Abstract
During development and regeneration, proliferation of tissue-specific stem cells is tightly controlled to produce organs of a predetermined size. The molecular determinants of this process remain poorly understood. Here, we investigate the function of Yap1, the transcriptional effector of the Hippo signaling pathway, in skin biology. Using gain- and loss-of-function studies we show that Yap1 is a critical modulator of epidermal stem cell proliferation and tissue expansion. Yap1 mediates this effect through interaction with TEAD transcription factors. Additionally, our studies reveal that α-catenin, a molecule previously implicated in tumor suppression and cell density sensing in the skin, is an upstream negative regulator of Yap1. α-catenin controls Yap1 activity and phosphorylation by modulating its interaction with 14-3-3 and the PP2A phosphatase. Together, these data identify Yap1 as a determinant of the proliferative capacity of epidermal stem cells and as an important effector of a ‘crowd control’ molecular circuitry in mammalian skin.
INTRODUCTION
During mammalian development, proliferation and cell death of tissue-specific progenitor and stem cells (SCs) needs to be tightly monitored and controlled to produce organs of a predetermined size. Experimental manipulation of SC numbers or activity during embryogenesis can thus have striking effects on the final size of certain organs (Depaepe et al., 2005; Kim et al., 2005; Stanger et al., 2007). Such exquisite regulatory mechanisms also orchestrate homeostasis of adult tissues and regenerative processes where the final shape and size of organs can be restored after cellular loss. While a number of signaling molecules have been implicated in controlling SC proliferation, little is known about endogenous mechanisms that ‘sense’ or provide information about organ size. It is also unclear how these mechanisms relay needs for tissue growth to its resident SCs and how these cues are translated into SC proliferation or apoptosis.
The Hippo signaling pathway was initially discovered in Drosophila as a potent mechanism that restricts tissue size by limiting cell proliferation and promoting apoptosis. At the core of this pathway is a kinase cascade composed of four tumor suppressors, including Hippo (Hpo) and Warts (Wts) and its regulatory proteins Salvador and Mats (Harvey et al., 2003; Lai et al., 2005; Pantalacci et al., 2003; Wu et al., 2003; Xu et al., 1995). The Hpo-Sav complex phosphorylates and activates the Wts-Mats complex, which in turn phosphorylates and inactivates the transcriptional co-activator Yorkie, Yki (Huang et al., 2005; Oh and Irvine, 2008). Yki phosphorylation prevents its nuclear translocation (Dong et al., 2007; Oh and Irvine, 2008; Zhao et al., 2007), where it acts as a co-activator for the TEAD/TEF family transcription factor Scalloped (Sd) (Wu et al., 2008; Zhang et al., 2008). This core set of Hippo signaling components is highly conserved in mammals, and orthologues of Hpo (Mst1/2), Sav (WW45), Wts (Lats1/2), and Yki (Yap1) exhibit similar biochemical properties in cultured cells (Dong et al., 2007; Oka et al., 2008; Zhao et al., 2007). The importance of Hippo signaling in mammalian organ size control has been studied extensively in the liver, where transgenic overexpression of Yap1, loss of Mst1/2, or Sav, leads to hepatomegaly (Camargo et al., 2007; Dong et al., 2007; Lu et al., 2010; Song et al., 2010; Zhou et al., 2009). However, to what extent these predicted epistatic and functional relationships are conserved and active in other tissues remains uncertain (Zhou et al., 2009).
Compared with the core kinase cascade regulating Yki phosphorylation, components acting upstream of this complex are less well defined. Earlier work in Drosophila has implicated the apical membrane-associated FERM-domain proteins Merlin (Mer), Expanded (Ex) and Kibra as pathway components upstream of Hpo (Baumgartner et al., 2010; Genevet et al., 2010; Hamaratoglu et al., 2006). Recent studies further implicated the apical transmembrane protein Crumbs (Crb) (Robinson et al., 2010) and the atypical cadherin Fat (Ft) (Hariharan, 2006) as modulators of the fly Hippo pathway. However, with the exception of the mammalian Merlin orthologue, NF2 (Zhang et al., 2010), the significance and relevance of these proteins in regulating the activity of Yap1 in vivo in mammals is largely unclear. The identification of physiological upstream regulators of mammalian Hippo signaling could provide important insights into the mechanisms sensing and controlling organ size.
The mammalian epidermis is a rapidly regenerating epithelial tissue whose maintenance depends on the self-renewing ability of epidermal SCs residing in the basal layer. After division, short-lived progenitor cells leave the basal layer and move through the suprabasal layers to the tissue surface as they terminally differentiate (Fuchs, 2007). Epidermal growth must be carefully balanced: inadequate proliferation results in thinning of the skin and loss of protection, and excessive growth is characteristic of hyperproliferative disorders. During development the epidermis must be able to sense needs for expansion and replace basal vacancies, and during regeneration cells must migrate and proliferate but also sense when to stop after wound closure. It has been postulated that adherens junctions (AJs), cadherin and catenin-based cell-adhesion structures, might have a function in ‘sensing’ epidermal cell density and restricting basal-cell proliferation (Lien et al., 2006b). For instance, loss of some, but not all, AJ components such as α-catenin or p120-catenin, triggers severe epidermal hyperproliferation and tumors in transgenic mice (Kobielak and Fuchs, 2006; Perez-Moreno et al., 2006; Vasioukhin et al., 2001). The mechanisms acting downstream of these complexes are not fully understood. Intriguingly, it has been recently found that NF2 can modulate epidermal development via an association with AJs, specifically α-catenin, and the Par3 polarity complex (Gladden et al., 2010). These findings raise the possibility that signaling cues that regulate cell density and cell polarity could converge on the regulation of downstream Hippo signaling components.
