Abstract
Spiking in central neurons depends on the availability of inward and outward currents activated by depolarization, and on the activation and priming of currents by hyperpolarization. Of these processes, priming by hyperpolarization is the least described. In the case of T-type Ca2+ current availability, the interplay of hyperpolarization and depolarization has been studied most completely in expression systems, in part because of the difficulty of pharmacologically separating the Ca2+ currents of native neurons. To facilitate understanding of this current under physiological conditions, we measured T-type current of isolated goldfish retinal ganglion cells with perforated-patch voltage clamp methods in solutions containing a normal extracellular Ca2+ concentration. The voltage-sensitivities and rates of current activation, inactivation, deactivation, and recovery from inactivation were similar to those of expressed α1G (CaV3.1) Ca2+ channel clones, except that the rate of deactivation was significantly faster. We reproduced the amplitude and kinetics of measured T currents with a numerical simulation based on a kinetic model developed for an α1G Ca2+ channel. Finally, we show that this model predicts the increase of T-type current made available between resting potential and spike threshold by repetitive hyperpolarizations presented at rates that are within the bandwidth of signals processed in situ by these neurons.
Keywords: T-type calcium current, kinetics, retina, native neuron, excitability
INTRODUCTION
Low-threshold, T-type Ca2+ current (IT) displays transient activation by depolarization when initiated from relatively hyperpolarized membrane potentials, and inactivation during sustained depolarization (for review, Huguenard 1996; Perez-Reyes 2003). This combination of properties enables IT to influence the shape and timing of action potentials in several ways. The most well known of these is low-threshold spiking, in which activation of IT triggers a brief burst of action potentials by transiently depolarizing cells. Secondly, the activation and steady-state inactivation profiles of IT often overlap near the resting potential of cells, forming a “window current” that can augment cell excitability (Williams et al. 1997). Thirdly, IT activated during normal action potentials can both shape spikes (Lambert et al. 1998) and influence spike frequency (Wolfart and Roeper 2002).
Although a T-type current underlies all of these effects, variations in timecourse and pharmacology in different systems suggested that there were multiple isoforms of IT (Huguenard 1996). This was confirmed by the recent isolation of three T-type Ca2+ channel clones, termed CaV3.1, CaV3.2 and CaV3.3 (or α1G, α1H and α1I, respectively, in the older nomenclature; for review, Perez-Reyes 2003). Whole-cell and single-channel recordings of these clones in expression systems have shown that they differ substantially in their rates of activation, inactivation and recovery from inactivation, while continuing to demonstrate the voltage-dependence that characterizes this channel family. These studies of expressed channels have provided an explanation for the variation in kinetic properties and pharmacology found in studies of natural IT (Huguenard 1996); and it has been possible, in a few instances, to match a specific cloned channel with the native channel in a particular cell (Satin and Cribbs 2000; Chemin et al. 2002). Still, accounting for the availability and influence of IT during spiking in particular cell types requires measurements of these properties from native channel populations under meticulous physiological conditions.
The best studied of the native systems are the various classes of thalamocortical cells that exhibit low-threshold spiking. IT was shown to support the low-threshold spike, both directly and in numerical simulations (Coulter et al. 1989; Crunelli et al. 1989; Suzuki and Rogawski 1989; Wang et al. 1991; Huguenard and Prince 1992; Huguenard and McCormick 1992; McCormick and Huguenard 1992). This and other work (Thomson 1988; Bal et al. 1995) have demonstrated that the ability of IT to support this behavior depends critically on the interaction of cell membrane potential and the timing and frequency of hyperpolarizing IPSPs.
To determine the properties that underlie this function and to examine the role of IT in another type of central neuron, we report here on the voltage-dependent control and kinetics of IT in retinal ganglion cells, a class of central neuron that has displayed this current in every species examined to date (rat: Karschin and Lipton 1989; Guenther et al. 1994; turtle: Liu and Lasater 1994; goldfish: Bindokas and Ishida 1996; cat: Huang and Robinson 1998; salamander: Henderson and Miller 2003). Activation threshold and inactivation kinetics, as well as some pharmacology, have been described for the IT in retinal ganglion cells of postnatal rat (Karschin and Lipton 1989; Guenther et al. 1994), turtle (Liu and Lasater 1994) and goldfish (Bindokas and Ishida 1996), but the other biophysical properties of these currents have yet to be studied. To ensure that we obtained the basal properties of the current: (1) we used isolated cells in short-term culture to avoid the influence of surrounding cells; (2) we used perforated patch methods to maintain cytoplasmic integrity; and (3) we measured currents in normal physiological Ca2+ concentration to avoid surface charge effects and augmented current amplitudes. Under these conditions we have found in goldfish retinal ganglion cells that the voltage-sensitivities and rates of current activation, inactivation, and recovery from inactivation resemble those of T-type currents from a number of other tissues, and that deactivation is 2-3 times faster. We could reproduce the amplitude and kinetics of IT activated by typical depolarizing voltage jumps with a kinetic model developed for an α1G (CaV3.1) Ca2+ channel clone. Moreover, we show that this model predicts the increase of IT that can be recorded between resting potential and spike threshold after volleys of hyperpolarizations, as might occur during recurrent inhibitory input.
MATERIALS and METHODS
The voltage-clamp currents described here were measured in retinal ganglion cell somata isolated from adult common goldfish (Carassius auratus). The methods used in this study have been described in detail elsewhere (Bindokas and Ishida 1996; Tabata and Ishida 1996, 1999; Hidaka and Ishida 1998). All animal care and experimental protocols were approved by the Animal Use and Care Administrative Advisory Committee of the University of California, Davis. Cells were dissociated by brief incubation of freshly dissected retinas in either protease-containing saline or, in a few instances, an enzyme-free, low-Ca2+ solution (Hayashida, Partida, and Ishida; unpublished), followed by thorough rinsing in saline supplemented with bovine serum albumin and trituration with a large-bore glass pipette. Cells were identified either by retrograde labeling, via the optic nerve, of rhodamine B isothiocyanate-coupled dextran (Tabata and Ishida 1996) or by the nucleolar expansion observed exclusively in ganglion cells subsequent to crush of the optic nerve (Ishida and Cohen 1988). We found no statistically significant difference between these cell groups in terms of the current properties reported here (activation threshold, kinetics, voltage-sensitivity of steady-state inactivation, rate of recovery from inactivation). Therefore, all data from these cells have been pooled. Voltage-gated Ca2+ current was recorded in perforated-patch mode (Horn and Marty 1988), using an Axopatch-1D patch-clamp amplifier (Axon Instruments, Union City, CA) and pCLAMP software (v. 8.1.01, Axon Instruments) for voltage-jump protocol generation and data acquisition.
Patch electrodes were pulled from borosilicate glass capillaries to tip resistances of ca. 5 MΩ. The tips of these pipettes were filled with either of two “pipette solutions.” One formulation (ca. 60% of experiments) contained (in mM): 15 Na methanesulfonate, 120 CsOH, 30 tetraethylammonium chloride (TEA), 1 ethyleneglycoltetraacetic acid (EGTA), 0.34 CaCl2, 2.6 MgCl2, and 5 HEPES; pH adjusted to 7.40 with methanesulfonic acid. The second formulation (ca. 40% of experiments) was the same except that TEA was replaced with 30 CsOH, 15 sucrose, and additional methanesulfonic acid to adjust pH. We observed no differences between the Ca2+ currents recorded with these pipette solutions. The osmolality of both solutions was 285–290 mOsmol/kg. The pipette shanks were filled with the same solution supplemented with a 1:200 dilution of a mixture of 2 mg amphotericin B and 3 mg Pluronic F-127 (Molecular Probes; Eugene, OR) in 60 μL DMSO.
The extracellular (“bath”) solution contained (in mM): 120 NaCl, 3 CsCl, 2.5 CaCl2, 1 MgCl2, 30 TEA-Cl, 10 glucose, 5 HEPES. The pH was adjusted to 7.40 with NaOH, and the osmolality was 290–300 mOsmol/kg. Just prior to use, the bath solution was supplemented with 1 μM tetrodotoxin.
Reagents were obtained from the following sources: Na methanesulfonate, methanesulfonic acid (Aldrich Chemical, Milwaukee, WI); CsOH, CsCl (ICN, Aurora, OH); NaCl, TEA, MgCl2, EGTA, HEPES, amphotericin B, and DMSO (Sigma Chemical, St. Louis, MO); CaCl2 (BDH; Dorset, England); tetrodotoxin (Sankyo, Tokyo, Japan).
Experiments were performed at 20–24 °C within 36 hr of cell isolation. Recordings were made from cells adhering to a glass coverslip attached to the bottom of a 35 mm tissue culture dish. Cells recorded the day of isolation or after overnight culture gave equivalent results; there was no drift in T-current density or properties with time in culture. The current monitor output of the patch-clamp amplifier was analog-filtered by the built-in 4-pole Bessel filter (Fc = 2 or 5 kHz) and digitally sampled (usually at 10 kHz). An agar bridge was used to ground the bath solution, and the membrane potentials reported here have been corrected for liquid junction potentials that arose from differences between the bath and pipette solutions. Linear leak subtraction was performed with the P/N protocol in pCLAMP, using holding and command potentials that were never more negative than −100 mV, and never more positive than −60 mV. Series resistance compensation was not used.
Data were analyzed in pCLAMP and Excel (Excel 2000; Microsoft Corp., Redmond, WA), and data curves were fitted in SigmaPlot (version 5.0.5; SPSS, Chicago, IL). Means are presented ± SEM.
