Abstract
Background
Although the etiology of the metabolic syndrome remains unclear, recent evidence suggests that dysregulation of brain serotonergic activity may partly underlie the covariation of risk factors comprising the syndrome. In addition, prior studies have shown polymorphisms in the serotonin 2A receptor (HTR2A) gene to be associated with two syndrome components, hypertension and central adiposity. We conducted a study to confirm associations of HTR2A polymorphisms with elevated blood pressure and central adiposity and tested for association between these polymorphisms and the metabolic syndrome.
Methods
The study sample included 934 unrelated individuals of European ancestry. We tested for association of two HTR2A polymorphisms, one in the promoter: (−1438[G/A]) and one in the first intron (2416 [C/T]), individually and as a diplotype, with elevated blood pressure, central adiposity, elevated fasting glucose, triglycerides, high density lipoprotein (HDL) cholesterol and presence of the metabolic syndrome, as defined by the American Heart Association/National Heart, Lung and Blood Institute (AHA/NHLBI) Scientific Statement Executive Summary.
Results
Confirming previous reports, elevated blood pressure (>130/85 mm Hg) was associated with both the −1438 GG and 2416 TT genotypes and the GG/TT diplotype (ORs = 1.39–1.76); high waist circumference was associated with −1438 GG genotype only (OR = 1.57). In addition, both the −1438 GG and 2416 TT genotypes, and the GG/TT diplotype, predicted presence of the metabolic syndrome (ORs = 1.44–1.77). Fasting glucose, triglyceride and HDL cholesterol were not associated with either polymorphism.
Conclusions
Elevated blood pressure, central adiposity, and the metabolic syndrome are associated with polymorphisms in HTR2A gene.
INTRODUCTION
The metabolic syndrome is defined by a constellation of correlated cardiovascular disease risk factors that includes elevated blood pressure, central adiposity, and altered glucose and lipid metabolism. Although it remains unclear what pathophysiologic processes may underlie the aggregation of these diverse phenotypes, several studies have shown low central nervous system (CNS) serotonergic responsivity (as indexed by standard neuropharmacologic challenges) to predict presence of the metabolic syndrome, heightened blood pressure, obesity, dyslipidemia, and insulin insensitivity or insulin resistance in non-patient samples.1–4 These findings are consistent with evidence that neurons releasing the neuro-transmitter serotonin (5-hydroxytryptamine, (or 5-HT) modulate a range of behavioral, autonomic, and neuroendocrine functions relevant to metabolism and blood pressure regulation.5–8
Recent studies suggest that polymorphic variation in the serotonin 2A receptor (HTR2A) gene may be associated with hypertension9 and abdominal obesity.10 However, no associations were observed between HTR2A polymorphisms and hypertension in smaller case control studies in Chinese11 and Japanese subjects.12 The purpose of the present study was to confirm initial genetic observations in a larger, nonpatient study sample and to determine if the metabolic syndrome itself (previously metabolic syndrome has not been examined in relation to HTR2A gene) is also associated with polymorphic variation in HTR2A.
METHODS
Study population
Subjects included in the present analyses were 934 unrelated individuals with self-identified non-Hispanic European ancestry participating in the University of Pittsburgh Adult Health and Behavior (AHAB) project. Participants were community volunteers recruited by mass-mail solicitation from Southwestern Pennsylvania. All subjects were between 30 and 54 years of age and without a history of atherosclerotic cardiovascular disease; cancer diagnosis or treatment within the past year; and did not use psychotropic, glucocorticoid, antihypertensive, diabetic, dyslipidemic, or cholesterol-lowering medications. The study protocol was approved by the University of Pittsburgh IRB, and participants provided written informed consent.
Risk factor assessments
Participants arrived at the laboratory between 0730 and 1030 hours following a 12-hour overnight fast. Subjects rested in a seated position for at least 10 minutes, after which two blood pressure measurements were obtained by trained staff using a mercury sphygmomanometer and an appropriately-sized cuff. Height, weight, and waist circumference (at the umbilicus) were measured and, following phlebotomy, fasting serum lipids and glucose was determined by methods described previously13 at the University of Pittsburgh, Heinz Nutrition Laboratory. Metabolic syndrome was defined by the American Heart Association/National Heart Lung and Blood Institute criteria14 as the presence of three or more of the following: 1) fasting serum glucose 100 mg/dL or higher; 2) serum triglycerides 150 mg/dL or higher; 3) serum high density lipoprotein (HDL) cholesterol below 40 mg/dL in men or below 50 mg/dL in women; 4) blood pressure 130/85 mmHg or higher; 5) waist circumference 102 cm or more in men and 88 cm or more in women.
