4+ subset and 4.7-fold greater than CD39−CD4+ T cells (Fig. 6A and B). The same FoxP3+CD39+CD4+ T cells from Salmonella vector-immunized mice showed no IL-10 (Fig. 6B). These data demonstrated Salmonella-CFA/I vaccination induces heterogenous protective CD39+CD4+ T cells in which FoxP3−CD39+CD4+ T cells are capable of producing TGF-β, whereas FoxP3+CD39+CD4+ T cells are responsible for IL-10 production.
FIGURE 6.
FoxP3+CD39+CD4+ T cells express IL-10. A, Representative histogram of IL-10 expression by Salmonella-CFA/I-induced FoxP3+CD39+, FoxP3− CD39+, and CD39−CD4+ T cells; gray histographs depict isotype control Ab. B, IL-10-specific MFI corresponding to FoxP3− and FoxP3+ subsets of CD39+CD4+ T cells in spleens and combined MLNs and LNs are depicted; *p < 0.001; **p < 0.05.
Salmonella-CFA/I-induced CD39 expression mediated by activation (phosphorylation) of cAMP-response element-binding protein (CREB)
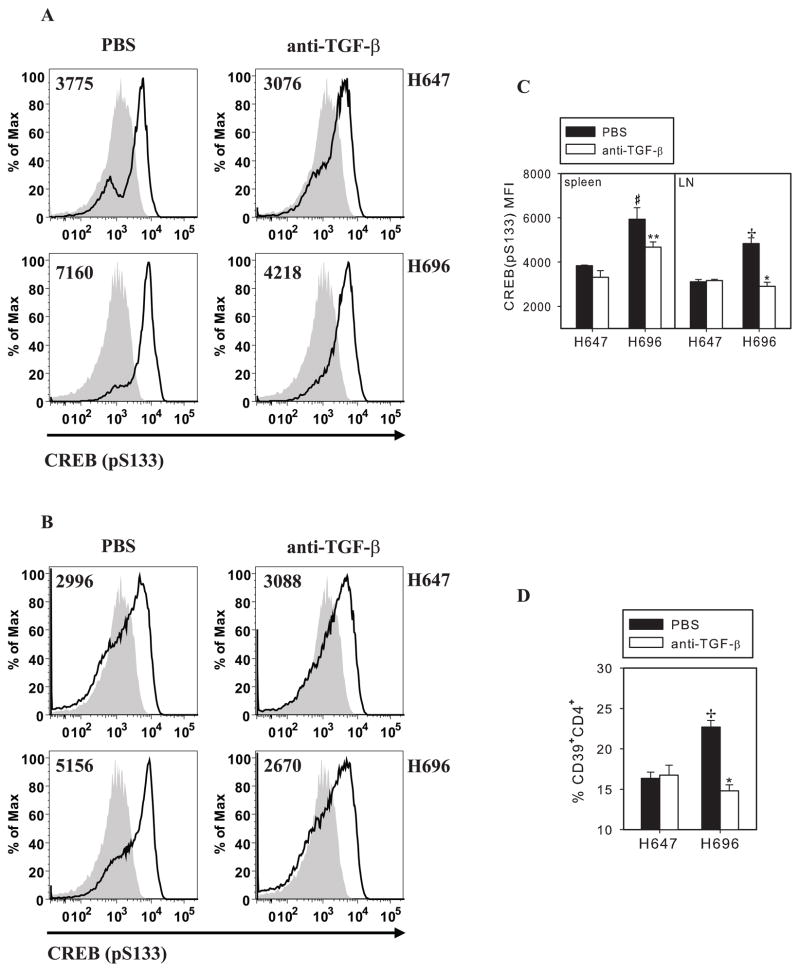
Recently, CD39 expression by mononuclear cells has been reported to be transcriptionally regulated through cAMP/CREB pathway (30). Accelerated phosphorylation of CREB by vascular smooth muscle cells treated with TGF-β has been also reported (31). To test if Salmonella-CFA/I-stimulated CD39 expression involves CRE-like sequence element transcriptional control and whether activation of CREB by phosphorylation of its serine 133 by the CD4+ T cells depends on TGF-β signaling, TGF-β was neutralized in vivo in Salmonella-CFA/I- or Salmonella vector-immunized mice. Starting the day of immunization, i.p. treatments were performed without or with anti-TGF-β mAb, and additional treatments were conducted every other day for a total of four doses. Subsequent mAb treatments, activated (phosphorylated) CREB by splenic and combined MLN and HNLN CD4+ T cells from individual mice was measured by flow cytometry. Analysis revealed that Salmonella-CFA/I-induced CD4+ T cells showed significant enhancement of activated CREB (CREB-pS133) by splenic (Fig. 7, A and C) and LN CD4+ T cells (Fig. 7, B and C). Comparing CREB (pS133) mean fluorescence-intensity (MFI) by CD4+ T cells from Salmonella-CFA/I- or Salmonella vector-immunized mice treated without or with anti-TGF-β mAb, in vivo TGF-β neutralization was found to inhibit phosphorylation of CREB by splenic and LN CD4+ T cells from Salmonella-CFA/I-immunized mice (Fig. 7, A–C), whereas TGF-β neutralization had no impact upon CREB phosphorylation by CD4+ T cells from Salmonella vector-immunized mice (Fig. 7, A–C). Interestingly, TGF-β neutralization reduced almost in half the percentage of CD39+ CD4+ T cells in LNs of Salmonella-CFA/I-immunized mice, while the frequency of CD39+CD4+ T cells in Salmonella vector-immunized mice remained unchanged (Fig. 7D). Thus, these data showed that Salmonella-CFA/I vaccine stimulates CD39+CD4+ regulatory T cells and requires the participation of TGF-β via cAMP/CREB pathway.
