Summary
Retinal pigment epithelial (RPE) cells are among the most active phagocytes in the body. Every morning, circadian shedding of outer segment fragments by photoreceptor cells activates a synchronized phagocytic response by RPE cells that is critical for vision. RPE cells require αvβ5 integrin receptors for particle binding that triggers engulfment. Here, we show that tetraspanins CD81 and CD9 reside in a complex specifically with αvβ5 integrin but not the engulfment receptors Mer tyrosine kinase and CD36 at the apical, phagocytic surface of RPE cells. Function blocking and RNA silencing of CD81 but not of CD9 specifically diminish particle binding. CD81 but not CD9 overexpression is sufficient to increase particle binding and surface levels of αvβ5 integrin. Wild-type and mutant RPE cells defective in particle engulfment equally reduce and increase particle binding in response to CD81 inhibition and CD81 overexpression, respectively. By striking contrast, neither CD81 inhibition nor CD81 overexpression has any effect on particle binding by RPE lacking αvβ5 integrin. These results identify a novel and important role for CD81 in phagocytosis. CD81 does not function as a binding receptor by itself but promotes outer segment particle binding through functional interaction specifically with αvβ5 integrin.
Keywords: Tetraspanin, Integrin, Phagocytosis, Binding, Receptor, Retinal pigment epithelium
Introduction
The retinal pigment epithelium (RPE) forms the outermost layer of the retina and consists of simple, cuboidal epithelial cells with unique plasma membrane polarity (Marmorstein, 2001). In the mammalian retina, each RPE cell underlies ~30 photoreceptor neurons all of which shed the aged, distal tip of their outer segment every morning stimulated by light and circadian rhythms (Young, 1967). RPE cells promptly and efficiently recognize and engulf shed photoreceptor outer segment fragments (POS) by receptor-mediated phagocytosis (Young and Bok, 1969). Thus, an individual post-mitotic RPE cell disposes of several thousand outer segment membrane disks once a day for decades. Synchronized RPE phagocytosis is critical for vision since its deficiency causes blindness in human patients and in animal models (Edwards and Szamier, 1977; Gal et al., 2000; Nandrot et al., 2004; Scott et al., 2001).
The molecular mechanism used by RPE cells to phagocytose POS belongs to a group of non-inflammatory clearance mechanisms used by other cell types to phagocytose apoptotic cells (Finnemann and Rodriguez-Boulan, 1999; Scott et al., 2001). These uptake pathways employ the integrin adhesion receptors αvβ3/αvβ5, Mer tyrosine kinase (MerTK; also known as Mertk or Mer) and the scavenger receptor CD36 (reviewed by Wu et al., 2006).
αvβ5 is the sole apical integrin receptor of the RPE in the mammalian eye and the only surface receptor shown thus far to be essential for POS binding by RPE cells (Finnemann et al., 1997; Nandrot et al., 2004). Furthermore, POS recognition by αvβ5 integrin activates a signaling pathway involving focal adhesion kinase (FAK) and MerTK that is required for internalization of bound POS (Finnemann, 2003). αvβ5 deficiency in β5 knockout (Itgb5−/−; hereafter referred to as β5−/−) mice abolishes early morning stimulation of FAK and MerTK and, therefore, the synchronized burst of RPE phagocytosis in the retina in response to photoreceptor shedding (Nandrot et al., 2004). Slow clearance of shed POS by β5−/− RPE suffices to prevent retinal accumulation of unengulfed POS in young mice. Nonetheless, lack of αvβ5 receptors causes accumulation of undigested POS components in the RPE cytoplasm and blindness in 1-year-old mice (Nandrot et al., 2004).
Tetraspanins are a large family of widely expressed four-transmembrane-domain proteins. They function to regulate the activity of surface receptors including integrins through assembly of cell-type-specific multi-protein complexes in specialized membrane microdomains (for recent reviews please see (Berditchevski, 2001; Hemler, 2005; Levy and Shoham, 2005; Yunta and Lazo, 2003). CD81 is the only tetraspanin to date shown to be highly expressed by RPE cells (Geisert et al., 2002). In 2-month-old CD81 knockout mice there is a small increase in RPE cell density suggesting that CD81 may play a role in regulating RPE cell proliferation during development (Song et al., 2004). Slightly shortened photoreceptor inner and outer segments in CD81 knockout mice could result from an imbalance in photoreceptor outer segment renewal in otherwise normal neural retina (Song et al., 2004). In mature retina, CD81 localizes to both apical and basolateral plasma membrane domains of post-mitotic RPE cells where it associates with PDZ domain proteins EBP50 and Sap97, respectively (Pan et al., 2007). Since other epithelial cells restrict CD81 to the basolateral surface (Yanez-Mo et al., 2001), we speculated that apical CD81 may be involved in a cell-type specific function that is unique to RPE cells. We have investigated whether CD81 plays a role in POS clearance. Our results demonstrate that functional interaction of CD81 specifically with apical αvβ5 integrin receptors regulates POS binding by RPE cells. These data identify a novel and essential role for CD81 in phagocytosis.
Results
Like αvβ5 integrin, tetraspanins CD81 and CD9 are expressed at the apical, phagocytic surface of RPE-J cells
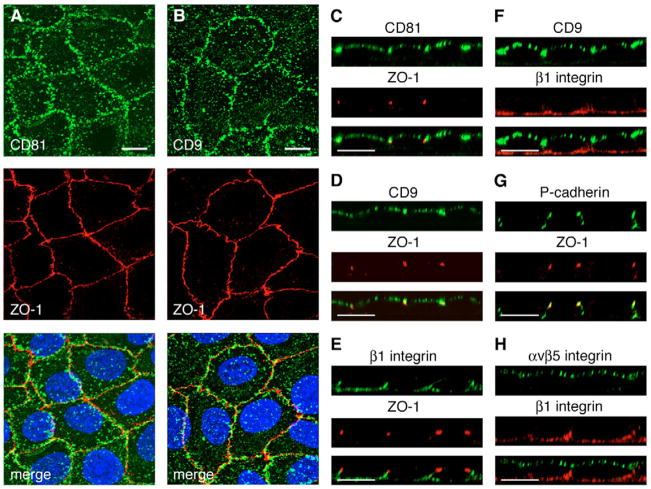
Immortalized RPE-J cells derived from rat RPE distribute their phagocytic machinery to the apical surface like RPE cells in the retina, and use αvβ5 integrin to bind POS in experimental phagocytosis assays (Finnemann, 2003; Finnemann et al., 1997). We hypothesized that tetraspanins with a function in POS uptake will also localize to the exposed, apical surface of these cells. We therefore labeled live RPE-J cells on ice with antibodies against extracellular epitopes of tetraspanins CD81 or CD9. Following fixation and permeabilization we incubated samples with ZO-1 antibodies to label tight junctions. Confocal microscopy showed prominent expression of both CD81 and CD9 in punctate structures in projections of x-y stacks (Fig. 1A,B). Z-axis scans revealed that both CD81 and CD9 were present at the apical side of the ZO-1-labeled tight junction protein (Fig. 1C,D). By contrast, β1 integrin expression, determined by antibody labeling of fixed cells was restricted to the basolateral plasma membrane below the ZO-1 marker and did not overlap with CD9 (Fig. 1E,F). Another basolateral marker protein, P-cadherin, also localized exclusively below the permeability barrier labeled by ZO-1 (Fig. 1G). As expected, antibody labeling of live cells detected the POS binding receptor αvβ5 integrin at the apical surface, unlike β1 integrin in the same cells (Fig. 1H). These experiments demonstrate that CD81 and CD9 are expressed at the apical, phagocytic surface of polarized RPE-J cells in culture. As we performed labeling on live cells with CD81 and CD9 antibodies, we do not rule out that either or both tetraspanins may additionally localize to the basolateral surface of RPE-J cells.
Fig. 1.
CD81 and CD9 are abundantly expressed at the apical, phagocytic surface of RPE cells. (A,B) Polarized live RPE-J cells on ice were labeled with CD81 or CD9 antibodies as indicated and shown in green, subsequently fixed and labeled with ZO-1 tight junction protein antibody (red). Nuclei are shown in blue in the merged fields. Maximal projections of representative whole cell x-y scans acquired at 0.2 μm intervals are shown. (C–H) Representative confocal x-z scans of polarized RPE-J cells co-labeled with antibodies to CD81, CD9, ZO-1, β1 integrin, P-cadherin or αvβ5 receptors as indicated. CD81, CD9 and αvβ5 were labeled in live cells on ice as in (A,B). ZO-1, β1 integrin and P-cadherin were labeled after fixation and permeabilization. Single and merged channels of the same field are shown with the labeled proteins indicated above each single channel picture. Bars, 10 μm.