Here we utilize gain- and loss-of-function models in mice to show that Yap1 is an essential regulator of epidermal maintenance and SC proliferative capacity. In addition, we identify α-catenin, an AJ component and a known tumor suppressor in the skin, as a crucial upstream negative regulator of Yap1 in epidermal cells in vitro and in vivo. Our work identifies a direct link between a signaling component of cell density-dependent AJs and a transcriptional regulator, and provides a paradigm for the regulation tissue expansion in response to extracellular cues.
RESULTS
Activation of Yap1 can expand the epidermal SC compartment
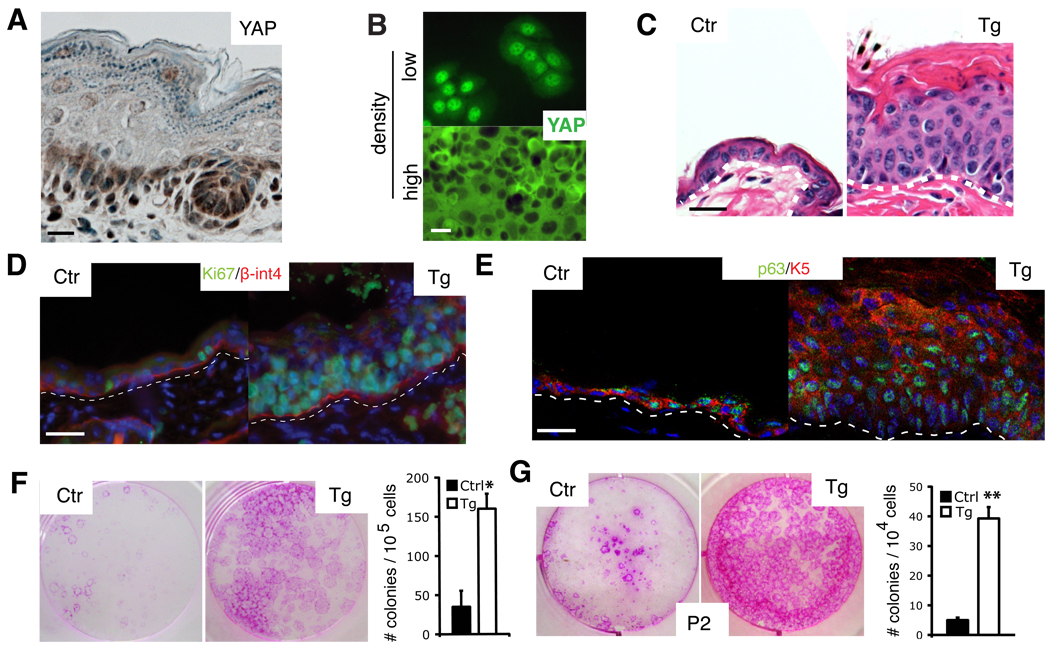
In newborn mouse epidermis, Yap1 has a dynamic sub-cellular localization pattern in the basal compartment, present in the nucleus in some cells and in the cytoplasm/membrane of others. Expression of Yap1 is reduced and in a more diffuse non-nuclear pattern in the suprabasal-differentiated cell layers (Fig. 1A). This pattern is also observed in cultured human keratinocytes in which high cellular densities lead to Yap1 re-localization out of the nucleus (Fig. 1B). Thus, regulation of Yap1 sub-cellular localization might be important for the switch between proliferative and terminally differentiating compartments. To test this, we utilized transgenic mice carrying a doxycycline (Dox)-inducible allele of a mutated version of Yap1 (S127A). This mutation results in the enhanced nuclear localization of Yap1 (Camargo et al., 2007; Dong et al., 2007; Zhao et al., 2007). To restrict Yap1 activation to the epidermis we developed a binary genetic strategy based on the Cre-mediated activation of a reverse tetracycline-dependent transactivator selectively in the progeny of K14-expressing progenitors of stratified epithelia (Belteki et al., 2005; Dassule et al., 2000) (Fig. S1A). Mice carrying these transgenes will be referred as Tg hereafter. Eight days after Dox administration, adult Tg mice developed thickening and wrinkling of the skin. Histological analyses demonstrated that contrary to the typical one/two-cell layer common to adult mouse epidermis, Tg skin developed into a multilayered epithelium (Fig. 1C). Staining for the proliferation marker Ki-67 revealed a substantial increase in the number of proliferating basal cells and an extension of the proliferative domain into the suprabasal layers, normally quiescent in control skin (Fig. 1D). Tg epidermis contained an abundance of cells expressing progenitor markers such as K5 and K14, whereas expression of the differentiation markers K10 and loricrin was mostly abrogated (Fig. 1E, S1B). Additionally, the number of cells expressing p63, a SC marker and essential regulator of stemness in stratified epithelia (Senoo et al., 2007), was amplified in Tg skin (Fig. 1F). Tg skin failed to express hair follicle SC markers such as K15, or Sox9 (data not shown). These results suggested that activation of Yap1 induced expansion of an undifferentiated stem/progenitor cell population in the interfollicular epidermis. We then compared the clonogenic capacity of primary keratinocytes isolated seven days after Dox administration. Skin of Tg mice had a greater than 5-fold increase in the number of colony-forming cells compared to controls after Yap1 activation in vivo (Fig. 1E). Moreover, serial plating assays, typically used to distinguish self-renewing SCs versus shorter-lived progenitors, revealed that Yap1 activation enhanced the SC proliferative capacity by 10-fold in secondary and tertiary platings (Fig. 1G, S1C). Taken together, these experiments demonstrate that activation of Yap1 promotes the proliferation of epidermal stem and progenitor cells and that its regulation is critical in maintaining normal skin homeostasis.
Figure 1. Activation of Yap1 leads to epidermal stem cell expansion.