Computer simulations were carried out using the simulation software NEURON (ver. 4.3.1 and 5.2 by J. W. Moore, M. Hines and T. Carnevale; see Hines and Carnevale 1997, 2000). Since the dendrites and axons of our retinal ganglion cells were typically shorn off by the dissociation procedures, leaving just the isolated somata, we modeled the ganglion cell as a single spherical compartment, 20 μm in diameter.
Current flow through the T-channel in the open state (IT, units of A/cm2) was calculated with an equation,
as used in other studies (e.g., Hille 1984; Huguenard and McCormick 1992; De Schutter and Smolen 1998). Here, PCa is the maximum permeability (units of cm/sec); O is the fraction of channels in the open state (see below); z is the valence (+2); [Ca2+]in and [Ca2+]out are Ca2+ concentrations inside and outside the cell; Vm is the membrane voltage; F, R, and T are the Faraday constant, gas constant, and absolute temperature, respectively. In this equation, the concentration-dependent rectification of current is described by the Goldman-Hodgkin-Katz constant field equation (Lewis 1979). Since our experiments utilized physiological concentrations of internal Na+ (15 mM) and extracellular Ca2+ (2.5 mM) and were confined to a limited range of negative membrane potentials, we could ignore monovalent cation permeability through the T channel (Fukushima and Hagiwara 1985; Lux et al. 1990). Similarly, as we used the same physiological concentration of external Mg2+ (1 mM) throughout, we did not explicitly incorporate the relatively minor effect of Mg2+ block on the T channel (Fukushima and Hagiwara 1985; Serrano et al. 2000). In the present simulations, an estimate of 7 × 10−6 cm/sec was used for PCa to produce 5 pA/pF of current density (see Results); and [Ca2+]in and [Ca2+]out were assumed to be constant at 100 nM and 2.5 mM, respectively. Within the time scale of our simulations, the amplitude and kinetics of calculated currents were not markedly altered by changes in [Ca2+]in between 10 nM and 1 μM (data not shown).
The fraction of channels in the open state was calculated using a Markovian kinetic model shown in Fig. 8A. The kinetic scheme was adapted from one developed for a cloned T-channel (Serrano et al. 1999). In brief, the channel occupies any of 12 states (five closed states, one open state, and six inactivated states). As membrane potential depolarizes, the probability of channel occupancy is higher for the states in the right-hand side of figure. The transitions through closed states (C1 through C5) and most of the inactivated-inactivated transitions (I1 through I5) are voltage-dependent. The closed-open transition (C5 to O) and one inactivated-inactivated transition (I5 to IO) are voltage-independent. The closed/open-inactivated transitions (C1-5/O to I1-O; parallel vertical transitions in figure) are also voltage-independent. However, for the first three closed-closed transitions (C1 through C4), the corresponding inactivation process is coupled allosterically to activation (cf. Kuo and Bean, 1994). Thus, inactivation develops with membrane depolarization, and saturates as the channel reaches the last two closed states and open state.
Fig. 8.
Kinetic model and simulated T current. (A) Markovian kinetic scheme developed originally by Serrano et al. (1999). C1 to C5 are the closed states; O is the open state; I1 to IO represent the inactivated states. kV and k−V are functions of membrane voltage, and the other transition rates (kO, k−O, kI and k−I) as well as the allosteric coupling factors (f and h) are constants. (B) Voltage-dependent activation and decay of inward current elicited by voltage steps from −92 mV to −62 through −37 mV, as simulated by the model (black). The corresponding superimposed experimental data were taken from Fig. 1A (gray). Time constant for the command voltage was set to 0.7 ms in the simulation. Model parameters were the same as Table 1 except k−V0 was 0.015. (C) Activation and deactivation of T current calculated by the model (black). The corresponding superimposed data were taken from Fig 3A (gray). The time constant for the command voltage was set to 0.25 ms in the simulation. The effect of this delay is shown by the thin black trace (marked *) that shows simulated current for instantaneous command steps. The model parameters were the same as Table 1 except k−V0 was 0.039.
In this model, there are a total of 10 free parameters to be estimated. The constraints on parameter estimation were as follows: (1) kV0, the rate of voltage sensor movement for activation, was selected to match the time-to-peak at a depolarized potential (−37 mV, Fig. 2A); (2) k−V0, the rate of voltage sensor movement for deactivation, was selected to reproduce V1/2 for the current activation curve (Fig. 5B); (3) VkV, Vk−V, the gating charge parameters associated with voltage sensor movement, were selected to generate the slope factor for the I-V relation (Fig. 5B); (4) kO, the channel opening rate, was set to match the time-to-peak at an intermediate potential (−52 mV, Fig. 2A); (5) k−O, the channel closing rate, was determined directly from the time constant of deactivation at a hyperpolarized potential (Fig. 3B); (6) kI, the inactivation rate, was taken directly from the time constant of the current decay at a depolarized potential, extrapolated from Fig. 2B; (7) k−I, the reverse rate of inactivation, was set to reproduce V1/2 for the steady-state inactivation curve (Fig. 5B); (8) f, the allosteric coupling factor for inactivation, was selected to yield the time constant of entry to inactivation measured near resting potential (Fig. 4C); (9) h, the allosteric coupling factor for recovery from inactivation, was selected to reproduce the time constant of recovery from inactivation measured at a hyperpolarized potential (Fig. 7B). When simulations were repeated to optimize the parameter values, the values of k−O and kI were fixed and kV0, k−V0, VkV, Vk−V and kO were adjusted first, and then k−I, f and h were tuned. The iteration and annealing were carried out manually until all constraints were reasonably satisfied.
Fig 2.
IT Activation and Inactivation Kinetics. (A) Time to peak current as a function of test voltage. (B) Time constant of decay of the current as a function of test voltage, as determined from a simple exponential fit. Filled circles plot mean; error bars represent ±1 SEM; n = 19 cells.
Fig. 5.
IT Steady-State Inactivation and Window Current. (A) Steady-State Inactivation: Inward currents elicited by 100-ms voltage steps to −42 mV from holding potentials that varied from −92 to −62 mV. Ehold was changed in 5-mV increments starting at −92 mV, as shown in the upper part of the figure. Each new membrane potential was held for 15 s before the test step to −42 mV. P/N subtraction was used to remove linear leak and capacitive currents. (Inset) Peak current vs. holding potential for this cell (open circles). The smooth line is a Boltzmann fit to these data, as described in the text. Peak current at each holding potential is normalized to the value obtained by the test step from −92 mV. (B) IT Window Current. Activated Current (Peak I-V): The filled circles plot the average normalized peak current vs. test voltage in 9 cells with the voltage protocol shown in Fig. 1. The peak current elicited by the step to −37 mV was used for normalization for each cell. Error bars represent ±1 SEM, but are mostly covered by the data markers in this figure. The smooth line through the data is a Boltzmann fit, as described in the text. Residual Current (Steady-state Inactivation): The open circles are average normalized peak current at one fixed test potential (−42 mV) vs. holding potential for the same 9 cells. The peak current produced from Ehold −92 mV was used for normalization of the data from each cell. Window current is the overlap region of the extrapolated Boltzmann fits and represents a voltage region in which IT could be persistently active.
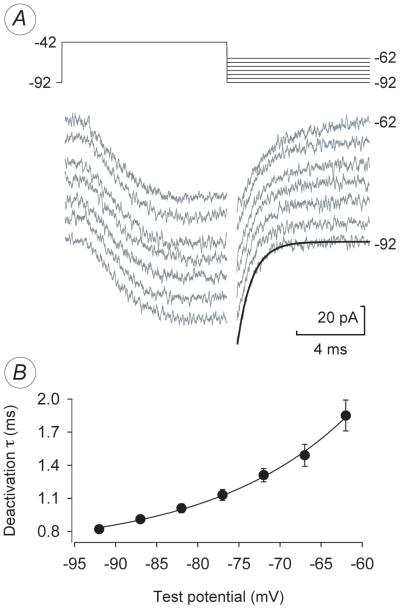
Fig. 3.
IT Deactivation Kinetics. (A) IT decay currents activated by the voltage protocol shown in the upper part of the figure. Each trace is the average of three trials with P/N leak subtraction. A period approximately equal to the charging time of this cell (0.25 ms for 30 Meg access × 8.4 pF capacitance, perforated patch) was blanked at the beginning of the repolarization. The traces were staggered to avoid overlap, and a sample exponential fit is shown in the lower right (−92 mV). Fitting was done in Clampfit. (B) Average deactivation time-constants as a function of voltage.
Fig. 4.