FIGURE 7.
Neutralization of TGF-β reverses Salmonella-CFA/I-enhanced phosphorylation of cAMP-response element-binding protein (CREB) and inhibits CD39 expression. Mice (3–4/group) were immunized with Salmonella-CFA/I (H696) or Salmonella vector (H647), as described. Beginning day 0, mice received 4 i.p. doses of 250 μg of TGF-β-specific mAb (1 mg total) every other day or sterile PBS. Immunofluorescent staining of spleen and combined MLN and HNLN cells was performed on day 14 post-immunization. Representative FACS histograms for activated CREB-specific fluorescence in A, spleens and B, LNs. C, Phosphorylated CREB-specific mean fluorescence intensity (MFI) from individual samples is shown; *p < 0.005, **p<0.05 as compared with PBS-treated Salmonella-CFA/I-immunized mice; +p<0.005, #p<0.05 as compared with corresponding Salmonella vector-immunized mice. D, Percentages of CD39 expressing CD4+ T cells in LNs. Mean frequencies of CD39 expressing CD4+ T cells ± SEM are shown; *p < 0.005 as. compared with PBS-treated Salmonella-CFA/I-immunized mice; +p<0.005 as compared with corresponding Salmonella vector-immunized mice. One of 4 experiments is represented.
Discussion
CIA is a rodent model disease widely exploited to develop new approaches for prevention and treatment of arthritis (32). Treatment with microbial products has been shown to prevent CIA (33) or reverse developing arthritis (34). In a similar fashion, we have shown that a single dose of a live bacterial vaccine, Salmonella-CFA/I, when given to DBA/1 mice 7 days prior CIA induction, prevents development of disease (10). To test its therapeutic potential, Salmonella-CFA/I was given to C57BL/6 mice previously challenged with CII. Two time points, post-CII challenge, were evaluated. By day 14 post-CII challenge, mice generally developed CD4+ T cell responses to CII (10); hence, it was hypothesized treatment at this time should interfere with the development of CII-specific effector CD4+ T cells and delays CIA onset or completely protects from clinical manifestation of arthritis. As such, protection against clinical disease similar to prophylactic immunization (10) with Salmonella-CFA/I was obtained, while control mice immunized with Salmonella vector developed a more severe arthritis than mice from PBS-treated group. In contrast, this therapeutic impact by Salmonella-CFA/I was weakened when mice were dosed on day 21 post-CII challenge and when first evidence of the clinical symptoms generally appears. Although statistically significant suppression of CIA incidence was observed, 60% of Salmonella-CFA/I-vaccinated mice developed disease. Nonetheless, disease severity by 5 weeks after post-CII challenge was still significantly less than disease for PBS- or Salmonella vector-treated groups.