A, Yap1 expression (brown) indicated by immunohistochemical staining on E18.5 murine wild-type epidermis. B, Immunofluorescence staining for Yap1 shows a dynamic localization pattern in human keratinocytes that is cell density-dependent. C, H&E staining shows an abnormally thick epidermis and hyperkeratinization in adult Tg mouse skin after 8 days of doxycyclin (Dox) induction. D-E, Immunofluorescence analysis on frozen sections of adult epidermis shows an expansion of the proliferative Ki-67 positive compartment as well as the stem cell compartment (p63-positive, K5-positive) in Tg skin after 8 days of dox induction. Dashed line marks epidermal-dermal junction. F, Rhodamine B staining of primary mouse keratinocytes isolated from 8-day Dox-treated Tg mice, and cultured for 9 days on feeders, shows a significant expansion of the epidermal stem cell compartment demonstrated by a higher colony-forming efficiency in Tg skin. G, Rhodamine B staining and colony counts show a significant increase in the self-renewal capacity of colony-forming progenitors after Dox administration measured by serial replating assays (shown is passage 2, P2). * p< 0.05, **p value < 0.01. Scale bars represent 20 µm. See also Supplementary Figure S1A–C.
Activation of Yap1 can lead to squamous-cell carcinoma-like tumors
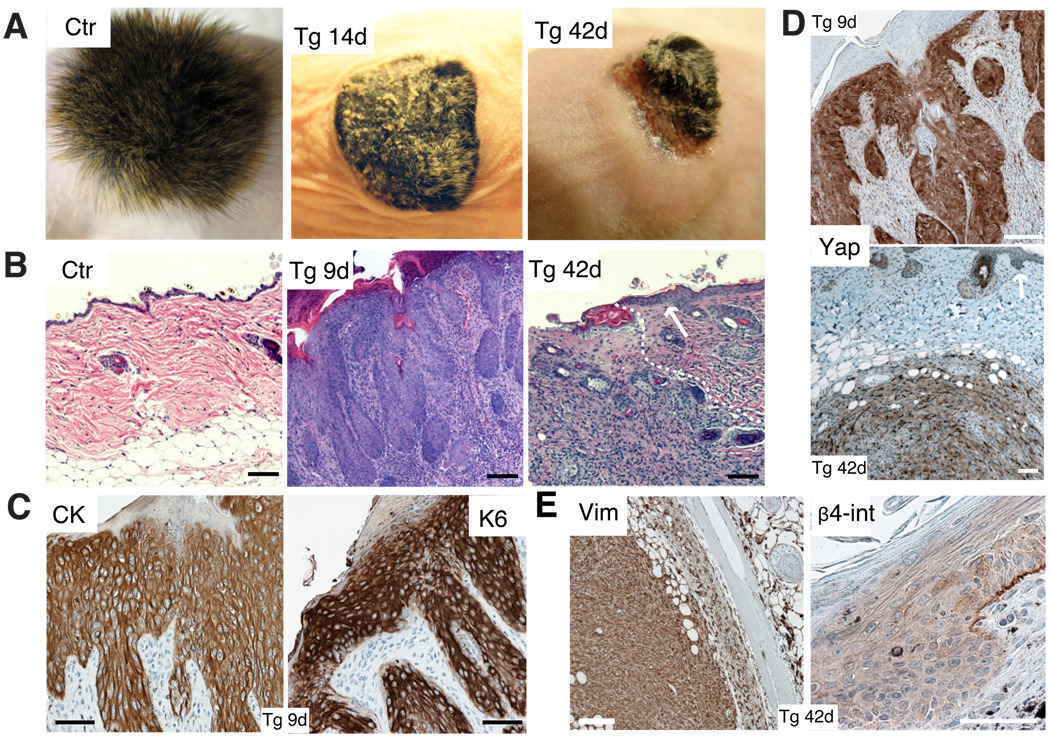
Tg mice became ill 8 days after Dox induction, likely because of similar dysplastic changes in mucosal tissues such as the tongue (Fig. S1D). In order to assess the long-term effects of Yap1 activation, newborn Tg and control skin was grafted onto Nude-mouse recipients followed by Dox administration. Epidermal thickening, hyperkeratosis, and stunted hair growth were evident in Tg-grafted mice shortly after grafting. Beginning at 20 days, Tg grafts developed large tumor-like masses that subsequently ulcerated (Fig. 2A). Histological analyses revealed hyperplasia and multiple epidermal invaginations into the dermis as early as 9 days after grafting (Fig. 2B). These lesions were epidermal in nature as judged by staining with a pan-cytokeratin antibody and by their expression of keratin 6, which is expressed anomalously in hyperproliferative states (Fig. 2C). Donor origin of the tumors was confirmed by their expression of the Yap1 transgene (Fig. 2D, S1E). Overall, the morphological perturbations resembled those of squamous cell carcinoma (SCC) in situ in human (Fig. 2B). By 42 days, full dermal invasion was evident and this was associated with the rupture of the basement membrane (Fig. 2E). At this stage, tumors resembled human sarcomatoid SCC, characterized by the presence of invasive atypical proliferating spindle cells intermingled with keratinized centers (Fig. 2B, S1F–G). As predicted from their morphology, invading cells stained mostly negative for keratin markers but expressed the mesenchymal marker vimentin, indicating a potential epithelial-mesenchymal transition, a common occurrence in advanced human SCC (Fig. 2E). Tumors were also assessed for their renewal by performing secondary transfers by subcutaneous injection into Nude mice. All transplantations gave rise to large masses of similar morphology as their primary counterparts (Fig. S1H). Our results here demonstrate that proper regulation of Yap1 activity is essential for tumor suppression in the epidermis.
Figure 2. Activation of Yap1 leads to tumor formation.