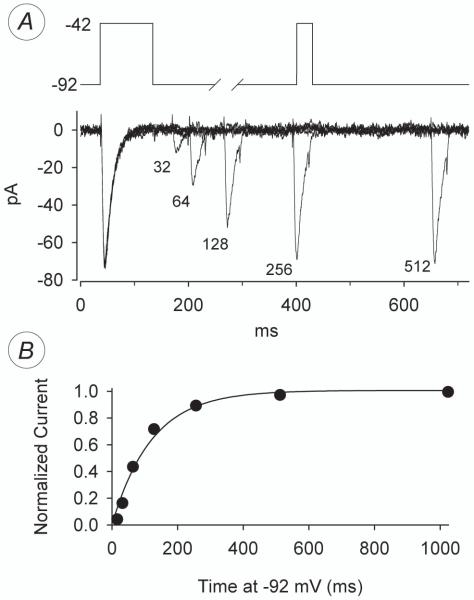
Inactivation of IT at −62 mV. (A) Overlaid raw current traces produced by the voltage protocol presented in the upper part of this figure. From Ehold −92 mV, a control current was elicited by a 50-ms test step to −42 mV; after 2.5 s at −92 mV, voltage was stepped to −62 mV for varying lengths of time (denoted by the paired slashes) before another 50-ms test step to −42 mV to check for residual current. Capacitive currents were clipped from these traces manually rather than by P/N subtraction. (B) The currents elicited by the test step to −42 mV, at higher resolution, after the indicated times at −62 mV. (C) Plot of the peak current of each test step divided by its matched control vs. time at −62 mV (circles) for this cell. The smooth line is a simple exponential fit; the time-constant for onset of inactivation in this cell was 103 ms.
Fig. 7.
Recovery from Depolarized Potential IT Inactivation. (A) Overlaid currents produced by the voltage protocol diagrammed in the upper part of this figure. From Ehold −92 mV, IT was measured and subsequently inactivated by a 100-ms test step to −42 mV; cell voltage was then stepped back to −92 mV for varying lengths of time (indicated by the paired slashes) before testing the recovery of IT with another test step to −42 mV for 30 ms. With P/N subtraction. (B) Plot of the peak current activated by each test step, divided by its matched control, vs. time at −92 mV (circles); this is the average of three identical protocols, one of which is shown in part A. The smooth line is a simple exponential fit (τ 121 ms), described in the text.
When comparing the model to a specific data set, time constants for the rise and fall of command voltage steps were set equal to the charging time constant measured from that cell. In all simulations, the temperature and the calculation time step were set to 23 °C and 2–5 μsec, respectively.
RESULTS
We describe below the following properties of low-threshold Ca2+ current (IT) in retinal ganglion cells: voltage-dependence and kinetics of activation and deactivation, voltage-sensitivity of steady-state inactivation, rate of inactivation near resting potential, and rate of recovery from inactivation. To measure these properties, current was identified as IT on the basis of three features: (1) it was activated by step-wise depolarizations from holding potentials more negative than −65 mV, to test potentials more positive than −65 mV (Fig. 1); (2) it inactivated markedly, if not entirely, when the holding potential was shifted to values more positive than −60 mV (Fig. 5A); and (3) it was kinetically transient, i.e. it decayed during test depolarizations of 100 ms (Fig. 1). This T-type current is greatly reduced by lowering extracellular Ca2+ (data not shown), and it can be abolished by replacement of the extracellular Ca2+ with a mixture of 2.4 mM Co2+ and 0.1 mM Ca2+ (Bindokas and Ishida 1996). It is insensitive to the spider toxin ω-Aga-IIIA (Bindokas and Ishida 1996), so it is unlikely that any of it is the R-type Ca2+ current that sometimes shows overlapping voltage-dependence and kinetic properties (Randall and Tsien 1997). Most (ca. 75%) of the cells recorded from in this study exhibited low-threshold Ca2+ current meeting these criteria, as we have reported previously (Bindokas and Ishida 1996).
Fig. 1.

IT Currents. Ca2+ currents elicited by 100-ms depolarization from a holding potential(Ehold) of −92 mV. The voltage steps were increased in 5-mV increments starting at −62 mV, as shown in the upper part of the figure. P/N subtraction was used to remove linear leak and capacitive currents. (Inset) Peak current vs. test voltage for these data (filled circles). The smooth line through the data points is a Boltzmann fit, described in the text. The peak current was normalized to the value obtained at −37 mV.
All of the currents reported here were recorded under conditions designed to minimize contamination by other voltage-gated currents, optimize recording stability, and avoid space clamp artifacts due to neurites. Voltage-gated Na+ current was blocked by inclusion of 1 μM tetrodotoxin in the bath (Hidaka and Ishida 1998); hyperpolarization-activated cation current (Ih) was blocked by the presence of 3 mM Cs+, and the absence of K+, in the bath (Tabata and Ishida 1996); outward K+ currents were minimized by use of a Cs+-based, K+-free pipette solution, and by inclusion of 30 mM TEA in the bath (Tabata and Ishida 1999); activation of high-threshold Ca2+ current and of TEA-resistant Cs+ efflux were avoided by use of sufficiently negative test potentials (Bindokas and Ishida 1996; Tabata and Ishida 1996, 1999); and leak-like Cl− current was minimized by use of perforated-patch recording mode (Tabata and Ishida 1999). Perforated-patch mode was also used to avoid drifts in the voltage-sensitivity of gating that occurred during ruptured-patch recording (Munckton, Pignatelli and Ishida, unpublished observations) and allay concerns about possible “rundown” (Wan et al. 1996). We restricted our investigation to voltages negative to −35 mV to avoid contamination by high-threshold Ca2+ current (see below). Recordings showing signs of inadequate space clamp (in particular, delayed activation or distorted I-V relations) were discarded from the data set. For this reason, we did not study cells bearing neurites longer than 10 μm or so, and we did not attempt to compare the properties of currents recorded from neurite-free cell bodies versus cells with significant amounts of neurites. Instead, we limited our analyses to the amplitude and kinetics of currents where we felt our control of membrane potential was the best possible. As such, our study is concentrated on somatically-expressed T-current, and we cannot say anything about currents derived from other cell locations (Ahlijanian et al. 1990; Baldridge 1996; Henderson and Miller 2003). Even with all these precautions, about 1/2 of all cells with low-threshold current were discarded because of the presence of slower kinetic components in the voltage range we used to characterize IT. This left 103 cells that formed the basis for the following analysis.
Activation Range
The range of membrane potentials that activate IT was measured by the voltage-jump protocol shown at the top of Fig. 1. Holding potential was set to the most negative value at which stable recordings were routinely obtained (−92 mV), and cells were depolarized to test potentials between −62 and −37 mV. Current vs. voltage (I-V) curves were constructed by plotting the peak amplitude of the Ca2+ current, versus the test potential at which the Ca2+ current was activated. To facilitate comparison of data collected from different cells, current amplitudes were normalized to the peak amplitude obtained in response to the step to −37 mV recorded in each cell. The normalized I-V relation for the data in Fig. 1 is plotted in the inset in Fig. 1, and fitted by Marquardt-Levenberg regression to the Boltzmann equation:
where I is peak current at each test voltage, Imax is peak current at −37 mV, V is the test voltage, V1/2 is the midpoint of the Boltzmann fit, and Vc is the voltage for an e-fold change around V1/2 (i.e., slope factor). For this cell, V1/2 is −53 mV and Vc is 4.3 mV.
In most cells, we observed the onset of a slowly inactivating inward component at test potentials more positive than ca. −37 mV. This portion of the whole-cell current was considered to be distinct from low-threshold Ca2+ current, because it was high-threshold and was only partially reduced in amplitude by depolarized holding potentials (see Bindokas and Ishida 1996). Because high-threshold Ca2+ currents were larger when elicited from a holding potential of −92 mV than from −62 mV, even in cells with no demonstrable IT, we could not subtract high-threshold current elicited from −62 mV to remove contamination, by high-threshold current, from IT elicited from −92 mV. Moreover, we found previously that there was not a satisfactory mix of blockers that could cleanly isolate IT from the other Ca2+ currents in goldfish retinal ganglion cells (Bindokas and Ishida 1996). Hence, we exploited the differential voltage-dependence of IT activation to assess IT properties by using currents collected only at command potentials that did not elicit noticeable amounts of high-threshold Ca2+ current, as in other studies of native cells (e.g., Carbone and Lux 1987; Biagi and Enyeart 1991). In most cells, the current elicited by the step to −37 mV is probably not quite the maximum for IT. The Boltzmann fit in Figs. 1 and 5B suggests the true maximum current would occur between −35 and −30 mV. A more direct measure of channel activation would be to normalize peak current by the instantaneous current (e.g., Herrington and Lingle 1992; Serrano et al. 1999). However, our inability to isolate IT across a wider voltage range and the inherent limitations of perforated patch recording prevented us from determining instantaneous currents. Compared to Figs. 1 and 5B, we would expect the channel activation curve to be broader and show attainment of maximum activation at a slightly more positive potential, as shown by the simulated activation curve corrected for the calculated Ca2+ driving force (dashed line) in Fig. 9C (see below). These curves also indicate that the error introduced by our use of the peak I-V relation is small at the hyperpolarized potentials where both channel activation and total T current are small.
Fig. 9.
Inactivation profiles of simulated T current. (A) Entry to inactivated state near the resting potential, simulated by the model (black). The corresponding experimental data were taken from Fig. 4B (light red). Time constant for the command voltage was set to 0.4 ms in the simulation. (B) Recovery from open-state inactivation, simulated by the model (black). The corresponding data were taken from Fig. 7A (light red). The time constant for the command voltage was set to 0.33 ms in the simulation. For both parts A and B, the model parameters were those in Table 1, except that kI was 0.082, f was 0.224 and h was 0.196. (C) Steady-state inactivation and normalized I-V curves, simulated by the model (black lines). The corresponding data were replotted with marks (light red) from Fig. 5B. Model parameters were as given in Table 1. A simulated channel activation curve, corrected for the calculated Ca2+ driving force, is shown by the dashed line.