Timing of intervention was also important in reversing the development of the inflammatory CD4+ T cells. Treatment on day 14 resulted in nearly 2-fold reduction of IFN- γ and IL-17, whereas this dampening of inflammatory CD4+ T cells was lost when mice were treated on day 21 post-CII challenge, as evidenced by no differences in IFN-γ and IL-17 production versus control diseased animals. Since IFN-γ responses have been reported to be elevated during early and late stages of CIA in C57BL/6 mice (23, 24), Salmonella-CFA/I not suppressing IFN-γ when treated on day 21 post-CII-challenge correlates with higher disease incidence. IL-17 contributes to arthritis pathogenesis supporting osteoclastogenesis via induction of RANKL expression on CD4+ T cells and osteoblasts (35). In addition, IL-17 enhances IL-1β and IL-6 production by infiltrating polymorphonuclear leucocytes (36). In this study, maximum clinical protection by Salmonella-CFA/I was associated with its capability to down regulate the development of Th17 cells.
Although the Th2-type cytokine, IL-4, is not typically detected in mice with CIA (17, 32), it has been shown to play an essential role in protection against CIA. IL-4 −/ − mice fail to be tolerized by intravenous CII prior CIA induction (26), and in a similar fashion, IL-4 neutralization reverses tolerization by CII-specific splenocytes (26) or reverses protection by Salmonella-CFA/I-induced CD4+ T cells (10). IL-4 can suppress rheumatoid inflammation by inhibiting Th1 cells development and stimulating inducible regulatory FoxP3+CD4+ T cells (37). However, as reflected by the clinical disease, stimulation of CII-specific Th2 cells as a result of day 21 immunization with Salmonella-CFA/I was insufficient to counteract CII-specific Th1 and Th17 cells, as accomplished with day 14 intervention.
IL-10 has been described as an important regulatory cytokine mediating antigen-specific regulatory CD4+ T cells (11), and IL-10 deficiency increases susceptibility to CIA (38). IL-10 production was stimulated in mice treated with Salmonella-CFA/I on either day 14 or day 21 when compared to mice treated with PBS or Salmonella vector. Notably, CII-specific CD4+ T cells from mice vaccinated on day 21 showed >10-fold increase in IL-10 production than cells from day 14-vaccinated mice. Although these findings inversely correlated with clinical disease and IL-17 production, these results implicated the importance of IL-4 and/or possibly other regulatory cytokines for protection again CIA.
TGF-β was not detected in PBS-treated CIA mice after following the disease course, but treatments with Salmonella vector or Salmonella-CFA/I induced its secretion by CD4+ T cells. Protection from CIA in day 14 Salmonella-CFA/I-treated mice correlated with 2-fold increased production of TGF-β compared with Salmonella vector-treated mice. At the same time, the diminished anti-inflammatory effect of Salmonella-CFA/I in day 21 treatment group coincided with significant decrease in TGF-β production.
In an attempt to discern the responsible regulatory T cell subset induced by Salmonella-CFA/I, CD25− and CD25+CD4+ T cells were found to be suboptimally protective against CIA (10). Protection against CIA was found not to be CD25-dependant, as in this study and in our previous studies (10, 17); rather, protection was CD39-dependant. A kinetic analysis of CD39+CD4+ T cell frequency induced upon immunization with Salmonella-CFA/I revealed 2-fold increases in the percentages of CD39+CD4+ T cells in MLNs and HNLNs beginning as early as day 7 post-vaccination. Only a modest elevation in the number of CD39+CD4+ T cells by Salmonella-CFA/I-immunized mice was found, correlating with a 5-fold increase in apyrase activity measured by CD4+ T cells.
Extracellular ATP-AMP-adenosine balance is an important regulatory mechanism in control of autoimmunity (17, 39). Regulatory CD4+ T cells expressing CD39 have been shown to protect against CIA (17). The relevance of regulatory CD39+CD4+ T cells has been shown for other human autoimmune diseases, including multiple sclerosis. These patients have been shown to have a deficiency of these regulatory T cells (39), and their CD39− CD25+CD4+ T cells failed to suppress IL-17 production by responder T cells in vitro.