Genetic analysis
Blood samples for genotyping were collected in 10mmol/L EDTA, and DNA was isolated following previously described protocols.15 Two single nucleotide polymorphisms (SNPs) in the HTR2A gene on chromosome 13q14-21 were genotyped using polymerase chain reaction (PCR) amplification and fluorescence polarization. assay16
Rationale for SNP selection
Since previous reports have found genetic variants in the promoter and promoter proximal regions to be associated with single components of the metabolic syndrome10,11 we chose to focus on the same area of the gene. Prior research has shown the C-allele of the common HTR2A T102C polymorphism to be more prevalent in elderly hypertensives than among age-matched nor-motensive controls.10 The T102C locus has no known functionality but is in complete linkage disequilibrium (LD) with the potentially functional promoter polymorphism, G–1438A,10,17 which has been shown to modulate transcriptional efficiency in reporter gene constructs.18 In particular, the −1438 G allele is associated with diminished gene promoter activity relative to the alternate −1438 A allele. Hence we chose to investigate only the promoter polymorphism −1438(G/A) (dbSNP: rs6311) here, and not the T102C polymorphism. In addition, we chose a second tag SNP, 2416(C/T) polymorphism (dbSNP: rs2070040) in intron 1 of the HTR2A gene, which is not in perfect LD with the −1438 promoter polymorphism19 to obtain wider coverage of the promoter proximal region of interest.
The primer sequences for −1438G/A polymorphism were 5′-GCCCTTTTGTGCAGATTCCCA-3′ (forward) and 5′-CCTGAGCCTATGTGGCCAAT-3′ (reverse), with the following conditions: 95°C for 5 minutes, followed by 35 cycles of denaturation at 95°C for 30 seconds, annealing at 53°C for 15 seconds, extension at 72°C for 30 seconds, and a final extension at 72°C for 5 minutes. The FP-TDI allele-specific detection primer was 5′-TCCTCGGAGTGCTGTGAGTGTC-3′. For the 2416(C/T) polymorphism, the primer sequences were 5′-ATTAGCAAGCTCCCGGGTGA-3′ (forward) and 5′-CACCTCAGCCCACCCTCCAA-3′ (reverse) under the following conditions: 94°C for 10 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds annealing at 54°C for 30 seconds and extension at 72°C for 1 minute and then at 72°C for 10 minutes. The FP-TDI allele specific detection primer was 5′-TCAGGACCTTCTCTAAAGCTCTCCAG-3′. Genotypes were read on the Analyst HT high throughput screening system (LJL Biosystems) and assigned using the Allele Caller software package. Fourteen subjects were run in duplicate to test for genotyping errors.
Allele frequencies and Hardy Weinberg equilibrium were ascertained using Genepop software.20 The program STRUCTURE21,22 was used to evaluate presence of genetic substructure in the sample, using 15 additional SNPs (rs1022106, rs1335995, rs1439564, rs1502812, rs1860300, rs548146, rs705388, rs715994, rs720517, rs722743, rs730899, rs734204, rs9059966, rs1328994, rs1485405, SNPs chosen from HapMap, www.hapmap.org) and a model with admixture, correlated allele frequencies, individual α parameter, and independent FST for all subpopulations. We tested models with 1, 2, and 3 subpopulations using a burn-in of 40,000 followed by 80,000 repetitions and compared the likelihoods of models fitting the data.
Statistical analysis
Associations between unadjusted outcome variables and HTR2A genotypes were examined using χ2 goodness of fit tests. Presence/absence of elevated blood pressure, high waist circumference, elevated blood glucose, elevated serum triglycerides and lower HDL cholesterol were examined by logistic regression for association with HTR2A genotypes, after adjusting for age, sex, BMI, and years of education. Phenotypes that showed a positive association in logistic regression were further evaluated in linear regression models to examine associations between polymorphisms and quantitative phenotypes, using the same covariates as before. HTR2A genotypes were evaluated for association with metabolic syndrome (present/absent) by χ2 tests and logistic regression analyses. SPSS v13 was used for all statistical analyses.