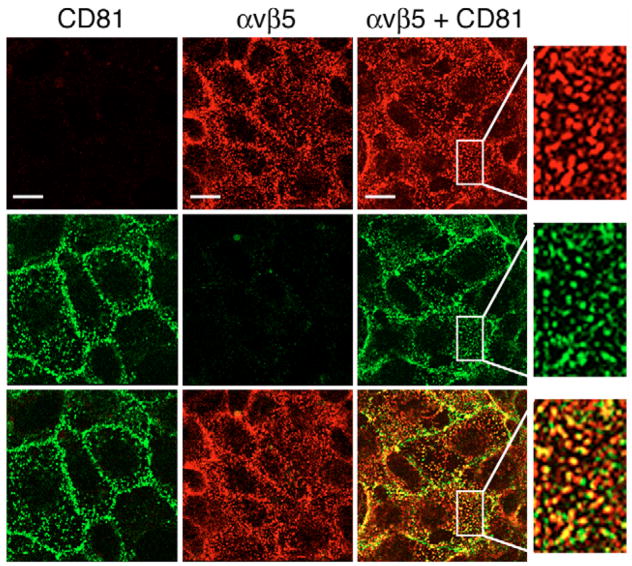
To directly compare the localization of αvβ5 and CD81, we next recorded single x-y plane scans of the apical surface of RPE-J cells following double labeling of live chilled cells with antibodies to CD81 and to αvβ5 receptors. These experiments revealed that CD81 partially co-localized with αvβ5 in puncta at the apical, phagocytic surface of RPE-J cells (Fig. 2). Similar double labeling experiments for CD9 and αvβ5 were not possible because all antibodies available to us for both antigens are raised in mouse.
Fig. 2.
CD81 partially colocalizes with αvβ5 integrin receptors at the apical surface of RPE cells. Live RPE-J cells were labeled with either CD81 antibody Eat2 (left column) or αvβ5 receptor antibody P1F6 (middle column), or both antibodies (right column). All samples received both secondary antibodies and were examined at both corresponding wavelengths to ensure labeling specificity of secondary antibodies. CD81 signals are shown in green in the middle row, αvβ5 signals are shown in red in the top row. The bottom row shows the merged signals of upper and middle rows. Panels show representative single x-y plane confocal microscopy scans of the RPE-J apical surface. Areas with focus on the apical surface of cells showed considerable colocalization of CD81 and αvβ5, appearing in yellow in the merged images. Selected areas are enlarged and shown in the fourth column to better illustrate co-localization. Bars, 10 μm.
CD81 and CD9 reside in a complex specifically with αvβ5 integrin but not with MerTK or CD36 in RPE cells
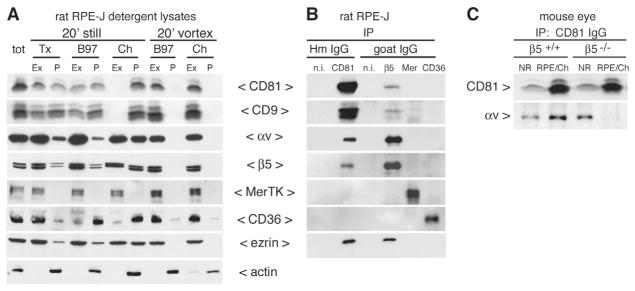
Next, we sought to further address whether CD81 and CD9 co-partitioned with the phagocytic machinery of the RPE, using biochemical methods. First, we compared extractability of relevant proteins by overlaying resting RPE-J cells with lysis buffers that contained 1% of either Triton X-100, Brij97, or CHAPS detergent on ice for 20 minutes (Winterwood et al., 2006). Tetraspanins and associated proteins are typically extracted from cellular membranes by buffers containing Triton X-100 or by Brij97 whereas their association with membrane lipids are largely maintained in CHAPS (Hemler, 2003). Indeed, we found that CD81 and CD9 in RPE-J cells were partially extracted by Triton X-100 and by Brij97 but not by CHAPS (Fig. 3A, panels 20′ still). When we tested partitioning of RPE phagocytic receptors in the same samples we found that αv and β5 integrin subunits were also more soluble in Triton X-100 and Brij97 than in CHAPS (Fig. 3A, panels 20′ still). By contrast, the engulfment receptor MerTK was fully soluble regardless of detergent (Fig. 3A, panels 20′ still). CD36 was largely soluble in Triton X-100 but mostly insoluble in Brij97 and CHAPS (Fig. 3A, panels 20′ still). Quantification of solubility of all proteins tested is provided in Table S1 in supplementary material. Notably, when we incubated the cells in the same detergent buffers for 20 minutes with vortexing, all proteins of interest were largely solubilized by 1% Brij97 or CHAPS (Fig. 3A, panels 20′ vortex). Hence, vortexed Brij97 and CHAPS extracts contain the entire cellular CD81, CD9, and phagocytic receptor proteins of RPE-J cells.
Fig. 3.
CD81 resides in a complex with αvβ5 but not with MerTK or CD36 in RPE cells. (A) Polarized RPE-J cells were separated into extracted (Ex) and insoluble pellet (P) fractions by differential extraction with buffer containing 1% Triton X-100 (Tx), Brij97 (B97), or CHAPS (Ch) either still on ice or with vortexing as indicated. Equal volumes of fractions were analyzed by western blotting with antibodies as indicated to the right of the panels. (B) IPs from vortexed RPE-J Brij97 lysates (as in A) with antibodies to CD81 or phagocytic receptors β5 integrin, MerTK and CD36, and non-immune hamster and goat antibodies were probed sequentially in the order of panels shown (top to bottom) for precipitated receptors and for CD9 as indicated to the left of each panel. CD81 and β5 antibodies co-isolated CD81, CD9, αv, and β5. Because after sequential re-probing of blots ezrin antibody showed only very weak ezrin signals, receptor IPs were repeated and fresh blots probed with ezrin yielding robust bands representing co-precipitated ezrin specifically with CD81 and β5 integrin (panel ezrin). (C) Tissue samples of neural retina (NR) and corresponding RPE-choroid (RPE/Ch) isolated from individual β5+/+ and β5−/− mouse eyes were lysed in buffer containing Brij97. IPs from these tissue lysates with CD81 antibody co-precipitated αv integrin from β5+/+ neural retina and RPE/choroid and from β5−/− neural retina but not from β5−/− RPE/choroid (RPE/Ch) (αv panel). CD81 immunoblotting revealed that CD81 antibody precipitated similar amounts of CD81 from β5+/+ and β5−/− tissues (CD81 panel). Non-immune antibodies did not isolate any of the proteins of interest (data not shown).
Second, we used cleared Brij97 extracts of RPE-J cells obtained by vortexing, as analyzed above, to test association of tetraspanins with phagocytic receptors in immune complexes. Here, CD81 IgG immunoprecipitations (IPs) specifically co-isolated CD9, αv and β5 integrins, but not MerTK and CD36 (Fig. 3B). Reciprocal experiments immuno-isolating phagocytic receptors confirmed this result: we detected CD81 and CD9 specifically in IPs of β5 integrin, but not in IPs with non-immune, MerTK or CD36 IgG. In agreement with earlier results (Pan et al., 2007; Sala-Valdes et al., 2006), the apical microvillus protein ezrin associated with CD81. Furthermore, we detected ezrin in β5 but not in MerTK or CD36 IPs (Fig. 3B, ezrin). Thus, ezrin resides in a complex with CD81 and with αvβ5 in RPE-J cells. Finally, we analyzed CD81 protein complexes in neural retina (lanes NR) and in eyecup tissue mainly containing RPE and choroid (lanes RPE/Ch) (Fig. 3C). We immunoprecipitated similar amounts of CD81 from wild-type (β5+/+) and β5−/− tissue extracts suggesting that CD81 expression level is normal in β5−/−retina. We consistently immunoprecipitated more CD81 from RPE/choroid than from neural retina regardless of whether tissues were harvested from β5+/+ or β5−/− mice. Probing IPs with β5 integrin antibodies failed to detect β5 integrin in CD81 IPs from either β5+/+ tissue, probably because of the relatively low sensitivity of available β5 integrin antibodies for mouse β5. Neural retina of both β5+/+ and β5−/− mice showed similar levels of αv integrin in CD81 complexes (Fig. 3C, NR) indicating that αv integrin association with CD81 can occur independently of β5 integrin. By contrast, CD81 IPs contained αv integrin in RPE/choroid of β5+/+ but not of β5−/− mice despite similar amounts of CD81 being immunoprecipitated (Fig. 3C, RPE/Ch). Thus, αv integrin association with CD81 complexes in RPE/choroid depends on expression of the β5 integrin subunit. This result implies that CD81 in RPE/choroid tissue in vivo resides in complexes with αvβ5 rather than other αv-integrin-containing receptors. Taken together, the biochemical analysis supports the results obtained by confocal microscopy indicating that CD81 resides in protein complexes with αvβ5 integrin receptors in RPE cells.