Rheumatoid arthritis results in tissue damage, which can result in the release of extracelluar ATP (40,41). Extracellular ATP is implicated in secretion of proinflammatory cytokine, IFN-γ (42), whereas, in contrast, adenosine facilitates Treg cell-mediated suppression by cAMP (43, 44). In this study, the enhanced ATP-hydrolysis by Salmonella-CFA/I-stimulated CD4+ T cells support the hypothesis that induced regulatory CD39+CD4+ T cells, particularly, in the conversion of ATP by CD39 ecto-ATPase, play an important role in protection against CIA in mice. These studies suggested Salmonella-CFA/I stimulates CD39+CD4+ T cells with enhanced apyrase activity to hydrolyze extracellular ATP, preventing further tissue damage and proinflammatory cytokine stimulation.
To understand how these CD39+CD4+ T cells are induced, studies were conducted to ascertain the CD4+ T cell subsets stimulated by Salmonella-CFA/I. The induced CD39+CD4+ T cells were found to be functionally and phenotypically heterogenous, including their expression of FoxP3 and CD25, suggesting a cooperative interaction amongst these subsets. Notably, the CD39+CD4+ T cells, not CD39−CD4+ T cells, from Salmonella-CFA/I-immunized mice completely suppressed development of CIA, unlike the same cells generated after immunization with Salmonella vector that were unable to confer protection against autoimmunity. While the CD4+ T cells used for adoptive transfer were phenotypically similar, their activation status for CD39 apyrase activity was considerably different. The activity from Salmonella vector-dosed mice resembled untreated (PBS-dosed) mice, whereas the apyrase activity from Salmonella-CFA/I-dosed mice was considerably greater evidenced by the 5-fold increase.
To assess the relative contributions by FoxP-GFP− and FoxP3-GFP+ CD4+ T cells for protection, an adoptive transfer study was performed. Salmonella-CFA/I immunization increased the frequency of FoxP3+CD39+CD4+ and FoxP3−CD39+CD4+ T cells compared to immunization with Salmonella vector. Salmonella-CFA/I-induced FoxP3+CD4+ and FoxP3−CD4+ T cells or FoxP3-GFP−CD39+CD4+ and FoxP3-GFP+CD39+CD4+ were found to confer equal protection against CIA. Questioning how the FoxP3−CD4+ T cells provided protection, subsequent flow cytometry analysis of FoxP3−CD4+ T cell recipients revealed an adoptively transferred subset of FoxP3-GFP− cells converted in vivo to FoxP3-GFP+during resolution of CIA. Moreover, in these recipient mice, native FoxP3+ CD4+ T cells were also induced (Fig. 4D), suggesting the adoptively transferred FoxP3−CD4+ T cells may have stimulated recipient regulatory T cells. Nonetheless, the adoptively transferred donor regulatory CD4+ T cells were CD39+. Thus, the adoptively transferred Salmonella-CFA/I-induced FoxP3−CD39+CD4+ T cells during the disease course converted into FoxP3+ CD39+CD4+ T cells, suggesting the dynamic development of regulatory T cells.
The protective CD39+CD4+ T cells were found to be a heterogenous subset composed of segregated cytokine-producing cells. Cell analysis revealed Salmonella-CFA/I stimulated IL-10-producing FoxP3+CD39+CD4+ T cells and TGF-β-producing FoxP3−CD39+CD4+ T cells. Analysis of CD39−CD4+ T cells revealed little to no TGF-β or IL-10 production by these T cells further demonstrating the significance of CD39+CD4+ T cells for conferring protection against CIA. For optimal protection against CIA, both the Foxp3− and FoxP3+ CD39+CD4+ T cells were required since adoptive transfer with either subset could confer protection, but only suboptimally, resulting in disease incidence. This evidence suggested these two subsets work cooperatively and are interdependent. As with IL-10, TGF-β is an important immunosuppressive cytokine associated with several regulatory T cell subsets: Th3 (13), natural CD4+CD25+ T cells (13), and Foxp3−CD25lowCD4+ regulatory cells (45). TGF-β is protective against a number of autoimmune diseases (13, 46), and it is also certainly important for arthritis (46, 47), since genetically impaired TGF-β signaling markedly enhances disease severity (28), while treatments with recombinant TGF-β suppress arthritis (27). This protective effect by TGF-β is abated when mice already induced with CIA, are adoptively transferred with Salmonella-CFA/I-generated CD4+ T cells, and recipients are neutralized of their TGF-β (10). Thus, TGF-β has an integral role in mediating the protective effect by Salmonella-CFA/I.