A, Gross morphology of Ctr and Tg grafts 14 and 42 days after beginning of Dox treatment. Note hyperkeratosis and stunted hair growth in Tg 14d graft and ulceration of skin and subcutaneous tumor mass in Tg 42d graft. B, H&E staining of Ctr and Tg grafts 9 and 42 days after Dox induction show an invasion of underlying dermis by invasive keratinocytes derived from the Tg graft. Arrow points out to neighboring normal Nude mouse epidermis. White dashed line indicates border of carcinoma to normal Nude dermis.C, Expression of cyto-keratin (CK) and keratin 6 (K6) in Tg grafts treated 9 days with Dox as shown by immunohistochemistry. D, Immunohistochemistry for Yap1 on Tg grafts with 9d and 42d Dox treatment reveals epithelial origin of the tumor. Arrow points at Nude mouse epidermis. E, Immunohistochemistry for vimentin (Vim) and integrin-β4 (β4-int) on Tg grafts with 42 days Dox induction. Scale bars represent 100 µm. See also Supplementary Figure S1D–H.
Loss of Yap1 results in failure of skin expansion
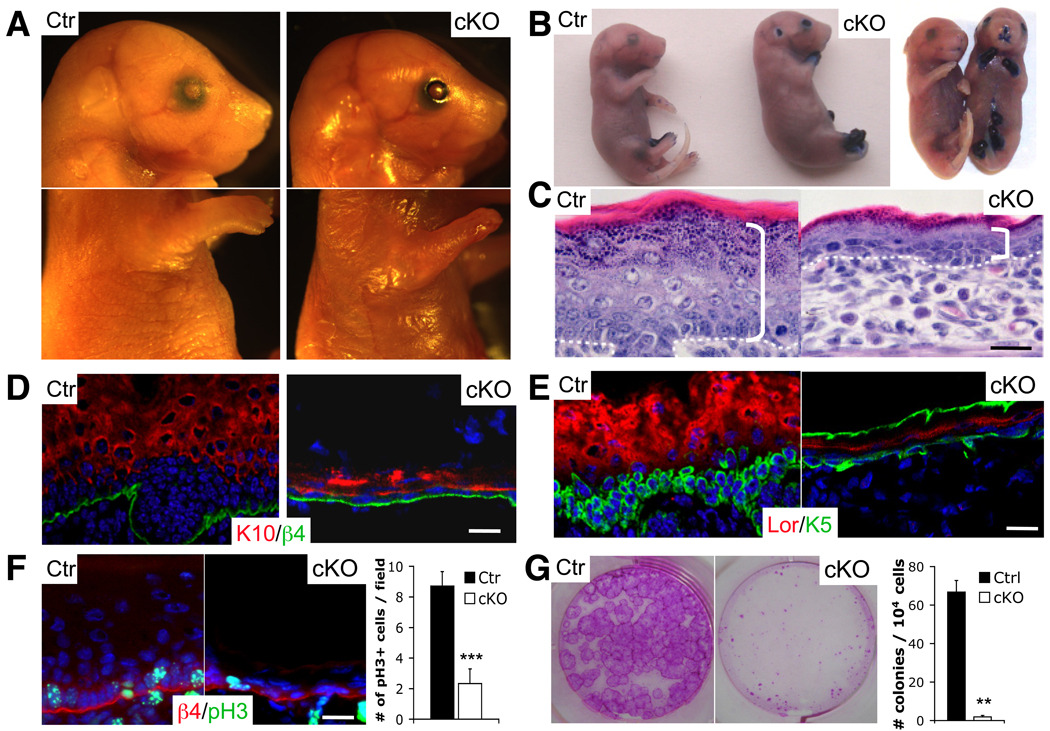
To test the role of endogenous Yap1 in epidermal biology, we generated a conditional floxed (fl) allele of Yap1 by gene targeting (Fig. S2A). Deletion of loxP flanked exons 1 and 2 of Yap1 after breeding to K14-Cre mice resulted in epidermis lacking Yap1 protein (Fig. S2B–D). K14-Cre Yap1(fl/fl) mice will be referred as cKO mice hereafter. cKO mice were either aborted or died shortly after birth. Gross examination of mutant embryos at E18.5 revealed thinner and fragile skin and absence of epidermal tissue covering the distal part of the limbs (Fig. 3A). Assessment of skin permeability using the toluidine blue dye revealed a complete loss of epidermal barrier function in the regions described and around the mouth and nose (Fig. 3B). Histological analyses of the proximal limb skin of cKO mice revealed a thinner epidermis, reduced stratum corneum, and disorganized epidermal architecture (Fig. 3C). Notably, the basal layer was hypoplastic and basal cells had lost their columnar organization and adopted a flat morphology, typical of suprabasal differentiated cells (Fig. 3C). These phenotypes were also observed in the back skin of cKO mice but were less severe.
Figure 3. Yap1 is required for the maintenance of the proliferative capacity of epidermal stem cells.
A, Gross morphology of representative control (Ctr) and cKO E18.5 embryos. Note absence of skin in distal limbs, eyes, and ears and overall thinner skin. B, Toluidine blue skin barrier assay in E18.5 mice reveal absence of epidermal barrier in limbs, ears, nose and mouth. C, H&E staining shows a decrease in epidermal thickness in cKO skin compared to the Ctr (indicated by bracket). Note the flat morphology of basal cells and disorganized epidermal architecture. Dashed line represents epidermal-dermal junction. D-E, Immunofluorescence on frozen E18.5 epidermis reveals signs of basal cell depletion in cKO skin. Note the reduction of keratin 10 (K10) negative, keratin 5 (K5) positive cells progenitor/stem cells juxatposed to the basement membrane (β4). Note background staining for K5 in stratum corneum of cKO. F, Marked reduction in number of proliferative basal cells in cKO limb skin as measured with an antibody against phospho-histone H3 (pH3). Results represent measurements of at least 5 different fields in 3 different animals. G, Reduction in the number of colony-forming progenitors in E18.5 skin. Rhodamine B stain and colony counts were performed at day eight after plating and are representative of two independent experiments. Data are presented as mean ± standard deviation (error bars). **p value < 0.01, *** p< 0.005. Scale bars represent 20 µm. See also Supplementary Figure S2A–F.