Phagocytic binding of POS by RPE cells requires CD81 but not CD9
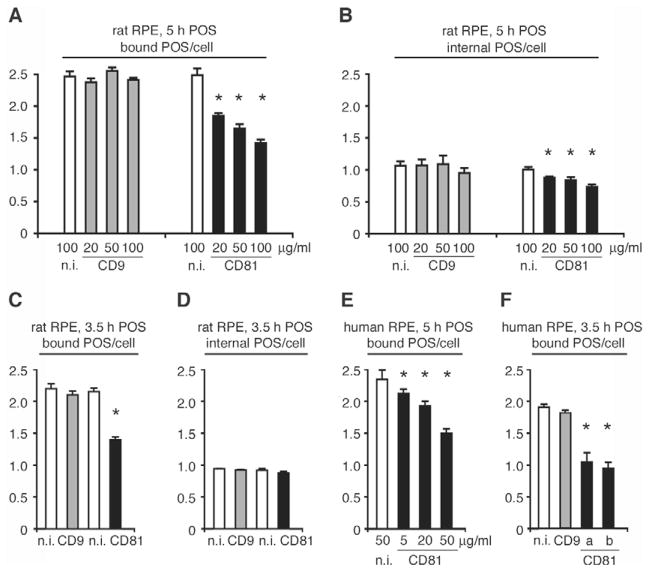
To determine whether CD81 and/or CD9 may play a role in RPE phagocytosis, we first tested the effects of antibodies to their extracellular domains on POS binding and internalization by RPE-J cells. CD81 monoclonal antibody (mAb) 2F7 is known to block CD81-dependent T cell development in live cell assays (Boismenu et al., 1996). CD9 mAb RPM.7 recognizes the large extracellular loop of rat CD9 and is the same antibody we used earlier for surface binding of CD9 in live cells (Fig. 1) (Azorsa et al., 1999). We challenged RPE-J cells with FITC-conjugated POS for 5 hours in the continuous presence of mAb 2F7, mAb RPM.7, or appropriate non-immune antibodies and quantified bound and internalized POS. We used an established procedure for FITC-POS quantification that is based on fluorescence scanning of fixed RPE-J cell samples on coverslips mounted on glass slides (for details please see Materials and Methods). This assay allows quantification of either total (bound plus internal) FITC-POS or of internal FITC-POS alone. Bound POS are calculated by subtracting internal from total POS counts. CD9 mAb RPM.7 at 20–100 μg/ml had no effect on POS binding or internalization by RPE-J cells (Fig. 4A,B, gray bars). By contrast, CD81 mAb 2F7 reduced POS binding in a dose-dependent manner from 25% at 20 μg/ml to 43% at 100 μg/ml (Fig. 4A, black bars, see figure legend for statistical analysis). CD81 mAb 2F7 also decreased POS internalization by RPE-J cells by up to 27% (Fig. 4B, black bars). In order to test whether POS internalization decreased as a consequence of decreased POS binding rather than as a direct effect of CD81 antibody on POS engulfment, we tested the effects of CD81 and CD9 inhibition at an earlier time point. Indeed, 50 μg/ml CD81 mAb equally decreased POS binding by RPE-J cells at 3.5 hours of POS challenge (by 35%) but had no effect on POS internalization (Fig. 4C,D). Next, we performed similar POS uptake assays using the human RPE-derived cell line D407, which retains phagocytic activity towards POS (Finnemann et al., 2002) and expresses apical surface CD81 (data not shown). To selectively target CD81 in D407 cells, we first used the function-blocking human CD81 mAb clone I.3.3.22 (Bertaux and Dragic, 2006). Consistent with our findings using RPE-J cells, co-incubation with mAb I.3.3.22 at 5 to 50 μg/ml decreased POS binding by up to 37% compared to non-immune IgG at 5 hours of phagocytic challenge (Fig. 4E, black bars). To further test the specificity of this effect, we compared the effects of CD81 mAb I.3.3.22 with mAbs MM2/57 and JS-81, known to block the functions of human CD9 and human CD81, respectively, at 3.5 hours of POS challenge (Cormier et al., 2004; Takeda et al., 2003). Fig. 4F shows that at the same concentration of 50 μg/ml, both CD81 mAb I.3.3.22 and JS-81 decreased POS binding by human D407 RPE cells by 46% and 51% (black bars), respectively, whereas CD9 mAb had no effect (gray bar). None of the antibodies significantly altered POS internalization by D407 RPE cells regardless of duration of the assay (data not shown). D407 RPE cells may compensate for decreased numbers of bound POS by increasing their internalization rate. Taken together, the antibody inhibition experiments suggest a role specifically for CD81 but not CD9 in the initial step of RPE phagocytosis, POS binding.
Fig. 4.
Immuno-blocking CD81 mainly decreases surface-binding of POS by RPE cells. (A–D) Rat RPE-J cells were challenged with POS for 3.5 or 5 hours as indicated, in the presence of rat CD9 mAb RPM.7 (gray bars), rat and mouse CD81 mAb 2F7 (black bars), or non-immune IgG (white bars). (E) Human D407 RPE cells were challenged with POS for 5 hours in the presence of human CD81 mAb clone I.3.3.22 (black bars). (F) D407 cells were challenged with POS for 3.5 hours in the presence of human CD9 mAb MM2/57 (gray bar), human CD81 mAb clone I.3.3.22 or JS-81 (black bars a, b, respectively) or non-immune IgG (white bar). Antibody concentrations are indicated in μg/ml below each bar. Pre-incubation with antibody before addition of POS had no additional effect on POS binding (data not shown). Results are presented as mean ± s.d., n=3, of bound (A,C,E,F) and internalized (B,D) POS per RPE cell. In A and B Student’s t-test was used to compare samples receiving tetraspanin antibody with samples receiving appropriate non-immune control antibody at the same concentration. Asterisks denote significant differences with P<0.05. (A,B) In 5-hour assays, CD81 antibody significantly inhibited both POS binding (P<0.01) and internalization (P<0.05) by RPE-J cells. CD9 antibody had no significant effect (P>0.05). (C,D) In 3.5-hour assays, CD81 antibody significantly inhibited only POS binding by RPE-J cells (P<0.01). CD9 antibody had no effect (P>0.05) and internalization was unaffected by either antibody (P>0.05). (E,F) Two different CD81 antibodies significantly inhibited POS binding by D407 cells (P<0.02). All other conditions did not cause significant changes (P>0.05). RPE-J cells without antibody bound 2.5 POS and internalized 1.1 POS per cell on average during a 5 hour assay. D407 cells without antibody bound 2.4 POS and internalized 1.4 POS per cell during a 5-hour assay. Hence, non-immune IgG did not significantly alter control uptake.
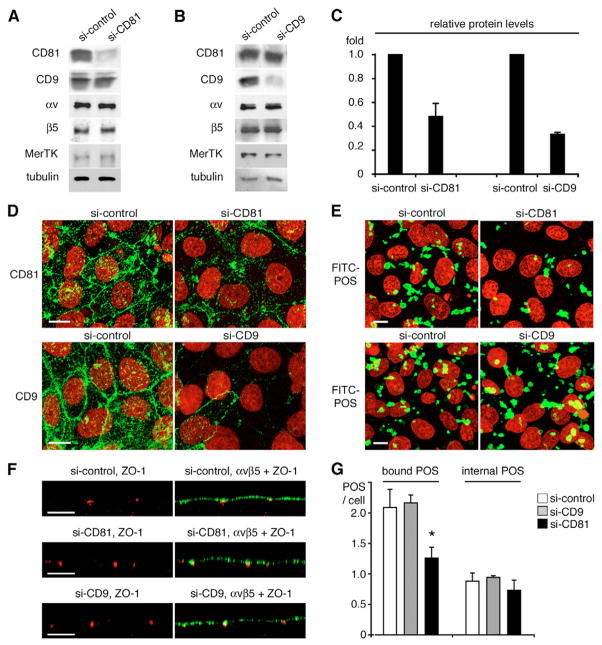
To more directly determine the relevance of CD81 in this process, we next compared the effects of depleting either CD81 or CD9 from RPE-J cells on POS phagocytosis. Transient transfections with siRNA complementary to the coding regions of either rat CD81 (si-CD81) or rat CD9 (si-CD9) reduced protein levels of CD81 by 52% and of CD9 by 67% compared with transfection with non-targeting siRNA (si-control; Fig. 5A-C). Both siRNAs specifically downregulated the targeted tetraspanin without altering levels of other relevant proteins (Fig. 5A,B). To determine whether tetraspanin si-RNA transfection affected protein localization at the apical surface of transfected cells, we labeled paraformaldehyde-fixed, unpermeabilized RPE-J cells with antibodies to CD81 and CD9. Si-CD81 and si-CD9-transfected cells revealed weakened labeling for the respective targeted tetraspanin (Fig. 5D). Thus, transfections with both si-RNAs succeeded in depleting the targeted tetraspanin from the phagocytic surface of RPE cells. Fluorescence microscopy of CD81− and CD9-depleted RPE cells after phagocytic challenge with FITC-POS revealed fewer total (bound and internal) FITC-POS in si-CD81-treated compared to si-control- or si-CD9-treated RPE-J cells following 3.5 hours of POS challenge (Fig. 5E). This was not due to gross changes in cell polarity as depletion of either tetraspanin did not alter subcellular distribution of ZO-1 or of αvβ5 integrin (Fig. 5F). Since CD81 may play a role in regulating RPE proliferation (Clarke and Geisert, 1998; Geisert et al., 2002), we also counted cell numbers following si-control and si-CD81 transfection. We found that CD81 depletion did not change density of RPE cells in culture within 48 hours post transfection (si-control samples: 59.67±2.08 cells per 14,000 μm2, si-CD81 samples: 59.33±4.04 cells per 14,000 μm2; mean ± s.d., n=3). Finally, fluorescence scanning quantification of POS phagocytosis assays showed that CD81 depletion reduced POS binding compared to si-control treatment on average by 45% which roughly correlated with the reduction of CD81 expression in si-CD81 treated cells (Fig. 5G, bound POS/cell, black bar, see figure legend for statistical analysis). By contrast, CD9 depletion had no effect on POS binding (Fig. 5G, bound POS/cell, gray bar). Neither si-RNA treatment altered POS internalization (Fig. 5G, internal POS/cell). Thus, similar to inhibiting CD81 with blocking antibodies, reducing CD81 protein expression in RPE cells specifically inhibits the recognition/binding step of POS phagocytosis.