While clearly TGF-β can act directly to suppress autoimmunity (13, 27, 44, 48), it may also have an indirect impact upon regulatory T cell development. Inquiring how Salmonella-CFA/I could stimulate CD39 expression, activation of CREB has been reported to mediate CD39 expression (30). In a separate study, TGF-β has been shown to potently induce CREB phosphorylation by vascular smooth muscle cells (31). To assess CREB’s possible role following Salmonella-CFA/I immunization, a significant increase in activated CREB (pS133) was detected relative to mice dosed with Salmonella vector. Moreover, this enhanced CREB (pS133) expression was eliminated in mice neutralized of their TGF-β, implicating CREB transcriptional control of regulatory CD39+CD4+ T cells in Salmonella-CFA/I-immunized mice is TGF-β-dependent. Neutralization of TGF-β also inhibited CD39 expression by CD4+ T cells from Salmonella-CFA/I-immunized mice, further supporting the critical role of this regulatory cytokine in the development of regulatory CD39+CD4+ T cells. What is unclear, thus far, is whether the source of TGF-β-producing cells is a regulatory T cell subset. Analysis of Salmonella-CFA/I-immunized FoxP3-GFP transgenic mice revealed that the source of TGF-β is from the FoxP3− CD39+CD4+ T cells, and of these, most were of the CD25− subset. It is unclear whether these FoxP3− T cells were previously FoxP3+; possibly, this FoxP3− CD39+CD4+ T cell subset could have been a regulatory T cell or be a Th2 cell subset, since both are capable of producing TGF-β. Alternatively, this cell subset may be a memory cell, as suggested by studies with naive mouse CD4+ T cells (49) or in humans (41, 50); however, these memory cells tend to be proinflammatory and do not produce TGF-β (41, 49, 50). When anti-CD3 plus anti-CD28 are co-stimulated in the presence of TGF-β, the murine FoxP3− CD39+CD4+ T cells inhibit the conversion of FoxP3+ CD39+CD4+ T cells (49), which seems to differ from our results implicating a helper function by FoxP3− CD39+CD4+ T cells as providing TGF-β to enhance the numbers and activity of FoxP3+ CD39+CD4+ T cells to suppress autoimmunity. In fact, TGF-β neutralization resulted in the loss of the induced regulatory CD39+ T cells, supporting the notion that these FoxP3− T cells stimulate CD39 cell expression in a CREB-dependent fashion.
In conclusion, this work demonstrated the observed live Salmonella-CFA/I vaccine can protect against CIA following immunization during the induction phase of disease. Vaccination on day 14 post-CII-challenge inhibited development of collagen II-specific pathogenic Th1 and Th17 cells with concomitant stimulation of regulatory and Th2 cells. As such, Salmonella-CFA/I induced the development of IL-10 producing regulatory FoxP3+ CD39+CD4+ T cells, as well as an adjunct TGF-β+ FoxP3− CD39+CD4+ T cell subset. The protective potential of the vaccine-induced regulatory CD39+CD4+ T cells was supported by the enhanced apyrase activity evident by increased extracellular ATP hydrolysis by CD4+ T cells from Salmonella-CFA/I-immunized mice. Similar T cells isolated from Salmonella vector-immunized mice lacked this enhanced apyrase activity, and thus these cells were ineffective against disease resolution when adoptively transferred into CIA mice. In contrast, adoptive transfer of Salmonella-CFA/I-induced regulatory CD39+CD4+ T cells conferred protection against CIA. These results showed that the TGF-β-producing FoxP3− CD25− CD39+CD4+ T cells support the stimulation of the Salmonella-CFA/I-induced regulatory CD39+ CD4+ T cells since TGF-β neutralization results in reduced CREB activation and compromises the stimulation of these regulatory T cells.