At E18.5 mutant cells expressing K10, an early differentiation marker, were nearly juxtaposed to the basement membrane in contrast to control skin where the basal layer was more pronounced (Fig. 3D). Staining of cKO skin with progenitor markers demonstrated their abnormal morphology and reduction in numbers (Fig. 3E). Staining with the proliferation marker phospho-histone H3 (pH3) revealed a greater than 3.5-fold decrease in the number of proliferating basal cells in proximal limb skin of cKO mice (Fig. 3F, S2E). No discernible difference in apoptotic cells was detected as judged by staining with active caspase-3 (data not shown). To directly address whether Yap1 deletion had an effect on the proliferative potential of epidermal SCs, we compared the clonogenic assays. These experiments demonstrated a greater than 50-fold decrease in the number of colony-forming cells and a decrease in colony size in cKO epidermis (Fig. 3G, S2F). This reduction in proliferative potential is likely the cause of the absence of skin in distal limb areas, where due to growth demands during development, the proliferation rate at E18.5 is more than 3-fold that of back skin (Fig. S2E). These results indicate that Yap1 is required for the maintenance of epidermal proliferative potential during development, and together with our gain-of-function experiments suggests that levels of active Yap1 are a critical and physiological determinant of epidermal SC proliferative capacity.
TEAD interaction is critical for the role of Yap1 in the epidermis
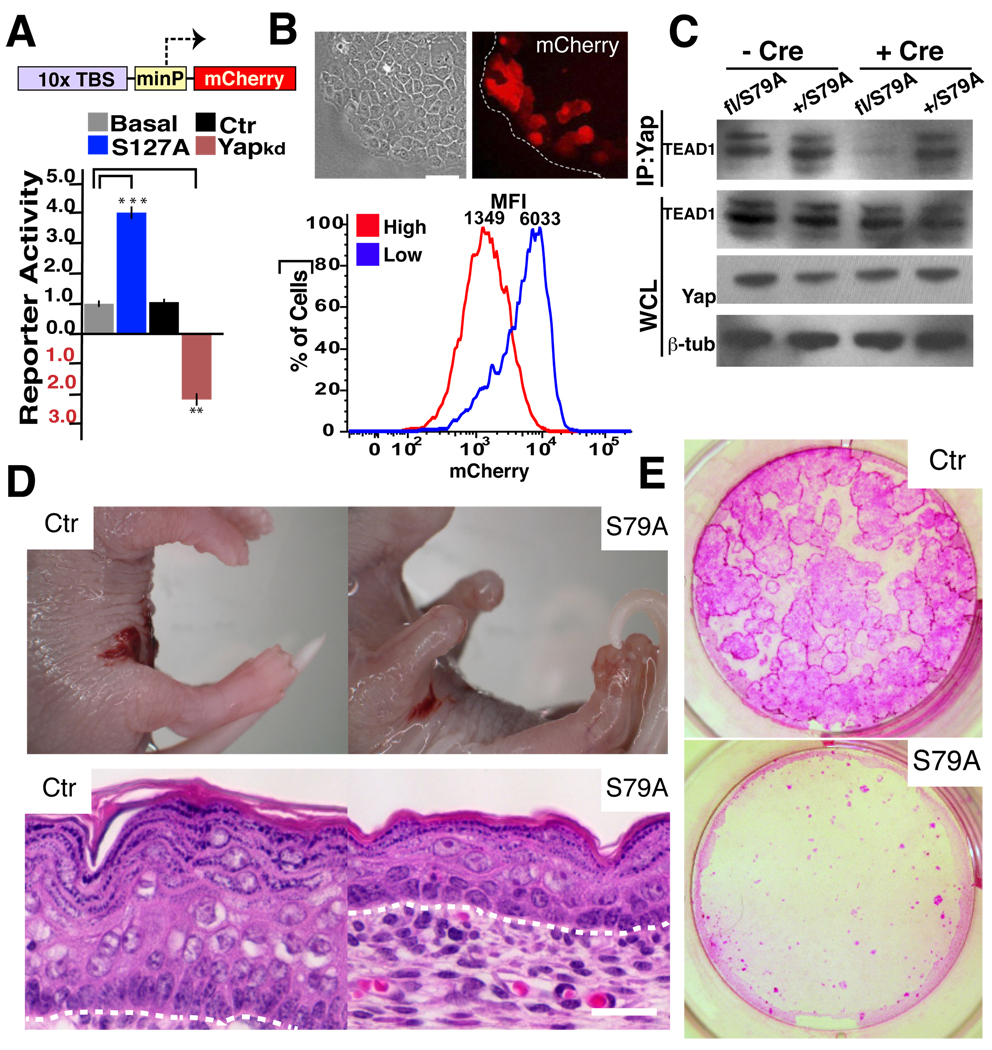
We next explored whether the ‘canonical’ Hippo pathway components played a role in regulating Yap1 in the epidermis. In mammals, the transcription factors TEAD1–4 have been shown to interact with Yap1 and to mediate some of its proliferative effects in vitro (Vassilev et al., 2001; Zhao et al., 2008). To test if TEAD-factors were also involved in the regulation of epidermal biology, we generated a reporter construct carrying multimerized copies of the consensus TEAD DNA-binding sequence (TBS) to monitor their endogenous activity (Fig. 4A). TBS reporter expression in a HaCaT keratinocyte cell line was highly responsive to Yap1, as its overexpression augmented reporter signal and its knockdown (KD) reduced reporter activity (Fig. 4A). Proliferating cells in the periphery of colonies expressed the highest levels of the reporter, and reporter signal decreased when these cells were cultured at high densities, thus mimicking the observed pattern of Yap1 activity (Fig. 4B).