Fig. 5.
CD81 depletion diminishes POS binding. RPE-J cells were transfected with either control siRNA (si-control), CD9-specific or CD81-specific siRNA (si-CD9 and si-CD81 respectively). 48 hours after transfection, protein expression (A–D) or phagocytic activity (E,F) of transfectants were evaluated. (A,B) Whole cell lysate representing equal numbers of cells were analyzed by western blotting with antibodies as indicated. (C) Quantification of western blots shows that CD81 and CD9 protein levels were reduced to 0.48±0.11-fold and 0.33±0.02-fold, respectively, by si-CD81 or si-CD9 transfection compared to si-control (mean ± s.d., n=3). (D) Transfectants, as indicated, were fixed with 4% paraformaldehyde before labeling with CD81 or CD9 antibodies (green). X-y maximal projections of representative images were acquired as described for Fig. 1. (E) Transfected cells were challenged with FITC-POS for 3.5 hours. X-y maximal projections of representative images show total FITC-labeled POS (green) taken up by the cells. Cell nuclei are shown in red in D and E. (F) Live transfectants were labeled on ice with αvβ5 antibody P1F6 and fixed before labeling with ZO-1 antibodies. X-z scans of representative areas show ZO-1 only or overlay of αvβ5 and ZO-1 as indicated. Bars, 10 μm. (G) POS phagocytosis assays were analyzed by fluorescence scanning to quantify bound and internalized POS. Bars represent mean ± s.d., n=3, bound and internalized POS per RPE cell as indicated (white bars: si-control; gray bars: si-CD9; black bars: si-CD81). CD81 siRNA significantly reduced the numbers of bound POS compared to si-control (asterisk, Student’s t-test, P<0.02). CD9 siRNA had no significant effect (P>0.05).
CD81 overexpression is sufficient to increase POS binding by RPE cells
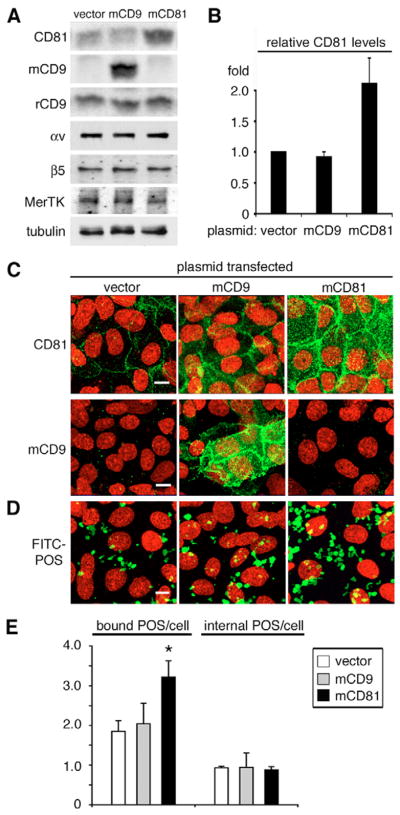
To further determine whether CD81 protein expression level directly correlated with POS phagocytic binding we tested the effect of increased apical, phagocytic surface CD81 on RPE phagocytosis. Transient transfection of RPE-J cells with plasmid encoding mouse CD81 specifically increased CD81 protein levels by about twofold (Fig. 6A,B). Transient transfection with plasmid encoding mouse CD9 resulted in de novo expression of mouse CD9 detected with mouse CD9 mAb (Fig. 6A). Immunoblotting with rat CD9 mAb RPM.7 showed that endogenous rat CD9 did not change upon CD9 or CD81 plasmid transfection. Overexpression of either tetraspanin did not affect protein levels of phagocytic receptors αv and β5 integrin or MerTK (Fig. 6A). Similar to CD81 depletion, transient CD81 overexpression did not change RPE-J cell density (vector transfectants: 61.33±4.16 cells per 14,000 μm2, mouse CD81 transfectants: 59.33±3.06 cells per 14,000 μm2; mean ± s.d., n=3). Immunofluorescent staining of RPE-J cells that were paraformaldehyde-fixed but not permeabilized demonstrated that transfection increased levels of CD81 and CD9 at the apical, phagocytic surface compared to cells transfected with empty vector (Fig. 6C). Fluorescence microscopy also illustrated that CD81 overexpression visibly enhanced FITC-POS total uptake compared to CD9 overexpression or control transfection (Fig. 6D). Quantification of POS uptake confirmed that CD81 overexpression increased POS binding by 74% on average compared to vector control, whereas CD9 overexpression had no effect (Fig. 6E, see figure legend for statistical analysis). Neither CD81 nor CD9 overexpression changed the amount of internalized POS (Fig. 6E). Hence, increasing CD81 levels was sufficient to promote POS recognition by RPE cells. Taken together, our three independent experimental approaches, function blocking, protein downregulation and protein overexpression, identify a novel function specific to the tetraspanin CD81 in POS recognition/binding by RPE cells.
Fig. 6.
CD81 overexpression increases POS binding by RPE cells. RPE-J cells were transfected with plasmids encoding mouse CD81 (mCD81), CD9 (mCD9), or empty vector as indicated. 48 hours after transfection, tetraspanin expression (A–D) and phagocytic activity of transfectants (E) were evaluated. (A) RPE proteins, as indicated, were analyzed by immunoblotting of transfectant lysates from equal numbers of cells. CD81 antibody Eat2 recognized both endogenous rat and transfected mouse CD81 protein. Mouse CD9 antibody KMC (mCD9) and rat CD9 antibody RPM.7 (rCD9) recognized exogenous mouse and endogenous rat CD9, respectively. (B) Quantification of western blots shows that CD81 transfection increased CD81 protein level to 2.1±0.4 fold of empty vector transfected cells (mean ± s.d., n=3). mCD9 plasmid transfection did not change CD81 expression. (C) Transfected CD81 and CD9 localized to the apical surface of transfected RPE-J cells. Cells were fixed with 4% paraformaldehyde before labeling CD81 and mouse CD9 (mCD9) as indicated (green). Panels show x-y maximal projections acquired as described for Fig. 1 of representative fields. (D) Transfected cells were challenged with FITC-POS for 3.5 hours. Maximal projections of representative fields show total (bound plus internal) FITC-POS (green) taken up by the cells. Cell nuclei were stained with DAPI (red) in C and D. Bars, 10 μm. (E) 3.5-hour POS phagocytosis assays were analyzed by fluorescence scanning to quantify bound and internal POS. Bars represent mean ± s.d., n=3, bound POS and internalized POS per RPE cell transfected with empty vector (white bars), CD9 (gray bars), or CD81 (black bars) expression plasmids. CD81 overexpression significantly increased the numbers of bound POS but not of internal POS as compared to vector control (asterisk, Student’s t-test, P<0.03). CD9 transfection had no effect (P>0.05).