Power calculations performed using QUANTO 1.123,24 indicate 86% to 99% power to detect small (R2 = 0.01) to medium (R2 = 0.04) effect sizes assuming a single gene effect on quantitative phenotypes in our sample.
RESULTS
Sample characteristics are presented in Table 1. Metabolic syndrome was present in 18% (172) of the 934 individuals included in this study. In the entire sample, allele frequencies at the −1438 locus were 0.57 (G-allele) and 0.43 (A), respectively, and 0.59 (C) and 0.41 (T) at the 2416 locus. The distribution of genotypes at both loci conformed to Hardy-Weinberg equilibrium (−1438[G/A]: GG = 299, GA = 449; AA = 166 [P = .84]; 2416[C/T]: CC = 329; CT = 449; TT = 156 [P = .85]), and the two polymorphisms were in strong LD (r2 = 0.443). No evidence of genetic substructure was detected in the sample (log probability of data for k = 1, 2, and 3 subpopulations in STRUCTURE were −16892, −17798 and −18068 respectively), no further adjustments were made to control for stratification.
Table 1.
Subject Sample Characteristics
| Characteristic | N = 934 |
|---|---|
| Mean ± SD | |
| Age (years) | 44.2 ± 6.8 |
| Female (%) | 52.1 |
| Education in years | 16.1 ± 2.8 |
| Percentage with Metabolic Syndrome | 18.4 |
| Waist circumference (cm) | 89.9 ± 15.3 |
| Systolic BP (mm Hg) | 114.7 ± 12.7 |
| Diastolic BP (mm Hg) | 77.4 ± 9.1 |
| HDL Cholesterol (mg/dL) | 54.1 ± 14.9 |
| Triglycerides (mg/dL) | 121 ± 83.2 |
| Glucose (mg/dL) | 95.02 ± 14.8 |
χ2 goodness of fit tests indicated a significant association of elevated BP (≥ 130/85 mmHg) with 2416 C/T genotype (χ2 = 9.95; degrees of freedom (df) = 2, P = .007) and a suggestive association with −1438 A/G genotype (χ2 = 5.53; df = 2, P = .06) (Table 2). In tests for allelic associations, the −1438 G allele and 2416 C allele were associated significantly with elevated BP (P < .05 for both; Table 2). Logistic regression models including age, sex, education, and BMI as covariates showed elevated BP associated significantly with −1438 GG genotype (OR = 1.39 [95% CI: 1.00–1.95], P = .05), 2416 TT genotype (OR = 1.76 [95% CI: 1.19–2.6], P = .004), and the GG/TT diplotype (OR = 1.70 [95% CI: 1.16–2.48], P = .004). Fifteen percent of individuals (142/934) were homozygous for both the −1438 G-allele and the 2416 T-allele. In linear regression models evaluating BP as a continuous variable, systolic BP (SBP) was associated significantly with −1438 GG genotype (δR2 = 0.04, P = .037) and the GG/TT diplotype (δR2 = 0.04, P = .024), but was associated only marginally with 2416 TT genotype (δR2 = 0.03, P = .06). Diastolic BP (DBP) was associated significantly with −1438 GG genotype (δR2 = 0.05, P = .015), the 2416 TT genotype (δR2 = 0.04, P = .024) and the GG/TT diplotype (δR2 = 0.05, P = .017).
Table 2.