Figure 4. Yap1 function in the skin is mediated through TEAD factors.
A, A HaCaT keratinocyte cell line carrying multimerized TEAD-binding sequences (TBS) upstream of a minimal promoter (minP) and an mCherry reporter is responsive to expression of Yap1-S127A, whereas RNAi KD of Yap1 downregulates reporter levels. Cells that have been transfected with a scrambled control-RNAi display reporter activity at basal levels. B, TBS reporter dynamics mimic Yap1 activity/localization. Top panel, TBS-mCherry shows a dynamic fluorescence pattern with keratinocytes at the edge of a growing colony expressing higher mCherry levels. Bottom panel, reporter signal decreases significantly at high cellular densities. C, Physical interaction between Yap1 and TEAD1 is virtually ablated in Adenovirus-Cre (+Cre) treated Yapfl/S79A embryonic fibroblasts. Immunoprecipitation (IP) was performed with an anti-Yap1 antibody followed by immunoblotting for TEAD1. Loading control β-tubulin (β-tub). D, Gross morphology of control (Ctr) and K14-Cre Yapfl/S79A E18.5 embryos. Note absence of skin in distal limbs and thinner skin with flat basal cells. E, Reduction in the number of colony-forming progenitors and proliferative potential in the skin of E18.5 K14-Cre Yapfl/S79A mice as demonstrated by colony assays stained with Rhodamine B. Data are presented as mean ± standard deviation (error bars), **p < 0.01, ***p < 0.005. Scale bars represent 20 µm. See also Supplementary Figure S3A–C.
Given the functional overlap among the four mammalian TEAD proteins, we decided to test their role in epidermal biology in vivo by genetically disrupting their interaction with Yap1. Through homologous recombination in ES cells, we mutated the Serine at position 79 of Yap1 to Alanine (S79A) (Fig. S3A–B). The orthologue serine in human Yap1 (S94) is necessary to mediate a physical interaction with the TEAD proteins but not with other DNA-binding co-factors (Zhao et al., 2008). The predicted consequences of this mutation were tested in Yap1fl/S79A mouse embryonic fibroblasts after administration of Cre and found that while the overall levels of Yap1 protein were unchanged, its ability to interact with TEAD factors was mostly ablated (Fig. 4C). We next generated K14-Cre Yap1fl/S79A (S79A) mice, in which only the Yap1 mutant protein would be expressed in the epidermis of mice starting at E14.5. These mice died at birth and fully phenocopied cKO mice (Fig. 4D). S79A animals lacked epidermis in the distal part of the limbs, eyes and ears. Mutant limb skin was thinner, with a reduced number of proliferating basal cells lying flat above the basal membrane (Fig. 4D, S3C). Clonogenic assays demonstrated a depletion of SC activity similar to cKO mice (Fig. 4E). These results provide genetic evidence TEADS are likely to be the major transcriptional partners of Yap1 in epidermal proliferation.
α-catenin interacts with Yap1 and 14-3-3
The mammalian orthologues of the Drosophila hippo kinase, Mst1 and Mst2, function upstream of Yap1 to mediate its phosphorylation and inactivation (Zhao et al., 2007). Deletion of both Mst1 and Mst2 in the liver results in Yap1 activation and hepatomegaly, suggesting that these kinases can act as bona fide negative regulators in vivo (Song et al., 2010; Zhou et al., 2009). We assessed whether deletion of Mst1/Mst2 in the skin would lead to similar phenotypes as Yap1 activation. Surprisingly, the epidermis of mice carrying a skin-specific deletion of Mst1/Mst2 displayed no abnormalities and no evidence of hyperplasia in mice up to 5 months of age (Fig. S4A–B). This was accompanied by no changes in Yap1 S127 phosphorylation in Mst1/Mst2-null keratinocytes (Fig. S4C). Additionally, KD of Mst1/Mst2 in the HaCaT-TBS reporter line produced no change in reporter activity or Yap1 nuclear localization (Fig. S4D–E). Similarly, knockdowns of Lats1 and Lats2, the orthologues of Drosophila warts, did not lead to increased reporter activity or enhanced Yap1 nuclear localization (Fig. S4D–E). These results suggested that Yap1 might be regulated by alternative mechanisms in keratinocytes that are independent of the predicted ‘canonical’ Hippo pathway kinases. In order to identify potential alternative upstream regulators of Yap1 activity, we performed co-immunoprecipitation (co-IP) experiments followed by mass-spectrometry (MS) analysis in high-density HaCaT cultures. Unexpectedly, this screen revealed that the most common interaction partner of Yap1 in keratinocytes corresponded to α-catenin, an important tumor suppressor in stratified epithelia (Table S1).
We next validated our mass spectrometry data by characterizing the interaction of Yap1 with α-catenin. Endogenous co-IP experiments in confluent HaCaT cells and keratinocytes directly isolated from skin, and pulldowns following transfection of tagged forms of Yap1 and α-catenin in 293T cells further demonstrate the biochemical interaction (Fig. 5A–B, S5A). Confocal microscopy studies in primary human keratinocytes corroborate these findings and reveal that Yap1 can begin to co-localize with α-catenin shortly after induction of differentiation following calcium treatment (Fig. 5C). With more time in calcium or at high cell densities, nuclear Yap1 is depleted, with some of it co-localizing with α-catenin at the membrane and some accumulating in a cytoplasmic pool (Fig. S5B).