CD81 overexpression increases surface levels of αvβ5 integrin receptors
To determine the mechanism through which CD81 may contribute to POS binding, we assessed whether CD81 or CD9 overexpression altered properties of the other known phagocytic receptors of RPE cells. In detergent solubilization experiments performed like those described in Fig. 3A, we found that αv and β5 integrins, MerTK, and CD36 were equally susceptible to differential detergent extraction from RPE-J cells transfected with empty vector, CD81 or CD9 cDNA plasmids (data not shown). Next, we assessed levels of phagocytic receptors at the apical surface of RPE-J cells after transient transfection by immunoprecipitating exclusively surface-localized receptors. In parallel, we also immunoprecipitated total cellular receptors. We found that surface expression of CD81 increased, on average, by 58% with CD81 transfection but was not changed by CD9 transfection (Fig. 7, please see figure legend for statistical analysis). Surface levels of combined endogenous and transfected CD9 could not be directly tested because antibodies recognizing both mouse and rat CD9 are not available. Yet, surface levels of endogenous rat CD9 were constant and transfected mouse CD9 was isolated by surface IP (Fig. 7, panels rCD9 IP, mCD9 IP). IPs with P1F6 antibody recognizing αvβ5 heterodimers revealed that levels of αvβ5 receptors at the exposed, apical surface increased by approximately one third in cells overexpressing CD81 compared with control transfectants but not in cells overexpressing CD9 (Fig. 7, panels IP αvβ5, for quantification and statistics, see figure legend). This increase was significant, reproducible, and specific to αvβ5, as surface levels of MerTK and CD36 were unaffected by tetraspanin transfections (Fig. 7, IP MerTK and CD36 panels). Control experiments with antibody recognizing an intracellular epitope of β5 integrin did not isolate αv or β5 integrin by labeling of live cells indicating that our experimental protocol specifically detected surface-exposed proteins (Fig. 7, IP β5-cyt panels). In additional control experiments were performed to determine live surface and total lysate IPs, with antibodies to the extracellular domain of β1 integrin. We detected similar levels of β1 integrin in total lysate IPs of all three transfectants but not in the live surface IP (Fig. 7, panels IP β1). Thus, live cell antibody labeling was only successful for proteins with epitopes exposed at the apical cell surface. These results also suggested that RPE-J cells retained plasma membrane polarity and that permeability barriers shielded the basolateral marker protein β1 integrin from antibody binding, irrespective of tetraspanin transfection. Taken together, these experiments demonstrate that CD81 overexpression moderately but specifically increases levels of αvβ5 integrin receptors at the apical surface of RPE-J cells.
Fig. 7.
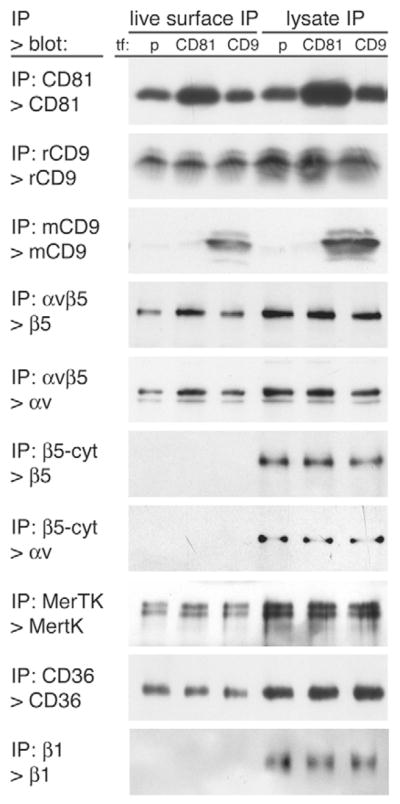
CD81 but not CD9 overexpression specifically increases surface levels of αvβ5 integrin receptors. Cells transfected with empty plasmid (p), CD81 or CD9 expression plasmids as indicated, (tf:) were either incubated live on ice with antibodies to receptor proteins for live surface IPs (left 3 lanes) or lysed and subjected to total lysate IP (right 3 lanes). Live surface and total lysate IPs were analyzed on the same gel and immunoblotted. Antibodies used for IP and immunoblotting detection are indicated to the left of each panel (IP>blot:). The experiment was performed four times independently. Band intensities of tetraspanin-overexpressing cells were compared with band intensities obtained from empty plasmid controls for each experiment and results averaged. CD81 transfection significantly (P<0.05) increased surface CD81 by 58±13%, total CD81 by 83±11%, surface αv by 36±5%, and surface β5 by 31±9% (mean ± s.d., all n=4). All other bands did not differ significantly from control IPs (average change <10%, P>0.05). Non-specific antibodies did not isolate any of the proteins tested in surface or lysate IPs (data not shown).
CD81 requires αvβ5 integrin to promote POS binding
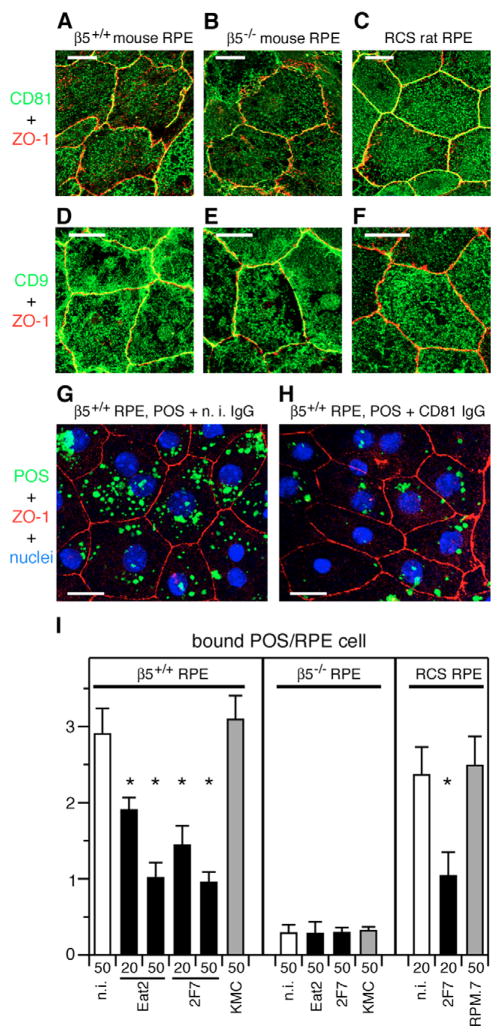
To further explore potential functional links between CD81 and αvβ5 integrin, we compared the role of CD81 in POS binding by primary RPE cells derived from β5+/+ and β5−/− mice and from RCS rats that lack MerTK. Lack of αvβ5 receptors reduces POS binding by β5−/− RPE cells in culture by ~80% (Nandrot et al., 2004). The molecular pathway that promotes the small but significant POS binding by β5−/− RPE has yet to be identified. RCS rat RPE cells use αvβ5 integrin to bind POS but do not internalize surface bound POS (Chaitin and Hall, 1983; Finnemann, 2003). Like RPE-J cells, both polarized β5+/+ and β5−/− mouse RPE as well as RCS rat RPE showed prominent apical surface expression of both CD81 and CD9 (Fig. 8A–F). The CD81 antibodies 2F7 and Eat2 each reduced POS binding by β5+/+ RPE cells by up to 64% in a dose-dependent manner (Fig. 8G–I, β5+/+, black bars). Furthermore, CD81 mAb 2F7 reduced POS binding by RCS RPE by 65% (Fig. 8I, RCS, black bar). By contrast, although β5−/− RPE expressed a similar level of apical CD81 as β5+/+ RPE, neither CD81 mAb further reduced their low, residual capacity to bind POS (Fig. 8I, β5−/−, black bars). Consistent with our results from RPE-J cells (Fig. 4), antibodies that specifically recognize mouse and rat CD9 did not affect POS binding by any of the primary cell samples (Fig. 8I, gray bars). These results show that POS binding by unpassaged β5+/+ mouse RPE and RCS rat RPE in primary culture is specifically sensitive to CD81 blockade. Furthermore, they suggest that CD81 function is probably irrelevant for the αvβ5-independent POS binding activity of β5−/− RPE.
Fig. 8.
CD81 antibody inhibits POS binding by β5+/+ mouse RPE and RCS (MerTK-deficient) rat RPE but not by β5−/− mouse RPE, whereas CD9 antibody has no effect. (A–F) Live, polarized, primary RPE from β5+/+ mice, β5−/− mice, and RCS rats were labeled with CD81 (A–C) or CD9 (D–F) antibody, fixed, and labeled with ZO-1 antibody to visualize tight junctions. A–F show x-y maximal projections acquired by confocal microscopy as described for Fig. 1. CD81 and CD9 apical surface labeling did not differ between samples prepared from animals of different genotype. (G,H) Total FITC-POS uptake by β5+/+ primary RPE after 1 hour of POS challenge was visibly reduced by CD81 antibody but not by non-immune (n.i.) antibody at 50 μg/ml. Representative fields show maximal x-y projections of FITC-POS, nuclei, and ZO-1 tight junctions. Bars, 10 μm. (I) Primary RPE cells were challenged with FITC-POS for 1 hour in the presence of non-immune (n.i.) IgG (white bars), CD81 (black bars) or CD9 (gray bars) mAbs at 20 or 50 μg/ml as indicated below each bar. Antibodies used were CD81 mAb clone Eat2 and 2F7, anti-mouse CD9 mAb KMC, anti-rat CD9 mAb RPM.7. Fluorescence scanning quantification of bound FITC-POS shows that CD81 antibodies inhibited POS binding by β5+/+ RPE and RCS rat RPE, but had no effect on the residual POS binding by β5−/−RPE (black bars). By contrast, CD9 antibodies did not change POS binding by any of the three types of RPE (gray bars). Bars represent mean number of POS bound per RPE cell ± s.d., n=3. Asterisk denotes significant inhibition of POS binding compared to appropriate non-immune antibody control at the same dose (P<0.01, Student’s t-test). None of the conditions affected POS internalization (data not shown).