Genotypic and Allelic Distribution of HTR2A Polymorphisms in 934 Subjects Included in Present Study
| Genotypic distribution | Hypertension
|
Central adiposity
|
Metabolic syndrome
|
|||
|---|---|---|---|---|---|---|
| Present N (%) | Absent N (%) | Present N (%) | Absent N (%) | Present N (%) | Absent N (%) | |
| −1438 (G/A) | ||||||
| GG | 90 (30) | 209 (70) | 178 (60) | 121 (40) | 68 (23) | 231 (77) |
| GA | 104 (23) | 345 (77) | 233 (52) | 216 (48) | 79 (18) | 370 (82) |
| AA | 37 (22) | 129 (78) | 85 (51) | 81 (49) | 21 (13) | 145 (87) |
| Statistic | χ2 = 53.5; P = 0.063 | χ2 = 4.986; P = 0.083 | χ2 = 7.61; P = 0.022 | |||
| 2416 (C/T) | ||||||
| CC | 75 (23) | 254 (77) | 177 (54) | 152 (46) | 53 (16) | 276 (84) |
| CT | 112 (25) | 337 (75) | 240 (53) | 209 (47) | 80 (18) | 369 (82) |
| TT | 56 (36) | 100 (64) | 86 (55) | 70 (45) | 41 (26) | 115 (74) |
| Statistic | χ2 = 9.95; P = 0.007 | χ2 = 0.131; P = 0.936 | χ2 = 7.6; P = 0.022 | |||
| Allelic distribution | ||||||
| −1438 | ||||||
| G | 284 (27) | 763 (73) | 589 (56) | 458 (44) | 215 (21) | 832 (79) |
| A | 178 (23) | 603 (77) | 403 (52) | 378 (48) | 121 (15) | 660 (85) |
| Statistic | χ2 = 4.45; P = 0.035 | χ2 = 3.91; P = 0.048 | χ2 = 7.58; P = 0.006 | |||
| 2416 | ||||||
| C | 262 (24) | 845 (76) | 594 (54) | 513 (46) | 186 (17) | 921 (83) |
| T | 224 (29) | 537 (71) | 412 (54) | 349 (46) | 162 (22) | 599 (78) |
| Statistic | χ2 = 7.793; P = 0.007 | χ2 = 0.042; P = 0.84 | χ2 = 5.98; P = 0.014 | |||
Genotype and allelic distributions shown are shown for unadjusted phenotypic values only. Significant P values are shown in bold.
Central adiposity (high waist circumference) showed significant allelic associations but not genotypic associations (Table 2). The −1438G allele was associated with central adiposity both before and after adjustment for age, sex, and education (OR = 1.57 [95% CI: 1.16–2.12], P = .003) (Table 2). However, neither the 2416 (C/T) polymorphism nor the GG/TT diplotype associated with central adiposity. Waist circumference, as a continuous measure was also associated with −1438 GG genotype (δR2 = 0.05, P = .023).
Elevated levels of serum triglyceride and blood glucose and lower levels of HDL cholesterol were not associated with the two HTR2A polymorphisms or diplotype.
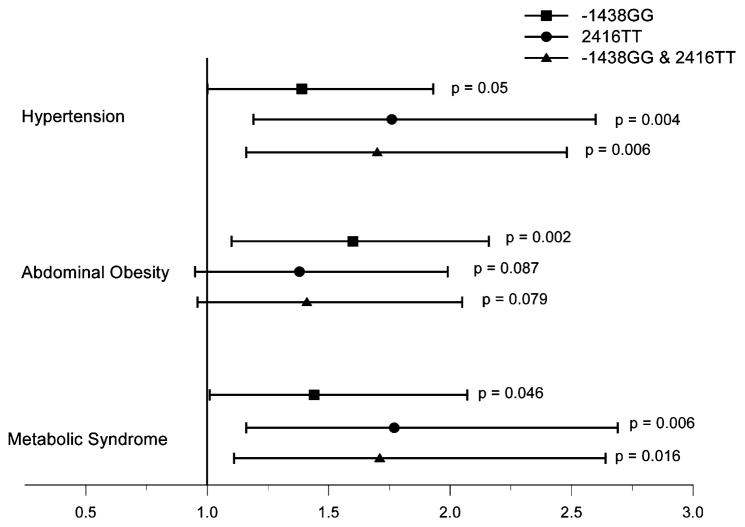
Prevalence of the metabolic syndrome varied significantly by genotypic distribution at both HTR2A loci (Table 2), with individuals homozygous for −1438 GG and 2416 TT genotypes showing higher prevalence of the syndrome (−1438(G/A): GG = 23%, GA = 18%, AA = 13% [χ2 = 7.6, P = .022]; 2416(C/T): CC = 16%, CT = 18%; TT = 26% [χ2 = 7.6, P = .022]). Age, sex, and education adjusted odds of meeting syndrome criteria were greater in −1438 GG homozygotes, relative to subjects carrying any −1438 A-allele (OR = 1.44 [95% CI: 1.01–2.07], P = .046) and in 2416 TT homozygotes compared to those carrying any 2416 C-allele (OR = 1.77 [95% CI: 1.16–2.69], P = .008]. The GG/TT diplo-type conferred increased risk for presence of the metabolic syndrome, compared to the aggregate of all other genotypes (OR = 1.71 [95% CI: 1.11–2.64], P = 0.016) (See also Figure 1).