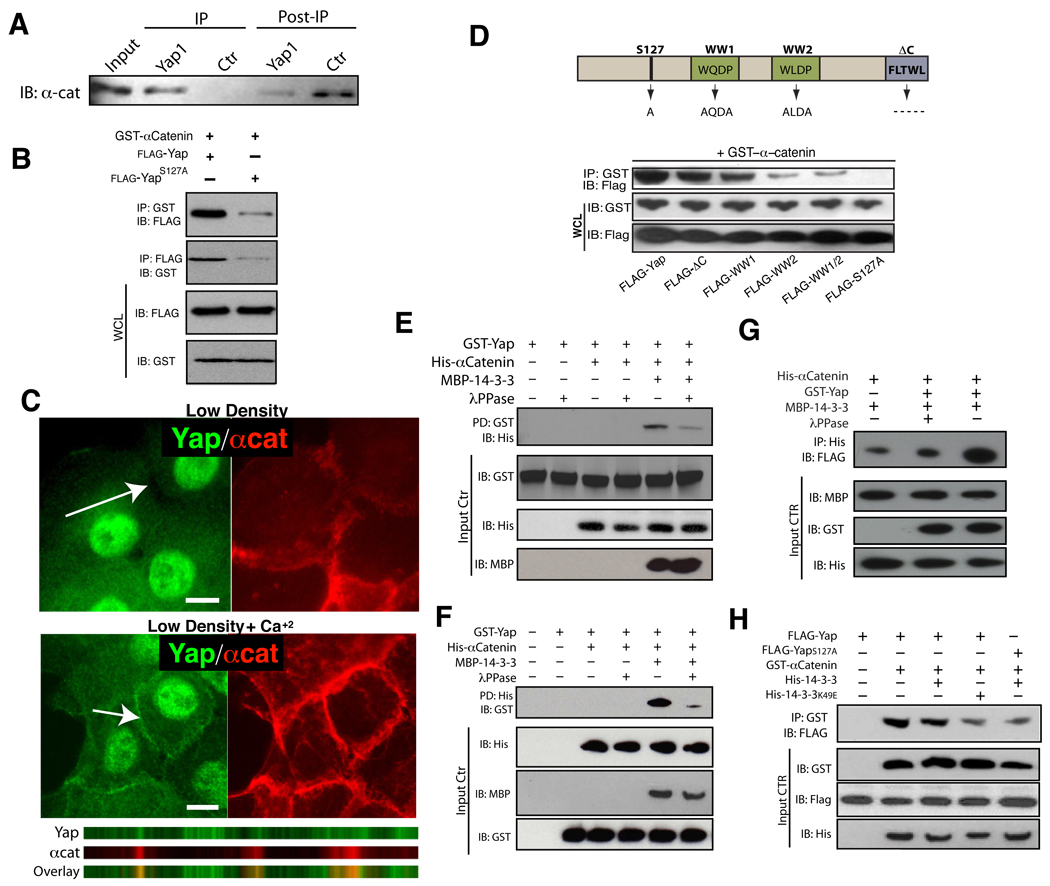
Figure 5. α-Catenin interacts with Yap1 and 14-3-3.
A, Confluent HaCaT cell lysates (Input) were used for an IP with an antibody against Yap1 or Pu.1 (Ctrl) and IP and post-IP material blotted with an antibody for α-catenin. B, 293T cells were co-transfected with the indicated plasmids followed by immunoprecipitation (IP) with either GST or FLAG-beads. Co-IPs demonstrate an association between full-length Yap1 and α-catenin and a largely diminished association between α-catenin and the YapS127A mutant. IB: Immunoblotting, WCL: Whole cell lysate. C, Calcium-induced differentiation in primary human keratinocytes leads to translocation of Yap1 into membrane regions that colocalize with α-catenin. scale bars = 10uM. D, Characterization of Yap1 domains important for the interaction with α-catenin.E–F, In vitro pulldown of recombinant Yap1 and α-catenin in the presence or absence of 14-3-3. G, In vitro pulldown of recombinant 〈-catenin and 14-3-3 in the presence of phosphorylated recombinant Yap1. H, Immunoprecipitation of Yap1 and α-catenin demonstrates that the biochemical interaction is dependent on 14-3-3. Scale bars depicted are 10 µm. See also Supplementary Figure S4A–E, S5A–B and Table S1.
Phosphorylation of Yap1 at Ser127 creates a 14-3-3 binding site that has been shown to be crucial for the regulation of its activity (Zhao et al., 2007). Remarkably, disruption of 14-3-3-mediated regulation by mutation of Yap1 Ser127 to alanine nearly abolished the interaction between Yap1 and α-catenin (Fig. 5B, D). The participation of 14-3-3 in Yap1 binding to α-catenin is further evidenced by the presence of a large number of 14-3-3 peptides found in our Yap1-mass spectrometry pulldowns (Table S1). We also find that the WW domains of Yap1, previously shown to be important for negative regulation by inhibitory kinases (Oka et al., 2008), are also required for its efficient interaction with α-catenin (Fig. 5D).
We next examined whether the interaction between α-catenin and Yap1 was direct using in vitro binding assays. Bacterially purified α-catenin and recombinant Yap1 purified from Sf9 cells were co-incubated and subjected to pulldown assays. Our results reveal a lack of direct interaction between these two proteins (Fig. 5E–F). Given that phosphorylation at Yap1 S127, and subsequent 14-3-3 binding is critical for the Yap1 and α-catenin association in cells, we tested whether 14-3-3 could allow for the formation of a tripartite complex in vitro. Indeed, the addition of bacterially purified 14-3-3 resulted in the association of Yap1 and α-catenin (Fig. 5E–F). As predicted, this association was dependent on Yap1 phosphorylation, as pre-treatment of phosphorylated recombinant Yap1 with lambda phosphatase resulted in loss of complex formation (Fig. 5E–F). Our results also provide evidence for a direct physical interaction between α-catenin and 14-3-3, which can be enhanced by the presence of phosho-Yap1 (Fig. 5G). Additionally, expression of a dominant negative 14-3-3 mutant (K49E) in mammalian cells leads to a reduction of Yap1 and α-catenin binding (Fig 5H). Thus, these data uncover α-catenin as a binding partner for Yap1, and demonstrate that 14-3-3 is critical for this biochemical interaction.