Finally, we overexpressed CD81 in β5+/+, β5−/− and RCS primary RPE to test dependence of CD81 function in POS uptake on αvβ5 and MerTK. Transient transfection specifically increased CD81 protein level in primary RPE 1.4 to 1.6 fold regardless of genotype (Fig. 9A,B). Such increase was sufficient to increase POS binding by β5+/+ RPE and by RCS RPE by 25% and 29%, respectively (Fig. 9C). Strikingly, CD81 overexpression had no effect on POS binding by β5−/− RPE (Fig. 9C). Thus, CD81 fails to increase POS binding in the absence of αvβ5 integrin. These data further confirm that CD81 is an important element of the RPE phagocytic mechanism. Moreover, they demonstrate that CD81 function in POS uptake requires the recognition receptor αvβ5 integrin but not the internalization receptor MerTK.
Fig. 9.
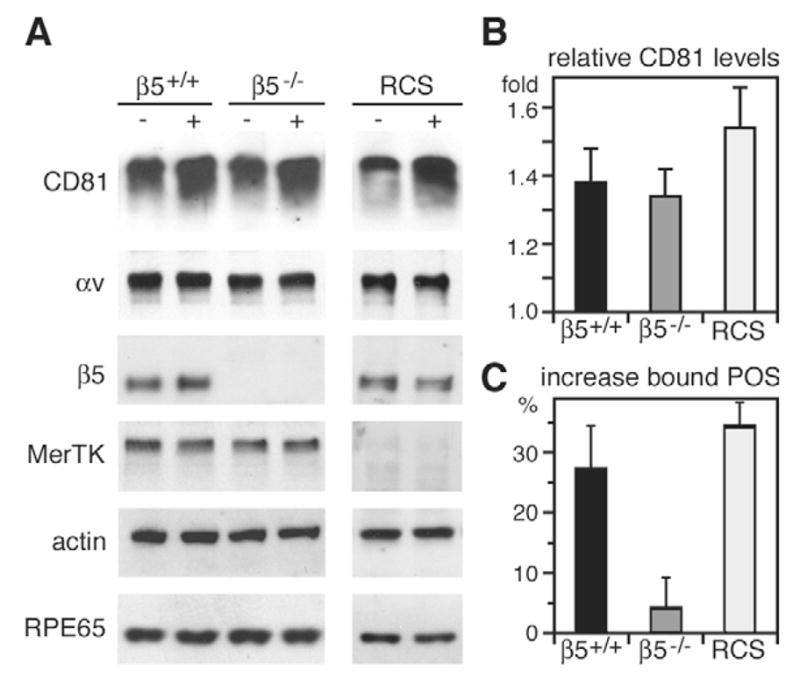
CD81 overexpression promotes POS binding only in the presence of αvβ5 integrin. Primary RPE isolated from β5+/+ mice, β5−/− mice, and RCS rats were transfected with CD81 expression plasmid or empty vector as control. 48 hours after transfection, we evaluated protein expression (A,B) and phagocytic activity (C) of transfectants were examined. (A) Total cell lysates, of equal numbers of cells, were analyzed by western blotting for proteins as indicated. (B) Quantification of western blots shows that CD81 plasmid transfection specifically increased CD81 protein to 1.4–1.6 fold of control cells. Bars show relative CD81 levels in β5+/+ (black), β5−/−(dark gray), and RCS RPE (light gray) (mean ± s.d., n=3). (C) Quantification of surface-bound POS following 1 hour of FITC-POS challenge shows that CD81 overexpression increased POS binding by β5+/+ and RCS RPE, but had no effect on β5−/− RPE. Bars represent mean ± s.d., n=3, percentage POS bound by CD81 overexpressing RPE cells compared to control transfected cells of the same genotype. POS binding by CD81 transfected β5+/+ and RCS RPE was significantly increased compared to respective control uptake (P<0.05, Student’s t-test). Remarkably, CD81 transfected β5−/− RPE did not differ in POS binding from control β5−/− cells (P>0.5).
Discussion
This study identifies a novel role for the tetraspanin CD81 in clearance phagocytosis. CD81 expression levels directly correlate with the binding activity of RPE cells towards POS particles. However, CD81 does not act as a bona fide particle binding receptor itself. Instead, CD81 requires αvβ5 integrin receptors to promote particle binding. These data provide the first evidence for a functional interaction between CD81 and αvβ5 integrin and for a role for tetraspanins in integrin-dependent phagocytosis. Like RPE cells, macrophages and dendritic cells bind apoptotic cells through αvβ3 or αvβ5 integrin for subsequent MerTK-dependent engulfment (Finnemann and Rodriguez-Boulan, 1999; Wu et al., 2005). Identification of the role of CD81 in RPE phagocytosis therefore strongly suggests that CD81 or similar tetraspanins fulfill equivalent functions in these related clearance mechanisms.
POS recognition by αvβ5 integrin stimulates FAK signaling in RPE cells that targets the engulfment receptor MerTK (Finnemann, 2003). Here, we show that CD81-dependent POS binding does not require MerTK. Furthermore, overexpressing CD81 increases POS binding but fails to increase POS engulfment. It is possible that exogenous CD81 promotes αvβ5-dependent POS binding that fails to couple to the POS engulfment machinery. However, it is important to emphasize that we do not know which molecule may be the limiting factor in forming the engulfment mechanism for extra bound POS. Thus, extra CD81 may increase αvβ5-dependent phagocytic signaling but fail to enhance engulfment because the engulfment machinery may be maximally active in response to POS in RPE with normal levels of CD81.
This is the first report that αvβ5 integrin resides in a complex with tetraspanins. Our co-localization studies demonstrate that these complexes localize to the apical, phagocytic surface of RPE cells. αv-CD81 co-precipitation in β5+/+ but not in β5−/− RPE/choroid tissue samples indicates that CD81-αvβ5 complexes exist and are probably relevant at the site of POS phagocytosis in vivo. Our CD81 immunoprecipitations from RPE-J lysates containing Brij97 co-isolated CD9 and ezrin, both of which are known to associate with CD81 (Charrin et al., 2001; Pan et al., 2007; Sala-Valdes et al., 2006; Stipp et al., 2001). The same CD81 immunoprecipitates also contained αv and β5 integrin subunits identifying a novel association of these proteins. Reciprocal immunoprecipitations using antibodies to the three phagocytic receptors of the RPE confirmed the specific association of CD81 and αvβ5. We failed to detect αv or β5 in a complex with CD81 in 1% Triton X-100 lysates (data not shown). Such susceptibility to Triton X-100 detergent disruption and resistance to Brij97 detergent suggests that CD81 associates with αvβ5 in the RPE in a level 2 interaction (Boucheix and Rubinstein, 2001; Hemler, 2003). The absence of the apical phagocytic receptors MerTK and CD36 demonstrates that our immunoprecipitations contained specific protein complexes rather than a representative mix of apical and/or phagocytic proteins. Both CD81 and αvβ5 receptors are widely expressed in different cell types in the body. It remains to be tested whether co-expression of the two receptors is sufficient to induce co-distribution or complex formation regardless of cellular context.
Tetraspanins form heterodimers with other tetraspanin family members to establish large protein complexes in membranes known as the tetraspanin web. Our data show that RPE cells express the tetraspanin CD9 in addition to CD81. Furthermore, CD9 co-immunoprecipitates with CD81 and, to a lesser extent, with β5 integrin from RPE cell lysates and shares the apical surface distribution susceptibility to detergent extraction with CD81 in RPE cells. In sperm-egg fusion, CD9 is known to closely functionally interact with CD81 (Kaji et al., 2002; Rubinstein et al., 2006). By contrast, we found no evidence that CD9 plays a role in RPE phagocytosis of POS. None of three independent experimental approaches, antibody inhibition, silencing and overexpression of CD9, affected binding or engulfment of POS. We conclude that facilitating POS recognition by αvβ5 integrin may be a function specific to CD81.