Acknowledgments
We thank Ms. Nancy Kommers for her assistance in preparing this manuscript.
Abbreviations used in this paper
- CFA/I
colonization factor antigen I
- CIA
collagen-induced arthritis
- CII
collagen II
- CM
complete medium
- CREB
cAMP-response element-binding protein
- FoxP3
forkhead box P3
- HNLNs
head and neck lymph nodes
- LNs
lymph nodes
- MLNs
mesenteric LNs
- PPs
Peyer’s patches
- Treg
regulatory T cells
Footnotes
This work is supported by U.S. Public Health Service Grant R21 AT-004312 and, in part, by Montana Agricultural Station and U.S. Department of Agriculture Formula Funds. Department of Immunology and Infectious Diseases’ flow cytometry facility was, in part, supported by NIH/National Center for Research Resources, Centers of Biomedical Excellence P20 RR-020185, and an equipment grant from the M.J. Murdock Charitable Trust.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat Immunol. 2007;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 2.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Immunol. 2007;7:305–310. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 3.Fontenot JD, Gavin MA, Rudensky AY. FoxP3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 5.Ochoa-Repáraz J, Riccardi C, Rynda A, Jun S, Callis G, Pascual DW. Regulatory T cells vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. J Immunol. 2007;178:1791–1799. doi: 10.4049/jimmunol.178.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 7.Rynda A, Maddaloni M, Mierzejewska D, Ochoa-Repáraz J, Maślanka T, Crist K, Riccardi C, Barszczewska B, Fujihashi K, McGhee JR, Pascual DW. Low-dose tolerance is mediated by microfold cell ligand, reovirus protein σ1. J Immunol. 2008;180:5187–5200. doi: 10.4049/jimmunol.180.8.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rynda A, Maddaloni M, Ochoa-Repáraz J, Callis G, Pascual DW. IL-28 supplants requirement for Treg cells in protein σ1-mediated protection against murine experimental autoimmune encephalomyelitis (EAE) PloS-ONE. 2010;5:e8720. doi: 10.1371/journal.pone.0008720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ochoa-Repáraz J, Rynda A, Ascón MA, Yang X, Kochetkova I, Riccardi C, Callis G, Trunkle T, Pascual DW. IL-13 production by regulatory T cells protects against experimental autoimmune encephalomyelitis independently of autoantigen. J Immunol. 2008;181:954–968. doi: 10.4049/jimmunol.181.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochetkova I, Trunkle T, Callis G, Pascual DW. Vaccination without autoantigen protects against collagen II-induced arthritis via immune deviation and regulatory T cells. J Immunol. 2008;181:2741–2752. doi: 10.4049/jimmunol.181.4.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, O’Garra A. IL-10-secreting regulatory T cells do not express FoxP3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- 12.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 13.Weiner HL. Induction and mechanism of action of transforming growth factor-β-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 14.Borsellino G, Kleinewietfeld M, Mitri DD, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell’Acqua ML, Rossini PM, Battistini L, Rötzschke O, Falk K. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 15.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppress effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 16.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kochetkova I, Golden S, Holderness K, Callis G, Pascual DW. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J Immunol. 2010;184:7144–7153. doi: 10.4049/jimmunol.0902739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bynoe MS, Viret C. Foxp3+ CD4+ T cell-mediated immunosuppression involves extracellular nucleotide catabolism. Trends Immunol. 2008;29:99–102. doi: 10.1016/j.it.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Virgilio FD. Purinergic modulation of interleukin-1β release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Y, Fisette PL, Denlinger LC, Guadarrama AG, Sommer JA, Proctor RA, Bertics PJ. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in RAW 264.7 macrophages. J Biol Chem. 1998;273:27170–27175. doi: 10.1074/jbc.273.42.27170. [DOI] [PubMed] [Google Scholar]