At the test potentials used here (between −67 and −37 mV), IT ranged in amplitude up to around 200 pA. However, large currents were unusual, and the median peak current at −37 mV was 35 pA (mean ± SEM: 44 ± 3.3 pA; n = 103). Division of the maximum current amplitude recorded in each cell by the membrane capacitance, yielded current densities of 3–5 pA/pF [5 ± 0.7 pA/pF for cells identified by retrogradely transported dextran (n = 9); 3 ± 0.3 pA/pF (n = 94) for cells identified morphologically (see Materials and Methods).
Because IT was small in most cells, background noise could interfere with our ability to measure the voltage at which current first activated. Typically, we needed ca. 3 pA of inward current to detect this point of threshold. To ensure the sensitivity of this determination, we confined our analysis of activation threshold to cells with larger peak current (>60 pA at −37 mV), such that the current required to identify the threshold was not more than 5% of the peak. The activation threshold in such cells was −61 ± 0.5 mV (n = 21). This is consistent with the I-V relation for the cell in Fig. 1, and for the average I-V relation of 9 other well-characterized cells shown in Fig. 5B (for these 9 cells: peak current −66 ± 9 pA at −37 mV; measured threshold −60 ± 0.8 mV). (The Boltzmann fit to the I-V plot for these cells meets the abscissa at around −70 mV (Fig. 5B), suggesting that small amounts of T current might activate at membrane potentials more negative than the threshold estimated above.) The test potential that elicited half-maximum current amplitudes (V1/2) in these 9 cells was −51 ± 0.5 mV, and near this voltage the current amplitude grew e-fold in amplitude every 4 ± 0.2 mV (Vc; see Fig. 5B).
Activation, Inactivation and Deactivation Kinetics
The rate at which IT rose in amplitude was measured by the time-to-peak (time between the onset of the command depolarization and the current peak) as a function of voltage, as shown in Fig. 2A. This yielded a smoothly increasing rate of rise with stronger depolarization. The rate at which IT fell in amplitude at these test voltages was estimated by the exponential time constant that fit the declining phase of currents (Fig. 2B). Stronger depolarizations produced faster decay of current until reaching a plateau at ca. −40 mV. The extrapolated limiting time constant for this inactivation was 15.4 ms.
To determine the rate of deactivation, IT was activated by a brief step to −42 mV, and the repolarization-induced decay of the current was recorded at potentials from −62 to −92 mV (Fig. 3A). Because deactivation proved to be a fairly rapid process, we restricted our analysis to cells in which the charging time of the cell (approximated by the product of access resistance and cell capacitance) was less than 0.5 ms. To reduce the access resistance and obtain the best quality measurements, some of these data were acquired in ruptured patch configuration immediately after patch rupture and before any significant change in the voltage-dependence of IT properties. Fig. 3B was generated from pooled perforated and ruptured patch data (7 ruptured and 5 perforated measurements as in part A in five cells). Deactivation was faster as the final membrane voltage was made more negative. The average deactivation time constant at −92 mV was 0.82 ms.
Rate of Inactivation Near Resting Potential
Little or no T-type current is available for activation if the holding potential is more positive than −65 mV. Because this is near the resting potential of spiking neurons (including retinal ganglion cells; see references cited below), we measured the rate at which IT inactivates (i.e., becomes unavailable for activation) at these voltages. To do this, we depolarized cells from a holding potential of −92 mV to a conditioning potential equal to a typical resting potential (−62 mV) using the voltage-jump protocol shown at the top of Fig. 4. To assess the rate of inactivation at this voltage, we measured the amount of Ca2+ current available for activation after conditioning depolarizations of various durations, by depolarizations to a fixed test potential (e.g., Figs. 4A and B). The amplitude of these currents was normalized to the amplitude of current activated from −92 mV, and plotted against the duration of the conditioning depolarization to −62 mV. In all of 3 cells tested, plots of this type were fitted by an exponential decline in amplitude, with an average time constant of 146 ± 25 ms (e.g., Fig. 4C).
Steady-State Inactivation
The voltage sensitivity of inactivation was measured by the voltage-jump protocol shown at the top of Fig. 5A. The holding potential was shifted to various values between −92 and −62 mV, and the amount of Ca2+ current available for activation was measured by depolarizing cells from each holding potential to a test potential of either −52 mV or −42 mV. Each holding potential was maintained for 15 s prior to initiating the test depolarizations, i.e., for a period well in excess of the inactivation time constant measured in Fig. 4. The current at each test potential from each holding potential was normalized to the current elicited by depolarizations from −92 mV to the same test potential, and fitted by least-squares regression to the Boltzmann equation (inset in Fig. 5A).
For the data in Fig. 5A, the conditioning potential that reduced the Ca2+ current to half-maximum amplitude (i.e., V1/2) was −74 mV. Near this voltage, the current amplitude fell e-fold every 3.9 mV. We found no dependence of the normalized currents on whether the holding potential was varied from −92 to −62 mV, or from −62 to −92 mV. The average steady-state inactivation of 9 cells (including this one) is plotted in Fig. 5B. Because we found no difference between the normalized currents collected at a test potential of −52 versus −42 mV, the average steady-state inactivation curve in Fig. 5B includes normalized current amplitudes that were measured at both test potentials. The average V1/2 for steady-state inactivation was −77 ± 0.9 mV and the average slope factor was 4 ± 0.2 mV. In three cells, IT was assessed from a holding potential of −102 mV. Currents activated by depolarizations from −102 mV were only ~3% larger than those activated in the same cells from −92 mV. The normalization error resulting from the use of −92 mV for the maximum current was therefore considered to be negligibly small.
Currents activating at membrane potentials more negative than the voltages that produce complete steady-state inactivation are termed “window” currents (Attwell et al. 1979). Superimposing the average peak I-V relation on the average steady-state inactivation curve provides an estimate of the voltage range for a window-type Ca2+ current in retinal ganglion cells (Fig. 5B). In the cells we recorded from, the overlap between these two curves was maximal at −64 mV. At this voltage, approximately 5% of IT is not inactivated (see Discussion).
Recovery from Inactivation
The normal resting potential of these retinal ganglion cells is in the range of −60 to −75 mV (Vaquero et al. 2001; Vaquero and Ishida, unpublished); similar values have been reported for carp, salamander, turtle, guinea pig, and cat retinal ganglion cells, of a variety of functional subtypes (Wiesel 1965; Murakami and Shimoda 1977; Baylor and Fettiplace 1979; Coleman and Miller 1989; Mittman et al. 1990; Demb et al. 2001; O’Brien et al. 2002). In this voltage region, only a few percent of IT is not inactivated. To estimate how quickly the availability of IT can increase, we measured the rate at which it recovers from inactivation at a hyperpolarized potential. Because this required us to compare test current amplitudes against control amplitudes, we used two different protocols to guard against effects of the control depolarization on the rate of recovery itself. In both, the holding potential was set to −92 mV, and IT was elicited by a test depolarization that started at various times after the end of a conditioning depolarization. In the first method, shown schematically at the top of Fig. 6A and with representative data in Figs. 6A and B, the conditioning potential was −62 mV (to mimic resting potential). The amplitude of the test IT was compared to that of IT elicited by a control depolarization presented before the conditioning depolarization. The second method, shown schematically at the top of Fig. 7A and with representative data in Fig. 7A, was the more widely used “two-pulse protocol”, in which the conditioning potential was set equal to the test potential, and the conditioning depolarization was used as the control depolarization. The rate of recovery from inactivation was estimated by normalizing the amplitude of current elicited by the test depolarization to that elicited by the control depolarization, and plotting this amplitude against the duration of the time elapsed between the end of the conditioning depolarization and the beginning of the test depolarization. Recovery, measured with either of the methods described above, followed an exponential time-course. In the example data shown here, the time constants of recovery were 76 ms (Fig. 6C) and 121 ms (Fig. 7B). Overall, there was no difference between the recovery time-course after inactivation at −62 mV (119 ± 11 ms, n = 8) or more depolarized potentials (112 ± 9 ms, n = 9). In three cells in which both protocols were performed, the time-constant was 116 ± 9 ms when inactivation was induced by depolarizations to −62 mV, vs. 104 ± 11 ms when inactivation was induced by depolarizations to −52 or −42 mV. Combining all data obtained by both protocols gives an average time-constant for recovery of 115 ± 7 ms (n = 17).
Fig. 6.
Recovery from Resting Potential IT Inactivation. (A) Overlaid currents elicited by the voltage protocol diagrammed in the upper part of this figure. From Ehold −92 mV, a control current was generated by a 50-ms test step to −47 mV; after 2.5 s at −92 mV, the potential was set at −62 mV for 3.0 s, and then stepped back to −92 mV for varying lengths of time (signified by the paired slashes) before testing the recovery of IT with another 50-ms test step to −47 mV. These traces are the average of two identical protocols performed in this cell, with P/N subtraction. (B) At higher resolution, the currents elicited by the test step to −47 mV after the indicated times at −92 mV. (C) Plot of the peak current activated by each test step, divided by its matched control, vs. time at −92 mV (circles) for this cell. The smooth line is a simple exponential fit (τ 76 ms), described in the text.
We generally observed that the recovery from inactivation was well-described by a single exponential time-constant. In some other preparations, the recovery from inactivation of IT is best described by two exponentials, with the faster component being most comparable to that observed here (Huguenard 1996). The amount of the slower component is increased by extending the length of the inactivating voltage step. However, we did not see evidence for a second recovery component in experiments comparing inactivation due to a 0.1 s step to −42 mV vs. 3 s at −62 mV. We did not test the effect of holding the potential for many s at depolarized potentials, and we explicitly avoided any such time-dependence in the determination of steady-state inactivation by holding at each potential for a long time.