Our experiments identify CD81 as a novel partner protein for αvβ5 integrin in POS particle binding, the initial step of POS phagocytosis by RPE cells. Our data on β5−/− RPE cells show that CD81 does not bind POS independently but requires αvβ5 integrin to promote POS binding by RPE cells. It remains a possibility that β5−/− RPE fails to activate endogenous and overexpressed CD81 for direct POS interaction. However, given our knowledge of tetraspanin function in other cell types, CD81 may not bind POS directly, but instead, regulate POS binding by altering activity of αvβ5 receptors. Earlier studies have shown that tetraspanins may modulate integrin function by (1) regulating integrin trafficking and recycling, (2) regulating integrin compartmentalization in the membrane, facilitating lateral protein and lipid associations, and (3) recruiting intracellular signaling molecules to integrin tail domains (reviewed by Berditchevski, 2001; Hemler, 2005; Levy and Shoham, 2005). Here, we found that CD81 overexpression in RPE cells was sufficient to increase apical surface levels not only of CD81 but also of αvβ5 receptors. Thus, CD81 may play a role in regulating availability of αvβ5 receptors for POS binding at the RPE cell surface. It remains to be shown whether CD81 affects steady-state surface levels of αvβ5 by altering receptor traffic or surface receptor retention. Furthermore, although specific and reproducible, the increase of αvβ5 receptors at the apical surface is relatively small and it remains to be shown whether these changes in αvβ5 surface levels account for the effect on RPE phagocytic activity by CD81 depletion and overexpression. CD81 may employ one or more of the mechanisms mentioned above to modulate αvβ5 receptor affinity and/or avidity for POS ligand binding in addition to altering αvβ5 surface levels. Experiments with cells devoid of CD81 will facilitate future studies to explore these intriguing possibilities.
Materials and Methods
Cells and tissues
Rat RPE-J cells (ATCC, Manassas, VA) were maintained in DMEM with 4% fetal bovine serum at 32°C (Nabi et al., 1993). Cells were seeded at 50% confluence and grown for 6 days before use. Human RPE D407 cells were provided by R. Hunt (University of South Carolina, Columbia, SC) and were maintained in DMEM with 3% fetal bovine serum at 37°C (Finnemann et al., 2002). Mutant RCS-rdy−/−-p−/−(MerTK−/−) rats (National Center for Research Resources, NIH, Bethesda, MD), β5−/− mice characterized previously (Huang et al., 2000; Nandrot et al., 2004) and wild-type (β5+/+) mice of the same genetic background (129T2/SvEmsJ) were housed and bred. All procedures involving animals were approved by the Weill Medical College Institutional Animal Care and Use Committee.
Primary RPE cultures were obtained from 10-day- to 12-day-old pups as described previously (Nandrot et al., 2004). Briefly, enucleated eyecups were sequentially incubated in 1 mg/ml hyaluronidase and 2 mg/ml trypsin (both Sigma, St Louis, MO) in Hank’s balanced saline solution (HBSS, Invitrogen, Carlsbad, CA) for 45 minutes each at 37°C. RPE sheets were collected from the choroid and plated in 96-well plates on serum-coated coverslips at a density of ~5000–10,000 cells/well. Cells were cultured in DMEM with 10% fetal bovine serum for 4–7 days before use.
Transfections
RPE cells in 96-well plates were transfected with plasmid DNA or siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Cells were used 48 hours after transfection. To silence CD81 and CD9 expression, we used a mixture of four different 21-nucleotide RNAs specifically targeting rat CD81 or CD9 (‘smartpool’) designed and synthesized by Dharmacon (Lafayette, CO). Pools of non-targeting siRNA with at least four mismatches to any human, mouse or rat gene were also from Dharmacon. For overexpression experiments, we used plasmid pcDNA3.1 (Invitrogen) as control and pEF6/V5-His-TOPO encoding mouse CD81 or CD9 (a gift from Gabriela Dveksler, USUHS, Bethesda, MD) (Waterhouse et al., 2002).
Phagocytosis assays
POS were isolated from fresh bovine eyes and covalently labeled with FITC (Invitrogen) according to established protocols (Finnemann et al., 1997). RPE cells were challenged with 10 POS per cell in DMEM with 5% delipidated fetal bovine serum (Biomeda, Foster City, CA). For antibody inhibition, RPE cells were incubated with a suspension of POS in the continuous presence of antibodies. Antibodies used recognized rat and/or mouse CD81 [2F7 (Southern Biotech, Birmingham, AL) and Eat2 (Biolegend, San Diego, CA), rat CD9 (RPM.7), mouse CD9 (KMC) (both BD Biosciences, San Jose, CA)], human CD81 [I.3.3.22 (Millipore, Billerica, MA) and JS-81 (BD Biosciences)] and human CD9 [MM2/57 (GeneTex, San Antonio, TX)]. Appropriate non-immune antibody controls were from Rockland (Gilbertsville, PA). Following POS incubation, RPE cells were washed three times with PBS containing 1 mM MgCl2 and 0.2 mM CaCl2 to remove excess POS and fixed in ice-cold methanol. Duplicate samples were incubated with Trypan Blue before fixation to quench FITC fluorescence derived from externally bound particles (Finnemann et al., 1997; Hed, 1986). FITC-POS fluorescence representing the amount of total or internal POS were recorded by fluorescence scanning of coverslips mounted on glass slides using a Typhoon Trio+ scanner (GE Healthcare Piscataway, NJ) and quantified using ImageQuant v1.2 [Molecular Dynamics, Sunnyvale, CA; for examples of these scans please see (Finnemann et al., 1997)]. Internal FITC-POS fluorescence was measured from Trypan Blue-treated samples, and total FITC-POS fluorescence was measured from parallel untreated samples. Surface bound FITC-POS fluorescence was calculated by subtracting internal from total FITC-POS fluorescence. All experiments included triplicates of each sample and were independently repeated at least three times. Fluorescence scanning averaged POS uptake by ~20,000 cells per sample. To convert fluorescence scanning counts to POS per cell (POS/cell) values and to ensure integrity of epithelial layers, scanned samples were also routinely evaluated by epifluorescence and laser confocal microscopy. Images were acquired as described below. POS/cell and cell densities were quantified by counting numbers of FITC-POS and DAPI-stained nuclei in at least three representative fields of at least 50 cells each.
Immunofluorescent staining and microscopy
For labeling of apical surface molecules in live cells, the cells were chilled and incubated in ice-cold HBSS with primary and secondary antibodies before fixation. Tetraspanin antibodies used were, clone Eat2 to detect mouse or rat CD81, and clones RPM.7 and KMC to detect rat and mouse CD9, respectively. αvβ5-heterodimer-specific antibody P1F6 (Covance, Richmond, CA) was used to detect apical cell surface αvβ5 integrin. For co-staining with junction or basolateral proteins, live-labeled cells were fixed in ice-cold 95% ethanol, 5% acetic acid before incubation with antibodies to ZO-1 (Invitrogen), P-cadherin (Redies and Muller, 1994), or β1 integrin (P&D Systems, Minneapolis, MN). To evaluate tetraspanin expression at the apical surface of transfected cells, cells were fixed in 4% paraformaldehyde before labeling. For colocalization of apical surface CD81 and αvβ5, the CD81 antibody Eat2 and the αvβ5-heterodimer-specific antibody P1F6 were sequentially used on chilled, live cells before paraformaldehyde fixation. Secondary Alexa Fluor-conjugated antibodies and DAPI, for nuclear staining, were from Invitrogen. Horizontal (x-y) and vertical (z) images were acquired using a Leica TSP2 confocal microscopy system. Representative maximal projections or single scan images were recompiled in Adobe Photoshop 7.0 in accordance with image manipulation guidelines issued by The Journal of Cell Science.
Immunoprecipitations
Cells were solubilized in HBSM buffer (20 mM Hepes, pH 7.4, 150 mM NaCl, 2 mM CaCl2, 5 mM MgCl2) containing 1% Brij97 detergent and protease inhibitors. Cleared lysates from 3×106 polarized RPE-J cells, neural retina and RPE/Ch isolated from one mouse eyecup were incubated with rotation with 4 μg receptor antibody for 2 hours at 4°C. To immunoprecipitate (IP) only apical surface proteins, live RPE cells were chilled and incubated on ice for 1 hour with receptor antibodies at 5 μg/ml in HBSS. Cells were washed with ice-cold HBSS before lysis in 1% Brij97 in HBSM buffer with protease inhibitors. Immune complexes were collected with protein G-agarose (Pierce, Rockford, IL). Beads were washed three times with lysis buffer and divided into two tubes during the last wash to elute half of each IP under reducing conditions and non-reducing conditions. Control IPs were performed with 4 μg of non-immune hamster or goat IgG. Antibodies used for IPs were CD81 Eat2, rat CD9 RPM.7, mouse CD9 KMC, β5 integrin cytoplasmic domain (Abcam, Cambridge, MA), β1 integrin, CD36 and MerTK (all R&D Systems) and αvβ5 P1F6.
Whole cell lysis, cell fractionations and immunoblotting
For analysis of whole cell lysates, cells were solubilized in HNTG buffer (50 mM Hepes, pH 7.4, 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1% Triton X-100 supplemented with 1 mM PMSF and 1% protease inhibitor cocktail; Sigma). Lysate representing equal numbers of cells were compared by immunoblotting.