FIG. 1.
Odds ratios and 95% confidence intervals for presence of hypertension, increased abdominal obesity, and metabolic syndrome in individuals carrying −1438(G/A) GG genotype (square), 2416(C/T) TT genotype (circle), and the GG/TT diplotype (triangle). All phenotypes were adjusted for age, sex, and years of education and, in case of hypertension, also adjusted for BMI.
DISCUSSION
In this study, genetic variation in the serotonin 2A receptor (HTR2A) gene was found to be associated with presence of the metabolic syndrome in a community sample of middle-aged, European American men and women. We further confirmed previous reports of an association between HTR2A variation and two key components of the metabolic syndrome, elevated blood pressure and central adiposity.10,11 Although prior research on BP identified the C-allele of the common HTR2A T102C polymorphism as more prevalent among elderly hypertensives than among age-matched normotensive controls, the T102C locus is in complete LD with the G–1438A polymorphism17 examined in this study (viz. 102C and −1438 G alleles co-occur). Homozygosity for the −1438G allele was associated with blood pressure 130/85 mm Hg or higher and allelic variation at this locus accounted for 4% to 5% of interindividual variability in the distribution of resting SBP and DBP after adjustment for demographic covariates (age, sex, education, and BMI). It is interesting that blood pressure has been associated with HTR2A polymorphisms in subjects of European ancestry,10 but not in individuals of Chinese or Japanese ancestry,11,12 possibly reflecting a population-specific effect of this polymorphism on blood pressure regulation. Positive findings at the promoter locus were corroborated at a second locus, C2416T in intron 1, which is not in perfect LD with the −1438 polymorphism19 (as opposed to the T102C locus) and is a tag SNP (as per analysis of the HAPMAP data on CEPH European Americans) located within our promoter proximal region of interest. Homozygosity for the 2416T allele predicted higher blood pressure levels and criterion elevation for inclusion in the metabolic syndrome, as did the GG/TT diplotype. The association between −1438 G allele and central adiposity (reported previously in a sample of 284 Swedish middle-aged men9) was also confirmed in this study, extending prior findings to a larger study sample of both men and women. Unlike blood pressure, however, neither the 2416 TT genotype nor the GG/TT risk diplotype was associated with central adiposity. Although the remaining components of the metabolic syndrome (ie, blood glucose, serum HDL cholesterol, and triglyceride levels) showed no genetic association, presence of AHA/NHLBI-defined metabolic syndrome was itself predicted by allelic variation at both loci and by the GG/TT diplo-type, with the latter conferring an estimated 71% increased risk of metabolic syndrome, relative to all other genotypes.
Although the mechanistic significance of these associations remains unclear, there is abundant evidence that serotonin and serotonergic neurotransmission play a role in cardiovascular regulation and metabolic function. Serotonin is a vasoactive monoamine possessing amphibaric properties indicated by its ability to elicit either vasoconstriction and arterial pressure elevation or vasodilation and hypotension, depending upon the site of application, concentration, time of observation and local factors.7,8,25,26 Peripherally, serotonin is synthesized by enterochromaffin cells lining the gut and, in the blood stream, is avidly sequestered in platelets. Circulating levels of serotonin remain quite low and although the release of serotonin during platelet activation has local effects on vascular tone, peripheral serotonin does not appear to have a major role in chronic regulation of arterial BP. Within the brain serotonergic responsivity in the hypothalamic-pituitary axis, as assessed by standard fenfluramine challenge, has been found to covary inversely with resting blood pressure in humans1 and is attenuated in spontaneously hypertensive rats, relative to normotensive controls.27 In this regard, the brainstem nuclei involved in BP regulation receive projections from serotonergic neurons, and the central application of serotonin and serotonergic drugs have well-documented acute effects on sympathetic nervous activity, vasopressin release and arterial BP.7,8 The vasoconstrictve and BP-elevating effects of serotonin, both peripherally and centrally, appear to be mediated by 5-HT2A receptors, whereas 5-HT1A receptor activation results in sympathoinhibition and vasodilation.26,28 Therefore, even in the absence of a known relationship between polymorphisms in the HTR2A gene and serotonin levels, their association with BP, as reported here and elsewhere,9 could reflect a role of serotonin in chronic BP regulation, most likely via central mechanisms.