A kinetic model for T-current in isolated retinal ganglion cells
The above experiments characterized the fundamental kinetic properties of the goldfish retinal ganglion cell IT. To assess whether these properties are consistent with those established for other T currents, and to provide a tool for predicting the behavior of IT in different circumstances, we developed a numerical model for IT using the simulation program NEURON (see Materials and Methods). Previous studies have shown that Hodgkin-Huxley models are valid as practical descriptions of whole-cell IT (Wang et al. 1991; Huguenard and McCormick 1992; see also Destexhe and Huguenard 2000), although they are not mechanistically accurate, especially in their description of inactivation processes. The recent cloning of T channels allowed detailed studies of their biophysical properties (Cribbs et al. 1998; Perez-Reyes et al. 1998; Lee et al. 1999a) and provided sufficient experimental data to develop plausible kinetic models of their behavior (Serrano et al. 1999; Frazier et al. 2001; Burgess et al. 2002). A similar kinetic model has also been applied to the IT of a native neuron (Kuo and Yang 2001). In the following sections, we show that, with one specific modification, the Markovian kinetic model previously developed by Serrano et al. (1999) for a cloned T channel can generate satisfactory fits to our experimental data, and that these fits extend over a variety of conditions.
Fig. 8A shows the kinetic scheme for gating of IT used in the present simulation. The behavior of this model is determined by 10 parameters (Table 1). The numerical values of some of these parameters were estimated directly from the present experimental data, whereas others were derived from iterative optimization of the overall model within the constraints imposed by our data (see Materials and Methods for the fitting procedure). The qualitative characteristics of these parameters are the same as those described by Serrano et al. (1999; especially their Fig. 14) except that the transition rate from O to C5 (k−O) was assumed to be voltage independent. This assumption reduced the number of free parameters in the model, while yielding satisfactory fits to our experimental data (see below). Fits between the simulations and experimental data did not improve when voltage-dependent rates of k−O (e.g., k−O=k−O0 exp(Vm/Vk−O)) were used in the simulations (results not shown). Hence, only the forward and backward rates of activation (kV and k−V) are voltage dependent variables, and the other transition rates (kO, k−O, kI and k−I), as well as the allosteric coupling factors (f and h), are constants.
Table 1.
The forward and backward rates of activation (kV and k−V) are given by kV = kV0 exp(Vm/VkV) and k−V = k−V0 exp(Vm/Vk−V). All 10 parameter values were derived from the present experimental data.
| parameter | estimated value (default) | units |
|---|---|---|
| kV0 | 3.7 | [1/ms] |
| k−V0 | 0.02 | |
| VkV | 25.5 | [mV] |
| Vk−V | −15.3 | |
| kO | 20 | [1/ms] |
| k−O | 1.65 | |
| kI | 0.062 | |
| k−I | 0.12×10−3 | |
| f | 0.305 | unitless |
| h | 0.226 |
Voltage-clamp simulations
To evaluate the model, calculated T currents were compared with actual data from the present experiments. For each data set, the time course of the current elicited by the appropriate voltage protocol was simulated, with minor adjustment of the values in Table 1 for each individual cell.
Figure 8 shows simulations of the activation and decay of IT during sustained depolarization (Fig. 8B) and the deactivation of IT following repolarization (Fig. 8C). The simulation results (black) superimpose well on the corresponding experimental data from Figs. 1 and 3, respectively (reproduced in gray). For Figs. 8B and C, the value of k−V0 was adjusted slightly, while the other parameters were fixed at the values in Table 1. Such adjustments could affect other properties of the current, but the changes were self-consistent. For example, although the adjustment applied in the simulation in Fig. 8C caused a shift in V1/2 for the I-V relation from −51 to ca. −46 mV for the simulated data, this value of V1/2 is close to the value measured experimentally in this particular cell (ca. −47 mV, data not shown).
The time course of entry into (Fig. 9A) and recovery from inactivation (Fig. 9B) were also reproduced well by the model. Figs. 9A and B show the simulation results (black) corresponding to the experimental data from Figs. 4 and 7, respectively (light red). For these, the values of kI, f and h were adjusted, and the same set of parameter values was used in both simulations. A small discrepancy between the simulation and experiment was seen in the amplitude of current available after 32 ms of recovery in part B. This might be due to an initial delay in the recovery phase, noted by others (Satin and Cribbs 2000; Kuo and Yang 2001; Burgess et al. 2002). Simulation of the other voltage protocol we employed (see Fig. 6A) reproduced almost the same time constant for recovery from inactivation (results not shown). The model also predicts that the apparent rate of recovery from inactivation will be slower at less hyperpolarized potentials. In particular, the model says the time constant for recovery will be 1.8 times longer at −77 mV than −92 mV. In two cells where such a comparison was made, the rate of recovery at −77 mV was 115 ± 7 ms vs. 78 ± 2 ms at −92 mV, 1.5 times longer.
Fig. 9C shows steady-state inactivation and I-V curves as generated by the model (black lines), corresponding to the average experimental data in Fig 5B (light red marks). The values used were those in Table 1 without modification. As shown in Fig. 9C, simulated curves for both inactivation and I-V gave reasonable fit to the experimental data and reproduced the “window” current region. A simulated channel activation curve is shown by the dashed line.
IT Availability
Since low threshold current is almost completely inactivated at resting potential, the availability of IT under physiological conditions will depend on hyperpolarizing inputs that bring about relief from inactivation (see Discussion below). Because ganglion cells are buffeted by a constant stream of excitatory and inhibitory inputs, and real hyperpolarizing influences are almost certainly transient (e.g., Sakai and Naka 1987), the availability of IT will depend not only on the magnitude of the net voltage shift, but also on the rates of deactivation and inactivation near the resting potential, and the rate of recovery from inactivation at hyperpolarized potentials. We therefore asked if the model utilized in this study, which reproduces the amplitude and timecourse of currents elicited by stepwise depolarizations, can be extended to describe the currents activated after a sequence of brief hyperpolarizations.
Fig. 10 presents a comparison of simulated and experimental data examining the availability of IT after cycling the membrane potential from −62 to −82 mV a variable number of times. The membrane potential was held at −62 and −82 mV for the same amount of time during each conditioning cycle (10, 30 or 50 ms in this example), and then IT was activated by a step from −82 to −42 mV, as shown schematically in the traces in the top row of Fig. 10. The frequencies of these conditioning cycles (generally 5-50 Hz) were chosen to be consistent with the timecourse of psychophysically measured temporal contrast sensitivity (Woodhouse and Barlow 1982), and the kinetics of GABA inhibitory postsynaptic currents (Protti et al. 1997). The simulated data (middle row) predict that the amount of IT activated by the step to −42 mV increases with additional cycles until a maximum value is reached, and that this value increases as the steps in the conditioning cycle are lengthened. When the step length is shorter, many more conditioning steps are needed to reach 90% of this limiting value, but the cumulative conditioning time to 90% is similar for each step length.
Fig. 10.
Priming and relief from inactivation. The availability of IT was assessed after a variable number of repetitive hyperpolarizing steps from −62 to −82 mV, as shown schematically in the top row. The membrane potential was set alternately at −82 and −62 mV for equal times (10, 30 or 50 ms) during each cycle; and the final test step was from −82 to −42 mV. The middle row presents simulations of the normalized Prediction for the test current as a function of the number of conditioning cycles (number shown next to trace). The traces labeled “Experiment” are currents recorded in response to the same protocol. The time axis represents the cumulative length of the protocols. The conditioning cycles were produced with the Conditioning Train option in Clampex, which does not record the currents elicited by the conditioning protocol. However, other experiments in which “conditioning” currents were saved showed little or no activation of IT by this procedure. The time constant for the command voltage was set to 1.48 ms in the simulation. The model parameters were those in Table 1, except that k−V0 was 0.018, kI was 0.13 × 10−3, f was 0.224 and h was 0.125. The prediction was made after adjusting the model parameters to reproduce the voltage-dependence of activation and inactivation for this particular cell, based on its measured activation and inactivation kinetics, rate of recovery from inactivation, and activation and steady-state inactivation curves. The experimental priming data were not used for this parameter adjustment.
Representative experimental data obtained by this protocol are shown in the bottom row of Fig. 10. There is good correspondence between the Prediction and Experiment traces in each column, suggesting that the model satisfactorily implements the “priming” of IT by recurrent membrane potential oscillation. We recorded such data from three cells with equivalent results. As a fraction of the peak current elicited by a step to −42 mV from a holding potential of −82 mV, the average limiting availability of IT for these cells increased from 0.29 ± 0.01 (n = 2) to 0.59 ± 0.03 (n = 3) for conditioning steps of 10 and 100 ms, respectively.
DISCUSSION
We have characterized and modeled the biophysical behavior of IT in goldfish retinal ganglion cells, with particular attention to the voltage-dependence and kinetics of activation, inactivation and recovery from inactivation. Below, we compare the kinetics of ganglion cell IT to that of the T channel clones, and discuss the role and interaction of membrane potential and IT in ganglion cells at rest and during light responses.