Differential extraction with Triton X-100, CHAPS and Brij97 was carried out exactly as described earlier (Winterwood et al., 2006). Briefly, polarized RPE-J cells in six-well plates were overlaid with 1 ml HBSM buffer containing 1% detergent and protease inhibitors and incubated on ice for 20 minutes with a gentle swirl every 5 minutes. Following retrieval of extracted proteins, the remaining cellular proteins were extracted by vortexing in 1 ml HNTG buffer with protease inhibitors. Equal volumes of both fractions from the same cells were analyzed side-by-side by immunoblotting.
Boiled samples were separated by SDS polyacrylamide electrophoresis before immunoblotting using standard protocols and ECL detection (PerkinElmer, Boston, MA). Antibodies used for immunoblotting were the same as for IPs (see above) and β5 integrin H-96 (Santa Cruz Biotechnology), αv integrin (BD Biosciences), ezrin and actin (both Sigma). Horseradish peroxidase-conjugated secondary antibodies were from Rockland. Scans of x-ray films were compiled in Photoshop 7.0. Band intensities were quantified using NIH image 1.63.
Supplementary Material
Acknowledgments
We thank Gabriela Dveksler and Richard Hunt for generously providing reagents. Mousumi Sircar provided excellent assistance preparing primary RPE cultures. This work was supported by National Institutes of Health grants R01-EY13295 and R24-EY15656. S.C.F. is the recipient of a William and Mary Greve Special Scholar Award by Research To Prevent Blindness, and of an Irma T. Hirschl Career Award.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/120/17/3053/DC1
References
- Azorsa DO, Moog S, Cazenave JP, Lanza F. A general approach to the generation of monoclonal antibodies against members of the tetraspanin superfamily using recombinant GST fusion proteins. J Immunol Methods. 1999;229:35–48. doi: 10.1016/s0022-1759(99)00102-7. [DOI] [PubMed] [Google Scholar]
- Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci. 2001;114:4143–4151. doi: 10.1242/jcs.114.23.4143. [DOI] [PubMed] [Google Scholar]
- Bertaux C, Dragic T. Different domains of CD81 mediate distinct stages of hepatitis C virus pseudoparticle entry. J Virol. 2006;80:4940–4948. doi: 10.1128/JVI.80.10.4940-4948.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boismenu R, Rhein M, Fischer WH, Havran WL. A role for CD81 in early T cell development. Science. 1996;271:198–200. doi: 10.1126/science.271.5246.198. [DOI] [PubMed] [Google Scholar]
- Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci. 2001;58:1189–1205. doi: 10.1007/PL00000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitin MH, Hall MO. Defective ingestion of rod outer segments by cultured dystrophic rat pigment epithelial cells. Invest Ophthalmol Vis Sci. 1983;24:812–820. [PubMed] [Google Scholar]
- Charrin S, Le Naour F, Oualid M, Billard M, Faure G, Hanash SM, Boucheix C, Rubinstein E. The major CD9 and CD81 molecular partner. Identification and characterization of the complexes. J Biol Chem. 2001;276:14329–14337. doi: 10.1074/jbc.M011297200. [DOI] [PubMed] [Google Scholar]
- Clarke K, Geisert EE. The target of the antiproliferative antibody (TAPA) in the normal and injured rat retina. Mol Vis. 1998;4:3. [PubMed] [Google Scholar]
- Cormier EG, Tsamis F, Kajumo F, Durso RJ, Gardner JP, Dragic T. CD81 is an entry coreceptor for hepatitis C virus. Proc Natl Acad Sci USA. 2004;101:7270–7274. doi: 10.1073/pnas.0402253101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RB, Szamier RB. Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science. 1977;197:1001–1003. doi: 10.1126/science.560718. [DOI] [PubMed] [Google Scholar]
- Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003;22:4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for αvβ3 and αvβ5 integrins, and protein kinase C regulates αvβ5 binding and cytoskeletal linkage. J Exp Med. 1999;190:861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. Proc Natl Acad Sci USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Leung LW, Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc Natl Acad Sci USA. 2002;99:3842–3847. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26:270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- Geisert EE, Abel HJ, Fan L, Geisert GR. Retinal pigment epithelium of the rat express CD81, the target of the anti-proliferative antibody (TAPA) Invest Ophthalmol Vis Sci. 2002;43:274–280. [PubMed] [Google Scholar]
- Hed J. Methods for distinguishing ingested from adhering particles. Meth Enzymol. 1986;132:198–204. doi: 10.1016/s0076-6879(86)32008-1. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- Huang X, Griffiths M, Wu J, Farese RV, Jr, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in β5-deficient mice. Mol Cell Biol. 2000;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K, Oda S, Miyazaki S, Kudo A. Infertility of CD9-deficient mouse eggs is reversed by mouse CD9, human CD9, or mouse CD81; polyadenylated mRNA injection developed for molecular analysis of sperm-egg fusion. Dev Biol. 2002;247:327–334. doi: 10.1006/dbio.2002.0694. [DOI] [PubMed] [Google Scholar]
- Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- Marmorstein AD. The polarity of the retinal pigment epithelium. Traffic. 2001;2:867–872. doi: 10.1034/j.1600-0854.2001.21202.x. [DOI] [PubMed] [Google Scholar]
- Nabi IR, Mathews AP, Cohen-Gould L, Gundersen D, Rodriguez-Boulan E. Immortalization of polarized rat retinal pigment epithelium. J Cell Sci. 1993;104:37–49. doi: 10.1242/jcs.104.1.37. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J Exp Med. 2004;200:1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Brown C, Wang X, Geisert EE. The developmental regulation of CD81 in the rat retina. Mol Vis. 2007;13:181–189. [PMC free article] [PubMed] [Google Scholar]
- Redies C, Muller HA. Similarities in structure and expression between mouse P-cadherin, chicken B-cadherin and frog XB/U-cadherin. Cell Adhes Commun. 1994;2:511–520. doi: 10.3109/15419069409014215. [DOI] [PubMed] [Google Scholar]
- Rubinstein E, Ziyyat A, Prenant M, Wrobel E, Wolf JP, Levy S, Le Naour F, Boucheix C. Reduced fertility of female mice lacking CD81. Dev Biol. 2006;290:351–358. doi: 10.1016/j.ydbio.2005.11.031. [DOI] [PubMed] [Google Scholar]
- Sala-Valdes M, Ursa A, Charrin S, Rubinstein E, Hemler ME, Sanchez-Madrid F, Yanez-Mo M. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J Biol Chem. 2006;281:19665–19675. doi: 10.1074/jbc.M602116200. [DOI] [PubMed] [Google Scholar]
- Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- Song BK, Levy S, Geisert EE. Increased density of retinal pigment epithelium in CD81−/− mice. J Cell Biochem. 2004;92:1160–1170. doi: 10.1002/jcb.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipp CS, Orlicky D, Hemler ME. FPRP, a major, highly stoichiometric, highly specific CD81− and CD9-associated protein. J Biol Chem. 2001;276:4853–4862. doi: 10.1074/jbc.M009859200. [DOI] [PubMed] [Google Scholar]
- Takeda Y, Tachibana I, Miyado K, Kobayashi M, Miyazaki T, Funakoshi T, Kimura H, Yamane H, Saito Y, Goto H, et al. Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J Cell Biol. 2003;161:945–956. doi: 10.1083/jcb.200212031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse R, Ha C, Dveksler GS. Murine CD9 is the receptor for pregnancy-specific glycoprotein 17. J Exp Med. 2002;195:277–282. doi: 10.1084/jem.20011741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterwood NE, Varzavand A, Meland MN, Ashman LK, Stipp CS. A critical role for tetraspanin CD151 in α3β1 and α6β4 integrin-dependent tumor cell functions on laminin-5. Mol Biol Cell. 2006;17:2707–2721. doi: 10.1091/mbc.E05-11-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Singh S, Georgescu MM, Birge RB. A role for Mer tyrosine kinase in αvβ5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci. 2005;118:539–553. doi: 10.1242/jcs.01632. [DOI] [PubMed] [Google Scholar]
- Wu Y, Tibrewal N, Birge RB. Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol. 2006;16:189–197. doi: 10.1016/j.tcb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Yanez-Mo M, Tejedor R, Rousselle P, Sanchez-Madrid F. Tetraspanins in intercellular adhesion of polarized epithelial cells: spatial and functional relationship to integrins and cadherins. J Cell Sci. 2001;114:577–587. doi: 10.1242/jcs.114.3.577. [DOI] [PubMed] [Google Scholar]
- Young RW. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967;33:61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunta M, Lazo PA. Tetraspanin proteins as organisers of membrane microdomains and signalling complexes. Cell Signal. 2003;15:559–564. doi: 10.1016/s0898-6568(02)00147-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.