Central serotonergic responsivity has also been shown to covary inversely with body mass, insulin resistance, dyslipidemia, and the full metabolic syndrome.2–4 In obese individuals, augmentation of serotonin release by the agonist, fenfluramine, may decrease autonomic sympathetic activity.29 Serotonin dysregulation is one mechanism that influences eating behaviors in conjunction with altered mood and impulse control.30,31 Genetic associations between HTR2A polymorphism and eating behavior,32,33 food preferences in adults,34 and lower energy and fat intake in children35 have also been reported. Together, these observations suggest that variation in brain serotonergic activity may contribute to the several correlated abnormalities subsumed by the metabolic syndrome and that these associations may be influenced by common genetic variation in serotonin signal transduction, as mediated by the 5-HT2A receptor. Furthermore, to the extent that commonly prescribed psychiatric medications have either favorable36 or unfavorable effects37 on insulin resistance, body weight, or the metabolic syndrome, the HTR2A polymorphisms studied here may moderate such effects.
In conclusion, we have confirmed associations of blood pressure and central adiposity and shown an association of presence of the metabolic syndrome with a well-studied and putatively functional HTR2A promoter polymorphism. Additionally, the present study has identified a HTR2A tag SNP showing strong association with the metabolic syndrome and blood pressure. The associations with the GG/TT diplotype highlight the potential importance of variation in the promoter proximal region of the HTR2A gene in cardiovascular and metabolic regulation. However, further studies are required to ascertain in vivo functionality and to identify other functional loci that are likely to be in LD with either locus examined here, in order to elucidate a mechanistic basis for observed associations with blood pressure, central adiposity, and the metabolic syndrome. Confirmatory studies are now also warranted using larger samples and more definitive genetic methodologies, such as family-based designs or, in populations with genetic stratification, application of genomic control procedures.38
Acknowledgments
This publication was supported by NIH grants HL076852/076858, PO1 HL040962 and RO1 HL065137.
References
- 1.Muldoon MF, Sved AF, Flory JD, Perel JM, Matthews KA, Manuck SB. Inverse relationship between fenfluramine-induced prolactin release and blood pressure in humans. Hypertension. 1998;32:972–975. doi: 10.1161/01.hyp.32.6.972. [DOI] [PubMed] [Google Scholar]
- 2.Muldoon MF, Mackey RH, Williams KV, Korytkowski MT, Flory JD, Manuck SB. Low central nervous system serotonergic responsivity is associated with the metabolic syndrome and physical inactivity. J Clin Endocrinol Metab. 2004;89:266–271. doi: 10.1210/jc.2003-031295. [DOI] [PubMed] [Google Scholar]
- 3.Muldoon MF, Mackey RH, Korytkowski MT, Flory JD, Pollock BG, Manuck SB. The metabolic syndrome is associated with reduced central serotonergic responsivity in healthy community volunteers. J Clin Endocrinol Metab. 2006;91:718–721. doi: 10.1210/jc.2005-1654. [DOI] [PubMed] [Google Scholar]
- 4.Horacek J, Kuzmiakova M, Hoschl C, Andel M, Bahbonh R. The relationship between central serotonergic activity and insulin sensitivity in healthy volunteers. Psychoneuroendocrinology. 1999;24:785–797. doi: 10.1016/s0306-4530(99)00026-8. [DOI] [PubMed] [Google Scholar]
- 5.Sanders-Bush E, Mayer S. 5-Hydroxytryptamine (serotonin) receptor agonists and antagonists. In: Hardman JG, Limbird LE, editors. Goodman, Gilman’s the pharmacological basis of therapeutics. New York: McGraw-Hill; 2001. pp. 269–290. [Google Scholar]