Comparison with cloned T-channel currents
We found that the rate of activation was strongly voltage-dependent, that the rate of inactivation reached a plateau with strong depolarization, and that deactivation and recovery from inactivation were fast. These are general characteristics of IT in many different native systems (Huguenard 1996) as well as the three primary cloned channels that produce T-type currents in expression systems (Perez-Reyes 2003). The kinetic parameters of IT of the goldfish retinal ganglion cell are plotted together with those of cloned rat and human T channels in Fig. 11. Goldfish IT kinetic parameters are comparable to these other values, although goldfish values tend to be among the fastest, especially for deactivation. This could reflect some unique property or accessory subunit association of the goldfish T channel (cf. Hobom et al. 2000), or it might simply reflect conditions used to make the different studies. Although the measurements shown in Fig. 11 were made under reasonably consistent ionic conditions, almost all the studies were done at room temperature, which is cold for the mammalian channels, but normal or a bit warm for the goldfish. Since the Q10’s of T-type current properties are generally > 2 (Coulter et al. 1989; Takahashi et al. 1991), mammalian T channels at appropriate physiological temperature would gate substantially faster, and their deactivation kinetics would be closer to that of the goldfish IT. The kinetics of both the goldfish and mammalian IT would then be comparable.
Fig. 11.

Kinetic parameters summary. Compares kinetic parameters obtained for IT from goldfish retinal ganglion cells with those obtained for rat and human T-channel clones in expression systems. Circles are values determined for α1G clones, squares for α1H and triangles for α1I. The heavy horizontal lines show the values for goldfish IT. τ (h) is the time constant for inactivation, τ (recov) the time constant for recovery from inactivation (at ca. −90 mV), t-t-p is time-to-peak (at ca. −40 mV), and τ (deact) is the time constant for deactivation (at ca. −90 mV). Note log scale for ordinate. Clone data were taken from Chemin et al. (2001, 2002), Cribbs et al. (2000), Klöckner et al. (1999), Monteil et al. (2000), Satin and Cribbs (2000), and Serrano et al. (1999).
Fig. 11 shows that the kinetic parameters of the IT subtypes tend to cluster in a characteristic manner. The parameters obtained for goldfish IT show significant overlap with the α1G and α1H subtypes, but not with α1I. Between the overlapping subtypes, two observations suggest that the goldfish retinal ganglion cell IT is of the α1G subtype: (1) the kinetics of recovery from inactivation are markedly faster for IT in this work and for α1G than for α1H (Fig. 11); and (2) the α1H subtype is blocked by Ni2+ at low concentrations (IC50 13 μM; Lee et al. 1999b), but both cloned α1G (IC50 250 μM; Lee et al. 1999b) and this IT (38% block at 100 μM; Bindokas and Ishida 1996) are relatively insensitive to Ni2+. In the only other retinal ganglion cells where Ni2+-sensitivity has been assessed, T-type current was reduced about 85% by 20 μM Ni2+ in Xenopus (Akopian and Witkovsky 1996). This higher sensitivity to Ni2+ suggests that this T current is produced by a different channel subtype, but the recording conditions of this study were sufficiently different that a direct comparison might not be valid.
Consistent with this similarity of T-current properties between the retinal ganglion cell and the cloned α1G subtype (Fig. 11), most of the parameter values of the kinetic model used in our simulations were roughly near the values for cloned α1G (compare Table 1 vs. the legend for Fig. 14 of Serrano et al. 1999). The rate constants for channel opening and closing were significantly different from those for α1G; these parameters needed to be larger to account for the relatively rapid deactivation of T current in the retinal ganglion cell. The same kinetic model has been applied successfully to the α1I T-type channel (Frazier et al. 2001). Although this subtype displays faster deactivation kinetics than α1G (Fig. 11), its other rate constants are substantially slower (Frazier et al. 2001). A simulation using rate constants similar to α1I exhibits an order of magnitude slower increase in IT availability during priming and yields a much poorer fit to our data (not shown). Overall, the kinetics and pharmacology of ganglion cell IT are in agreement with the properties of the α1G subtype, although we cannot exclude that other subtypes may be present.
Physiological Role
IT may contribute to setting the resting [Ca2+] level. In almost all cell types expressing IT, including the goldfish retinal ganglion cell, the extrapolated window current coincides with the approximate resting potential (Huguenard 1996). This suggests that a small residual Ca2+ influx due to an IT window could help set resting intracellular [Ca2+]. There are two different approaches we can use to estimate the Ca2+ influx due to persistently active IT in retinal ganglion cells. The “window” current arising from the overlap of the I-V and steady-state inactivation curves would be on the order of 0.1 pA. [i.e., 0.05 (fraction of non-inactivated IT at −64 mV) × 0.02-0.05 (approximate open probability of IT at −64 mV) × 51 pA (mean peak IT adjusted for driving force at −64 mV)] in goldfish retinal ganglion cells. Although a very small current, this would still bring in the equivalent of ~150 nM/sec homogeneously-distributed [Ca2+] in a 20 μm diameter cell (~12.5 pF, modeled as a sphere). This “window” current calculation formally assumes the independence of activation and inactivation. On the other hand, in the kinetic model we used to simulate our results (Serrano et al. 1999), activation and inactivation are not independent. In this scheme, inactivation is incomplete and 0.1-0.2% (simulated with our parameter values) of T channels remain open at all voltages where IT activates. Although different in mechanism and voltage dependence from the “window” current, the magnitude of persistent Ca2+ current calculated by the kinetic model is very similar to the “window” at −64mV (data not shown). In either case, with measured resting intracellular [Ca2+] of ~120 nM (Ishida et al. 1991), a continuous Ca2+ influx of this magnitude could contribute in setting [Ca2+] levels in goldfish retinal ganglion cells.
Another possibility is that T window (or persistent) current could contribute directly to a form of input signal amplification, as described in thalamocortical neurons (Williams et al. 1997). However, this phenomenon depends on IT being relatively large compared to the leak, and satisfying the relation that the maximum slope of dIT/dV in the vicinity of the window is greater than the leak conductance (Williams et al. 1997). Estimated leak conductance in goldfish retinal ganglion cells is 0.9 nS (Lee and Ishida, unpublished). A generous estimate of window dIT/dV is 0.1 pA/5 mV = 0.02 nS, well short of the threshold for this effect. It therefore seems unlikely that the amounts of T current we have recorded could contribute to excitability by this mechanism in retinal ganglion cells. Cells with more T current might be different.
Voltage- and time-dependent changes in the availability of IT might also play a role in spike generation. Two issues must be considered when assessing the possible contribution that IT could make: First, do ganglion cells hyperpolarize enough to prime IT? Second, after priming, how does IT compare in amplitude with other subthreshold currents in ganglion cells?
Because the resting membrane potential of isolated goldfish retinal ganglion cells is in the range of −60 to −75 mV (Vaquero et al. 2001; Vaquero and Ishida, unpublished), and because most IT is inactivated in this voltage range (Fig. 5), a mechanism to hyperpolarize ganglion cells, and thereby relieve the inactivation, must come into play for IT to contribute to excitability. Intracellular recordings have shown that suitably arranged stimuli hyperpolarize ganglion cells in various species by as much as 15 mV beyond their resting potential (Slaughter and Bai 1989; Ammermüller and Kolb 1995), and recent recordings have indicated that the reversal potential for these voltage changes is around −95 mV (e.g., Demb et al. 2001; Zaghloul et al. 2003). Assuming that these values are not peculiarities of the species used (salamander, turtle, and guinea pig), these results suggest that ganglion cells could hyperpolarize to membrane potentials that increase the availability of IT. Fig. 10 shows that a sequence of transient hyperpolarizations, mimicking repetitive inhibitory input, leads to partial relief from inactivation of IT in goldfish retinal ganglion cells. Hence, if ganglion cells hyperpolarized to −80 or −85 mV during such inhibitory input, our results predict that the availability of IT would increase to ~75% of maximal current.
Activation threshold for the inactivating Na+ current in goldfish retinal ganglion cells is about −55 mV and is not changed by prior hyperpolarization (Hidaka and Ishida 1998). Negative to −55 mV, IT, Ih, persistent Na+ and a linear leak are the only known currents; Ih activates at voltages negative to −70 mV, and persistent INa gates positive to about −65 mV (Tabata and Ishida 1996, 1999; Hidaka and Ishida 1998; Lee and Ishida, unpublished observations). We can therefore ask if IT contributes to changes in membrane potential after terminating a volley of hyperpolarizations, by combining the T-current properties found in the present study with our measurements of the current density, kinetics, and voltage-sensitivity of Ih, and the input conductance around −65 mV in the absence of any channel-blocking pharmacological agents. Calculation of the voltage expected in a cell body with these properties (Fig. 12A) shows that termination of a single 100-msec hyperpolarization leads to an off depolarization, like that recorded in various retinal ganglion cell preparations (Eng et al. 1990; Tabata and Ishida 1996; O’Brien et al. 2002), and that the off depolarization is approximately 30% smaller when IT is deleted from the calculation. Explicitly including a term for persistent INa had no qualitative effect on these simulated currents; removing IT from the simulation still reduces the amplitude and rate of the rebound depolarization (not shown). Fig. 12 also shows that the presence and absence of IT makes little difference to the depolarization after a single 50-msec pulse, but that a contribution appears after a volley of several 50-msec hyperpolarizations (Fig. 12B). This is consistent with the increase in the availability of IT with multiple short hyperpolarizations shown in Fig. 10. A fuller understanding of how this contributes to spiking in situ will require additional recording, as well as modeling that incorporates the mix and magnitude of all currents near spike threshold.
Fig. 12.