- 21.Tonetti M, Sturla L, Giovine M, Benatti U, De Flora A. Extracellular ATP enhances mRNA levels of nitric oxide synthase and TNF-α in lipopolysaccharide-treated RAW264.7 murine macrophages. Biochem Biophys Res Commun. 1995;214:125–130. doi: 10.1006/bbrc.1995.2265. [DOI] [PubMed] [Google Scholar]
- 22.Brand DD, Kang AH, Rosloniec EF. The mouse model of collagen-induced arthritis. Methods Mol Med. 2004;102:293–312. doi: 10.1385/1-59259-805-6:295. [DOI] [PubMed] [Google Scholar]
- 23.Inglis JJ, Criado G, Medghalchi M, Andrews M, Sandison A, Feldmann M, Williams RO. Collagen-induced arthritis in C57BL/6 mice is associated with robust and sustained T-cell response to type II collagen. Arthritis Res Ther. 2007;9:R113. doi: 10.1186/ar2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kai H, Shibuya K, Wang Y, Kameta H, Kameyama T, Tahara-Hanaoka S, Miyamoto A, Honda S, Matsumoto I, Koyama A, Sumida T, Shibuya A. Critical role of M. tuberculosis for dendritic cell maturation to induce collagen-induced arthritis in H-2b background of C57BL/6 mice. Immunology. 2006;118:233–239. doi: 10.1111/j.1365-2567.2006.02361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 26.Myers LK, Tang B, Stuart JM, Kang AH. The role of IL-4 in regulation of murine collagen-induced arthritis. Clin Immunol. 2002;102:185–191. doi: 10.1006/clim.2001.5162. [DOI] [PubMed] [Google Scholar]
- 27.Kuruvilla AP, Shah R, Hochwald GM, Liggitt HD, Pallandino MA, Thorbecke GJ. Protective effect of transforming growth factor β1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci USA. 1991;88:2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schramm C, Kriegsmann J, Protschka M, Huber S, Hansen T, Schmitt E, Galle PR, Blessing M. Susceptibility to collagen-induced arthritis is modulated by TGF-β responsiveness of T cells. Arthritis Res Ther. 2004;6:R114–119. doi: 10.1186/ar1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jun S, Gilmore W, Callis G, Rynda A, Haddad A, Pascual DW. A live diarrheal vaccine imprints a Th2 cell bias and acts as an anti-inflammatory vaccine. J Immunol. 2005;175:6733–6740. doi: 10.4049/jimmunol.175.10.6733. [DOI] [PubMed] [Google Scholar]
- 30.Liao H, Hyman MC, Baek AE, Fukase K, Pinsky DJ. cAMP/CREB-mediated transcriptional regulation of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) expression. J Biol Chem. 2010;285:14791–14805. doi: 10.1074/jbc.M110.116905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamiya K, Sakakibara K, Ryer E, Hom R, Leof EB, Kent KC, Liu B. Phosphorylation of the cyclic AMP response element binding protein mediates transforming growth factor β-induced downregulation of cyclin A in vascular smooth muscle cells. Mol Cell Biol. 2007;27:3489–3498. doi: 10.1128/MCB.00665-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmdahl R, Bockermann R, Bäcklund J, Yamada H. The molecular pathogenesis of collagen-induced arthritis in mice - a model for rheumatoid arthritis. Ageing Res Rev. 2002;1:135–147. doi: 10.1016/s0047-6374(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 33.Luross JA, Heaton T, Hirst TR, Day MJ, Williams NA. Escherichia coli heat-labile enterotoxin B subunit prevents autoimmune arthritis through induction of regulatory CD4+ T cells. Arthritis Rheum. 2002;46:1671–1682. doi: 10.1002/art.10328. [DOI] [PubMed] [Google Scholar]
- 34.Sugihara R, Yoshimura M, Mori M, Kanayama N, Hikida M, Ohmori H. Prevention of collagen-induced arthritis in DBA/1 mice by oral administration of AZ-9, a bacterial polysacchride from Klebsiella oxytoca. Immunopharmacol. 2000;49:325–333. doi: 10.1016/s0162-3109(00)00247-2. [DOI] [PubMed] [Google Scholar]