- 6.Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. Euro Psych. 2005;20:S302–S306. doi: 10.1016/s0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- 7.McCall RB. Neurotransmitters involved in central regulation of cardiovascular system. Prog Drug Res. 1996;46:43–113. doi: 10.1007/978-3-0348-8996-4_2. [DOI] [PubMed] [Google Scholar]
- 8.Ramage AG. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull. 2001;56(5):425–439. doi: 10.1016/s0361-9230(01)00612-8. [DOI] [PubMed] [Google Scholar]
- 9.Liolitsa D, Powell JF, Prince M, Lovestone S. Association study of the 5-HT(2A) receptor gene polymorphism, T102C and essential hypertension. J Hum Hypertens. 2001;15:335–339. doi: 10.1038/sj.jhh.1001177. [DOI] [PubMed] [Google Scholar]
- 10.Rosmond R, Bouchard C, Bjorntorp P. 5-HT2A receptor gene promoter polymorphism in relation to abdominal obesity and cortisol. Obes Res. 2002;10:585–589. doi: 10.1038/oby.2002.79. [DOI] [PubMed] [Google Scholar]
- 11.Yu BN, Wang A, Zhou G, Zhang W, Hu DL, Li Q, He YJ, Zhou HH. T102C genetic polymorphism of the 5-HT2A receptor in Chinese hypertensive patients and healthy controls. Clin Exp Pharmacol Physiol. 2004;31:847–849. doi: 10.1111/j.1440-1681.2004.04124.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto M, Jin JJ, Wu Z, Abe M, Tabara Y, Nagai T, Yamasaki E, Igase M, Kohara K, Miki T, Nakura J. Interaction between serotonin 2A receptor and endothelin-1 variants in association with hypertension in Japanese. Hypertens Res. 2006;29:227–232. doi: 10.1291/hypres.29.227. [DOI] [PubMed] [Google Scholar]
- 13.Muldoon MF, Nazzaro P, Sutton-Tyrrell K, Manuck SB. White-coat hypertension and carotid artery atherosclerosis: a matching study. Arch Intern Med. 2000;160:1507–1512. doi: 10.1001/archinte.160.10.1507. [DOI] [PubMed] [Google Scholar]
- 14.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spercus JS, Costa F. Diagnosis and Management of the Metabolic Syndrome. An American Heart Association/National Heart Lung and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 15.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:12–15. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Levine L, Kwok PY. Fluorescence polarization in homogeneous nucleic acid analysis. Genome Res. 1999;9:492–498. [PMC free article] [PubMed] [Google Scholar]
- 17.Spurlock G, Heils A, Holmans P, Williams J, D’Souza UM, Cardno A, Murphy KC, Jones L, Buckland PR, McGuffin P, Lesch KP, Owen MJ. A family based association study of T102C polymorphism in 5HT2A and schizophrenia plus identification of new polymorphisms in the promoter. Mol Psychiatry. 1998;3:42–49. doi: 10.1038/sj.mp.4000342. [DOI] [PubMed] [Google Scholar]
- 18.Parsons MJ, D’Souza UM, Arranz MJ, Kerwin RW, Makoff AJ. The −1438A/G polymorphism in the 5-hydroxytryptamine type 2A receptor gene affects promoter activity. Biol Psychiatry. 2004;56:406–410. doi: 10.1016/j.biopsych.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Veenstra-VanderWeele J, Kim SJ, Lord C, Courchesne R, Akshoomoff N, Leventhal BL, Courchesne E, Cook EH., Jr Transmission disequilibrium studies of the serotonin 5-HT2A receptor gene (HTR2A) in autism. Am J Med Genet. 2002;114:277–283. doi: 10.1002/ajmg.10192. [DOI] [PubMed] [Google Scholar]
- 20.Rousset F, Raymond M. Testing heterozygote excess and deficiency. Genetics. 1995;140:1413–1419. doi: 10.1093/genetics/140.4.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164(4):1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauderman WJ. Sample size requirements for matched case-control studies of gene-environment interaction. Stat Med. 2002;21:35–50. doi: 10.1002/sim.973. [DOI] [PubMed] [Google Scholar]