Simulation of depolarizing rebound after priming. A cell model was constructed incorporating IT (Fig. 8A), Ih (Tabata and Ishida 1996), leak current, and membrane capacitance. The Ih and leak currents were calculated with equations introduced previously (Tabata and Ishida 1996). The parameter values for Ih were the same as those estimated by Tabata and Ishida (1996). The parameter values for leak current were estimated from the input conductance measured at around −65 mV in the absence of any channel blockers (42 pS/pF; Lee and Ishida, unpublished). The specific membrane capacitance was assumed to be 1 μF/cm2. (A) Membrane voltage responses to single hyperpolarizing current pulses. The current pulse was injected into the cell model whose PCa was set to 7 × 10−6 cm/sec (i.e., default value, solid lines) or 0 cm/sec, (i.e., no T current, dashed lines). The pulse amplitude was 15 pA and the pulse duration was 50 ms (red lines) or 100 ms (black lines). (B) Membrane voltage response to single versus repetitive hyperpolarizing current pulses. The current pulse was injected once (red line) or nine times (black line), into the cell model whose PCa was set to the default value. The pulse amplitude was 15 pA. The pulse duration and inter-pulse interval were 50 ms and 30 ms, respectively, for the repetitive hyperpolarization.
In summary, we were able to record IT under physiological conditions and develop a model that replicates the behavior of IT under these conditions. This suggests that we can use the model to extend the range of IT behaviors that can be investigated and incorporate IT into broader models of retinal ganglion cell function. Previously published models of nerve impulse generation in retinal ganglion cells (e.g., Fohlmeister and Miller 1997) did not include hyperpolarization-dependent currents (IT and Ih) and did not address the effect of inhibitory hyperpolarizing inputs. To extend these sorts of models, our results suggest that the increased availability of IT at the conclusion of a hyperpolarizing sequence, in combination with concurrent activation of Ih, should lead to a depolarizing rebound at termination of the IPSPs. IT could thus function as a modulator of the interplay between excitation and inhibition in retinal ganglion cells, and the results of this study provide a specific timecourse and voltage range over which to look for this modulation.
ACKNOWLEDGEMENTS
This work was supported by NIH grant EY 08120 (to A.T.I.) and National Eye Institute Core grant P30 EY12576. We thank J. R. Huguenard and P. A. Pappone for comments on the manuscript, G. J. Partida for preparing the primary cell cultures used in these experiments, and K. E. Munckton and A. Pignatelli for participation in some initial recordings.
REFERENCES
- Ahlijanian MK, Westenbroek RE, Catterall WA. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron. 1990;4:819–832. doi: 10.1016/0896-6273(90)90135-3. [DOI] [PubMed] [Google Scholar]
- Akopian A, Witkovsky P. Activation of metabotropic glutamate receptors decreases a high-threshold calcium current in spiking neurons of the Xenopus retina. Vis Neurosci. 1996;13:549–557. doi: 10.1017/s0952523800008221. [DOI] [PubMed] [Google Scholar]
- Ammermüller J, Kolb H. The organization of the turtle inner retina. I. ON- and OFF-center pathways. J Comp Neurol. 1995;358:1–34. doi: 10.1002/cne.903580102. [DOI] [PubMed] [Google Scholar]
- Attwell D, Cohen I, Eisner D, Ohba M, Ojeda C. The steady state TTX-sensitive (“window”) sodium current in cardiac Purkinje fibres. Pflügers Arch. 1979;379:137–142. doi: 10.1007/BF00586939. [DOI] [PubMed] [Google Scholar]
- Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol. 1995;483:641–663. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge WH. Optical recordings of the effects of cholinergic ligands on neurons in the ganglion cell layer of mammalian retina. J Neurosci. 1996;16:5060–5072. doi: 10.1523/JNEUROSCI.16-16-05060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Fettiplace R. Synaptic drive and impulse generation in ganglion cells of turtle retina. J Physiol. 1979;288:107–127. [PMC free article] [PubMed] [Google Scholar]
- Biagi BA, Enyeart JJ. Multiple calcium currents in a thyroid C-cell line: biophysical properties and pharmacology. Am J Physiol Cell Physiol. 1991;260:C1253–C1263. doi: 10.1152/ajpcell.1991.260.6.C1253. [DOI] [PubMed] [Google Scholar]
- Bindokas VP, Ishida AT. Conotoxin-sensitive and conotoxin-resistant Ca2+ currents in fish retinal ganglion cells. J Neurobiol. 1996;29:429–444. doi: 10.1002/(SICI)1097-4695(199604)29:4<429::AID-NEU2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Burgess DE, Crawford O, Delisle BP, Satin J. Mechanism of inactivation gating of human T-type (low-voltage activated) calcium channels. Biophys J. 2002;82:1894–1906. doi: 10.1016/S0006-3495(02)75539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Lux HD. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Bourinet E, Nargeot J, Lory P. Alternatively spliced α1G (CaV3.1) intracellular loops promote specific T-type Ca2+ channel gating properties. Biophys J. 2001;80:1238–1250. doi: 10.1016/S0006-3495(01)76100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Monteil A, Perez-Reyes E, Bourinet E, Nargeot J, Lory P. Specific contribution of human T-type calcium channel isotypes (α1G, α1H and α1I) to neuronal excitability. J Physiol. 2002;540:3–14. doi: 10.1113/jphysiol.2001.013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PA, Miller RF. Measurement of passive membrane parameters with whole-cell recording from neurons in the intact amphibian retina. J Neurophysiol. 1989;61:218–230. doi: 10.1152/jn.1989.61.1.218. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Huguenard JR, Prince DA. Calcium currents in rat thalamocortical relay neurones: kinetic properties of the transient, low-threshold current. J Physiol. 1989;414:587–604. doi: 10.1113/jphysiol.1989.sp017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs LL, Gomora JC, Daud AN, Lee J-H, Perez-Reyes E. Molecular cloning and functional expression of CaV3.1c, a T-type calcium channel from human brain. FEBS Lett. 2000;466:54–58. doi: 10.1016/s0014-5793(99)01756-1. [DOI] [PubMed] [Google Scholar]
- Cribbs LL, Lee J-H, Yang J, Satin J, Zhang Y, Daud A, Barclay J, Williamson MP, Fox M, Rees M, Perez-Reyes E. Cloning and characterization of α1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res. 1998;83:103–109. doi: 10.1161/01.res.83.1.103. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Lightowler S, Pollard CE. A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J Physiol. 1989;413:543–561. doi: 10.1113/jphysiol.1989.sp017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schutter E, Smolen P. Calcium dynamics in large neuronal models. In: Koch C, Segev I, editors. Methods in Neuronal Modeling: From Ions to Networks. The MIT Press; Cambridge (Mass.): 1998. [Google Scholar]
- Demb JB, Zaghloul K, Haarsma L, Sterling P. Bipolar cells contribute to nonlinear spatial summation in the brisk-transient (Y) ganglion cell in mammalian retina. J Neuroscience. 2001;21:7447–7454. doi: 10.1523/JNEUROSCI.21-19-07447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destexhe A, Huguenard JR. Nonlinear thermodynamic models of voltage-dependent currents. J Comput Neurosci. 2000;9:259–270. doi: 10.1023/a:1026535704537. [DOI] [PubMed] [Google Scholar]
- Eng DL, Gordon TR, Kocsis JD, Waxman SG. Current-clamp analysis of a time-dependent rectification in rat optic nerve. J Physiol. 1990;421:185–202. doi: 10.1113/jphysiol.1990.sp017940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fohlmeister JF, Miller RF. Impulse encoding mechanisms of ganglion cells in the tiger salamander retina. J Neurophysiol. 1997;78:1935–1947. doi: 10.1152/jn.1997.78.4.1935. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Serrano JR, George EG, Yu X, Viswanathan A, Perez-Reyes E, Jones SW. Gating kinetics of the α1I T-type calcium channel. J Gen Physiol. 2001;118:457–470. doi: 10.1085/jgp.118.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y, Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther E, Rothe T, Taschenberger H, Grantyn R. Separation of calcium currents in retinal ganglion cells from postnatal rat. Brain Res. 1994;633:223–235. doi: 10.1016/0006-8993(94)91543-1. [DOI] [PubMed] [Google Scholar]
- Henderson D, Miller RF. Evidence for low-voltage-activated (LVA) calcium currents in the dendrites of tiger salamander retinal ganglion cells. Vis Neurosci. 2003;20:141–152. doi: 10.1017/s0952523803202054. [DOI] [PubMed] [Google Scholar]
- Herrington J, Lingle CJ. Kinetic and pharmacological properties of low voltage-activated Ca2+ current in rat clonal (GH3) pituitary cells. J Neurophysiol. 1992;68:213–232. doi: 10.1152/jn.1992.68.1.213. [DOI] [PubMed] [Google Scholar]
- Hidaka S, Ishida AT. Voltage-gated Na+ current availability after step- and spike-shaped conditioning depolarizations of retinal ganglion cells. Pflügers Arch. 1998;436:497–508. doi: 10.1007/s004240050664. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sinauer Associates; Sunderland (Mass.): 1984. p. 230. [Google Scholar]