- 35.Ju JH, Cho ML, Jhun JY, Park MJ, Oh HJ, Min SY, Cho YG, Hwang SY, Kwok SK, Seo SH, Yoon CH, Park SH, Kim HY. Oral administration of type-II collagen suppresses IL-17-associated RANKL expression of CD4+ T cells in collagen-induced arthritis. Immunol Lett. 2008;117:16–25. doi: 10.1016/j.imlet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Koenders MI, Kolls JK, Oppers-Walgreen B, van den Bersselaar L, Joosten LAB, Schurr JR, Schwarzenberger P, van den Berg WB, Lubberts E. Interleukin-17 receptor deficiency results in impaired synovial expression of interleukin-1 and matrix metalloproteinases 3, 9, and 13 and prevents cartilage destruction during chronic reactivated streptococcal cell-wall-induced arthritis. Arthritis Rheum. 2005;52:3239–3247. doi: 10.1002/art.21342. [DOI] [PubMed] [Google Scholar]
- 37.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor α-chain-binding cytokines IL-4, IL-13, induce forckhead box P3-expressing CD25+CD4+ regulatory T cells from CD25−CD4+ precursors. J Immunol. 2005;175:6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- 38.Finnegan A, Kaplan CD, Cao Y, Eibel H, Glant TT, Zhang J. Collagen-induced arthritis is exacerbated in IL-10-deficient mice. Arthritis Res Ther. 2002;5:R18–24. doi: 10.1186/ar601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O’Farelly C, Tubridy N, Mills KHG. CD39+FoxP3+ regulatory T cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J Immunol. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 40.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 41.Moncrieffe H, Nistala K, Kamhieh Y, Evans J, Eddaoudi A, Eaton S, Wedderburn LR. High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population. J Immunol. 2010;185:134–143. doi: 10.4049/jimmunol.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langston HP, Ke Y, Gewirtz AT, Dombrowski KE, Kapp JA. Secretion of IL-2 and IFN-γ, but not IL-4, by antigen-specific T cell requires extracellular ATP. J Immunol. 2003;170:2962–2970. doi: 10.4049/jimmunol.170.6.2962. [DOI] [PubMed] [Google Scholar]
- 43.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Jackson EK, Johnson JT, Gorelik E, Lang S, Whiteside TL. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J Biol Chem. 2010;285:27571–27580. doi: 10.1074/jbc.M110.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bopp T, Becker C, Klein M, Klein-Heßling S, Palmetshofer A, Serfling E, Heib V, Becker M, Kubach J, Schmitt S, Stoll S, Schild H, Staege MS, Stassen M, Jonuleit MH, Schmitt E. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Q, Lee J, Jankowska-Gan E, Schultz J, Roennburg DA, Haynes LD, Kusaka S, Sollinger HW, Knechtle SJ, VanBuskirk AM, Torrealba JR, Burlingham WJ. Human CD4+CD25low adaptive regulatory cells suppress delayed-type hypersensitivity during transplant tolerance. J Immunol. 2007;178:3983–3995. doi: 10.4049/jimmunol.178.6.3983. [DOI] [PubMed] [Google Scholar]
- 46.Nistala K, Wedderburn LR. Th17 and regulatory T cells: rebalancing pro-and anti-inflammatory forces in autoimmune arthritis. Rheumatol(Oxford) 2009;48:602–606. doi: 10.1093/rheumatology/kep028. [DOI] [PubMed] [Google Scholar]
- 47.Arce F, Breckpot K, Stephenson H, Karwacz K, Ehrenstein MR, Collins M, Escors D. Selective ERK activation differentiates mouse and human tolerogenic dendritic cells, expands antigen-specific regulatory T cells, and suppresses experimental inflammatory arthritis. Arthritis Rheum. 2011;63:84–95. doi: 10.1002/art.30099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gregg RK, Jain R, Schoenleber SJ, Divekar R, Bell JJ, Lee HH, Yu P, Zaghouani H. A sudden decline in active membrane-bound TGF-β impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol. 2004;173:7308–7316. doi: 10.4049/jimmunol.173.12.7308. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Q, Yan J, Putheti P, Wu Y, Sun X, Toxavidis V, Tigges J, Kassam N, Enjyoji K, Robson SC, Strom TB, Gao W. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant. 2009;9:2303–2311. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, Doherty G, Deaglio S, Koulmanda M, Gao W, Robson SC, Strom TB. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10:2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]