- 24.QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies. 2006 ( http://hydra.usc.edu/gxe)
- 25.Pilowsky PM, Minson JB, Arnolda LF, Chalmers JP. Brain serotonin and hypertension. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis and Management, Second Edition. New York: Raven Press; 1995. pp. 775–787. [Google Scholar]
- 26.Frishman WH, Grewall P. Serotonin and the heart. Ann Med. 2000;32:195–209. doi: 10.3109/07853890008998827. [DOI] [PubMed] [Google Scholar]
- 27.Stocker SD, Muldoon MF, Sved AF. Blunted fenfluramine-evoked prolactin secretion in hypertensive rats. Hypertension. 2003;42:719–724. doi: 10.1161/01.HYP.0000082807.71659.4C. [DOI] [PubMed] [Google Scholar]
- 28.Watts SW. Serotonin-induced contraction in mesenteric resistance arteries: signaling and changes in de-oxycorticosterone acetate-salt hypertension. Hypertension. 2002;39:825–829. doi: 10.1161/hy0302.104668. [DOI] [PubMed] [Google Scholar]
- 29.Kolanowski J, Younis LT, Vanbutsele R, Detry J-M. Effect of dexfenfluramine treatment on body weight, blood pressure and noradrenergic activity in obese hypertensive patients. Eur J Clin Pharmacol. 1992;42:599–606. doi: 10.1007/BF00265922. [DOI] [PubMed] [Google Scholar]
- 30.Steiger H. Eating disorders and the serotonin connection: state, trait and developmental effects. J Psychiatry Neurosci. 2004;29:20–29. [PMC free article] [PubMed] [Google Scholar]
- 31.Kaye WH, Bailer UF, Frank GK, Wagner A, Henry SE. Brain imaging of serotonin after recovery from anorexia and bulimia nervosa. Physiol Behav. 2005;86:15–17. doi: 10.1016/j.physbeh.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Hinney A, Remschmidt H, Hebebrand J. Candidate gene polymorphisms in eating disorders. Eur J Pharmacol. 2000;410:147–159. doi: 10.1016/s0014-2999(00)00812-8. [DOI] [PubMed] [Google Scholar]
- 33.Bruce KR, Steiger H, Joober R, Ng Y, Kin NM, Israel M, Young SN. Association of the promoter polymorphism −1438G/A of the 5-HT2A receptor gene with behavioral impulsiveness and serotonin function in women with bulimia nervosa. Am J Med Genet B Neuropsychiatr Genet. 2005;137:40–44. doi: 10.1002/ajmg.b.30205. [DOI] [PubMed] [Google Scholar]
- 34.Prado-Lima PS, Cruz IB, Schwanke CH, Netto CA, Licinio J. Human food preferences are associated with a 5-HT(2A) serotonergic receptor polymorphism. Mol Psychiatry. 2006;11:889–891. doi: 10.1038/sj.mp.4001872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herbeth B, Aubry E, Fumeron F, Aubert R, Cailotto F, Siest G, Visvikis-Siest S. Polymorphism of the 5-HT2A receptor gene and food intakes in children and adolescents: the Stanislas Family Study. Am J Clin Nutr. 2005;82:467–470. doi: 10.1093/ajcn.82.2.467. [DOI] [PubMed] [Google Scholar]
- 36.Breum L, Bjerre U, Bak JF, Jacobsen S, Astrup A. Long-term effects of fluoxetine on glycemic control in obese patients with non-insulin-dependent diabetes mellitus or glucose intolerance: influence on muscle glycogen synthase and insulin receptor kinase activity. Metab Clin Exp. 1995;44:1470–1476. doi: 10.1016/0026-0495(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 37.Henderson DC, Cagliero E, Copeland PM, Borba CP, Evins E, Hayden D, Weber MT, Anderson EJ, Allison DB, Daley TB, Schoenfeld D, Goff DC. Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysis. Arch Gen Psychiatry. 2005;62:19–28. doi: 10.1001/archpsyc.62.1.19. [DOI] [PubMed] [Google Scholar]
- 38.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55:997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]