- Hines ML, Carnevale NT. The NEURON simulation environment. Neural Comput. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- Hines ML, Carnevale NT. Expanding NEURON’s repertoire of mechanisms with NMODL. Neural Comput. 2000;12:995–1007. doi: 10.1162/089976600300015475. [DOI] [PubMed] [Google Scholar]
- Hobom M, Dai S, Marais E, Lacinova L, Hofmann F, Klugbauer N. Neuronal distribution and functional characterization of the calcium channel α2δ-2 subunit. Eur J Neurosci. 2000;12:1217–1226. doi: 10.1046/j.1460-9568.2000.01009.x. [DOI] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S-J, Robinson DW. Activation and inactivation properties of voltage-gated calcium currents in developing cat retinal ganglion cells. Neuroscience. 1998;85:239–247. doi: 10.1016/s0306-4522(97)00351-5. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol. 1996;58:329–348. doi: 10.1146/annurev.ph.58.030196.001553. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, McCormick DA. Simulation of the currents involved in rhythmic oscillations in thalamic relay neurons. J Neurophysiol. 1992;68:1373–1383. doi: 10.1152/jn.1992.68.4.1373. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. A novel T-type current underlies prolonged Ca2+-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci. 1992;12:3804–3817. doi: 10.1523/JNEUROSCI.12-10-03804.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida AT, Bindokas VP, Nuccitelli R. Calcium ion levels in resting and depolarized goldfish retinal ganglion cell somata and growth cones. J Neurophysiol. 1991;65:968–979. doi: 10.1152/jn.1991.65.4.968. [DOI] [PubMed] [Google Scholar]
- Ishida AT, Cohen BN. GABA-activated whole-cell currents in isolated retinal ganglion cells. J Neurophysiol. 1988;60:381–396. doi: 10.1152/jn.1988.60.2.381. [DOI] [PubMed] [Google Scholar]
- Karschin A, Lipton SA. Calcium channels in solitary retinal ganglion cells from post-natal rat. J Physiol. 1989;418:379–396. doi: 10.1113/jphysiol.1989.sp017847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U, Lee J-H, Cribbs LL, Daud A, Hescheler J, Pereverzev A, Perez-Reyes E, Schneider T. Comparison of the Ca2+ currents induced by expression of three cloned α1 subunits, α1G, α1H and α1I, of low-voltage-activated T-type Ca2+ channels. Eur J Neurosci. 1999;11:4171–4178. doi: 10.1046/j.1460-9568.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- Kuo C-C, Bean BP. Na+ channels must deactivate to recover from inactivation. Neuron. 1994;12:819–829. doi: 10.1016/0896-6273(94)90335-2. [DOI] [PubMed] [Google Scholar]
- Kuo C-C, Yang S. Recovery from inactivation of T-type Ca2+ channels in rat thalamic neurons. J Neurosci. 2001;21:1884–1892. doi: 10.1523/JNEUROSCI.21-06-01884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert RC, McKenna F, Maulet Y, Talley EM, Bayliss DA, Cribbs LL, Lee JH, Perez-Reyes E, Feltz A. Low-voltage-activated Ca2+ currents are generated by members of the CaVT subunit family (α1G/H) in rat primary sensory neurons. J Neurosci. 1998;18:8605–8613. doi: 10.1523/JNEUROSCI.18-21-08605.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-H, Daud AN, Cribbs LL, Lacerda AE, Pereverzev A, Klöckner U, Schneider T, Perez-Reyes E. Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family. J Neurosci. 1999a;19:1912–1921. doi: 10.1523/JNEUROSCI.19-06-01912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-H, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block α1H. Biophys J. 1999b;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CA. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lasater EM. Calcium currents in turtle retinal ganglion cells. I. The properties of T- and L-type currents. J Neurophysiol. 1994;71:733–742. doi: 10.1152/jn.1994.71.2.733. [DOI] [PubMed] [Google Scholar]
- Lux HD, Carbone E, Zucker H. Na+ currents through low-voltage-activated Ca2+ channels of chick sensory neurones: block by external Ca2+ and Mg2+ J Physiol. 1990;430:159–188. doi: 10.1113/jphysiol.1990.sp018287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992;68:1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- Mittman S, Taylor WR, Copenhagen DR. Concomitant activation of two types of glutamate receptor mediates excitation of salamander retinal ganglion cells. J Physiol. 1990;428:175–197. doi: 10.1113/jphysiol.1990.sp018206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteil A, Chemin J, Leuranguer V, Altier C, Mennessier G, Bourinet E, Lory P, Nargeot J. Specific properties of T-type calcium channels generated by the human α1I subunit. J Biol Chem. 2000;275:16530–16535. doi: 10.1074/jbc.C000090200. [DOI] [PubMed] [Google Scholar]
- Murakami M, Shimoda Y. Identification of amacrine and ganglion cells in the carp retina. J Physiol. 1977;264:801–818. doi: 10.1113/jphysiol.1977.sp011695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’tBrien BJ, Isayama T, Richardson R, Berson DM. Intrinsic physiological properties of cat retinal ganglion cells. J Physiol. 2002;538:787–802. doi: 10.1113/jphysiol.2001.013009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee J-H. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- Protti DA, Gerschenfeld HM, Llano I. GABAergic and glycinergic IPSCs in ganglion cells of rat retinal slices. J Neurosci. 1997;17:6075–6085. doi: 10.1523/JNEUROSCI.17-16-06075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall AD, Tsien RW. Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology. 1997;36:879–893. doi: 10.1016/s0028-3908(97)00086-5. [DOI] [PubMed] [Google Scholar]
- Sakai HM, Naka K-I. Signal transmission in the catfish retina. IV. Transmission to ganglion cells. J Neurophysiol. 1987;58:1307–1328. doi: 10.1152/jn.1987.58.6.1307. [DOI] [PubMed] [Google Scholar]
- Satin J, Cribbs LL. Identification of a T-type Ca2+ channel isoform in murine atrial myocytes (AT-1 cells) Circ Res. 2000;86:636–642. doi: 10.1161/01.res.86.6.636. [DOI] [PubMed] [Google Scholar]
- Serrano JR, Perez-Dashti SR, Reyes E, Jones SW. Mg2+ block unmasks Ca2+/Ba2+ selectivity of α1G T-type calcium channels. Biophys J. 2000;79:3052–3062. doi: 10.1016/S0006-3495(00)76540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano JR, Perez-Reyes E, Jones SW. State-dependent inactivation of the α1G T-type calcium channel. J Gen Physiol. 1999;114:185–201. doi: 10.1085/jgp.114.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter MM, Bai S-H. Differential effects of baclofen on sustained and transient cells in the mudpuppy retina. J Neurophysiol. 1989;61:374–381. doi: 10.1152/jn.1989.61.2.374. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Rogawski MA. T-type calcium channels mediate the transition between tonic and phasic firing in thalamic neurons. Proc Natl Acad Sci USA. 1989;86:7228–7232. doi: 10.1073/pnas.86.18.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata T, Ishida AT. Transient and sustained depolarization of retinal ganglion cells by Ih. J Neurophysiol. 1996;75:1932–1943. doi: 10.1152/jn.1996.75.5.1932. [DOI] [PubMed] [Google Scholar]
- Tabata T, Ishida AT. A zinc-dependent Cl− current in neuronal somata. J Neurosci. 1999;19:5195–5204. doi: 10.1523/JNEUROSCI.19-13-05195.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Ueno S, Akaike N. Kinetic properties of T-type Ca2+ currents in isolated rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 1991;65:148–155. doi: 10.1152/jn.1991.65.1.148. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Inhibitory postsynaptic potentials evoked in thalamic neurons by stimulation of the reticularis nucleus evoke slow spikes in isolated rat brain slices--I. Neuroscience. 1988;25:491–502. doi: 10.1016/0306-4522(88)90253-9. [DOI] [PubMed] [Google Scholar]
- Vaquero CF, Pignatelli A, Partida GJ, Ishida AT. A dopamine- and protein kinase A-dependent mechanism for network adaptation in retinal ganglion cells. J Neurosci. 2001;21:8624–8635. doi: 10.1523/JNEUROSCI.21-21-08624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X, Desilets M, Soboloff J, Morris C, Tsang BK. Muscarinic activation inhibits T-type Ca2+ current in hen granulosa cells. Endocrinology. 1996;137:2514–2521. doi: 10.1210/endo.137.6.8641205. [DOI] [PubMed] [Google Scholar]
- Wang X-J, Rinzel J, Rogawski MA. A model of the T-type calcium current and the low-threshold spike in thalamic neurons. J Neurophysiol. 1991;66:839–850. doi: 10.1152/jn.1991.66.3.839. [DOI] [PubMed] [Google Scholar]
- Wiesel TN. Recording inhibition and excitation in the cat’s retinal ganglion cells with intracellular electrodes. Nature. 1959;183:264–265. doi: 10.1038/183264a0. [DOI] [PubMed] [Google Scholar]
- Williams SR, Tóth TI, Turner JP, Hughes SW, Crunelli V. The ‘window’ component of the low threshold Ca2+ current produces input signal amplification and bistability in cat and rat thalamocortical neurones. J Physiol. 1997;505:689–705. doi: 10.1111/j.1469-7793.1997.689ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci. 2002;22:3404–3413. doi: 10.1523/JNEUROSCI.22-09-03404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse JM, Barlow HB. Spatial and temporal resolution and analysis. In: Barlow HB, Mollon JD, editors. The Senses. Cambridge University Press; Cambridge: 1982. [Google Scholar]
- Zaghloul KA, Boahen K, Demb JB. Different circuits for ON and OFF retinal ganglion cells cause different contrast sensitivities. J Neurosci. 2003;23:2645–2654. doi: 10.1523/JNEUROSCI.23-07-02645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]