α-catenin regulates Yap1 localization and activity
To test if AJs are functionally important for the non-nuclear localization and inactivation of Yap1 at high cellular densities, keratinocyte cultures were treated with the calcium chelator EGTA. After only 1 minute of EGTA treatment in sub-cellular localization of Yap1 turned predominantly nuclear (Fig. 6A, S5C). The rapid kinetics of these observations indicates that AJs play a functional role in inactivating Yap1, and that the same pool of Yap1 can rapidly re-localize to the nucleus. To more specifically dissect the role of individual AJ proteins in regulating Yap1 activity, we depleted their expression in HaCaT and 293T cell lines carrying the TBS-reporter. Remarkably, a robust activation of the reporter was observed after α-catenin KD, similar to levels obtained after depletion of the known upstream regulator NF2. A weaker but significant response was obtained after depletion of p120-catenin and no reporter activation was observed after KD of β-catenin, E-cadherin, P-cadherin or both E- and P-cadherins (Fig. 6B, S5D–E). α-catenin depletion activated the TBS reporter in several other cell lines tested, indicating a conserved phenomenon across epithelial cell types. (Fig. S5F). Silencing of α-catenin in confluent keratinocytes resulted in the Yap1 re-localization to the nucleus and the formation of a Yap1/TEAD nuclear complex (Fig. 6C–D). α-catenin depletion was also accompanied by a reduction in the inhibitory phosphorylation at Yap1-S127, and minimal changes in the activation status of the Mst and Lats kinases (Fig. 6E), suggesting that the regulatory effects of α-catenin might be independent of these kinases.
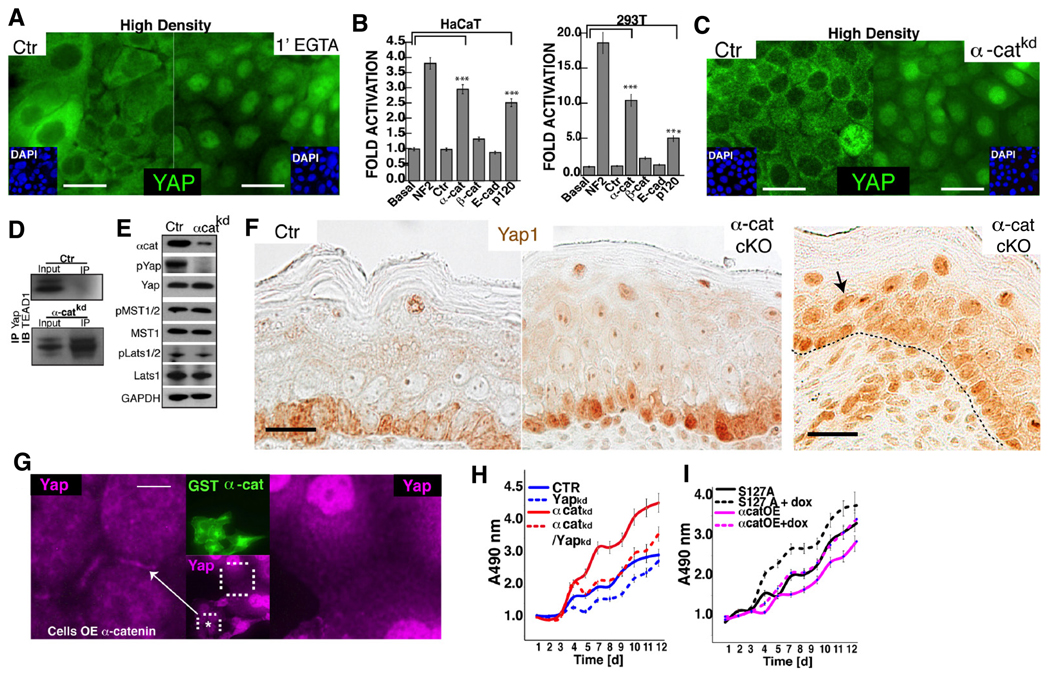
Figure 6. α-Catenin regulates Yap1 localization and activity.
A, Disruption of AJs in high-confluent keratinocytes with EGTA results in Yap1 nuclear localization after 1-minute of treatment. B, Knockdown (KD) of AJ proteins in either HaCaT or 293T cells carrying the TBS-reporter. KD of NF2 is shown as a positive control. C, KD of α-cat in confluent keratinocytes leads to Yap1 nuclear localization, and interaction with its nuclear partner Tead1 (D). E, siRNA-mediated depletion of α-cat in high density HaCaT cultures leads to loss of Yap phosporylation at serine 127 (pYap), and unchanged levels of activated Mst1/2 (pMst) or Lats1/2 (pLats). F, Immunohistochemistry for Yap1 in Ctrl and K14-Cre conditional α-catenin mutant (cKO) E18.5 epidermis. Note enhanced nuclear staining in basal and suprabasal cells of cKO mice. G, Immunofluorescence detection of Yap1 (purple) localization in low-density keratinocytes ectopically expressing GST-α-cat (green). Keratinocytes overexpressing α-cat show Yap1 localization to sites of cell-cell contact, whereas untransfected cells show nuclear staining and an absence of Yap1 staining at the cell membrane. H, Stable-knockdown of α-catenin (α-cat-KD) promotes hyperproliferation in HaCaT cells, however doxycycline (dox)-induced KD of Yap1 in α-cat-KD cells slows down the rate of cell proliferation to control (Ctr) levels. I, Ectopic expression of α-cat in a HaCaT cells suppresses cell proliferation (α-catOE). This growth inhibition is rescued by the expression of a Dox-inducible Yap1S127A mutant (Yap1S127A+Dox). Data are mean ± standard deviation (error bars), ***p < 0.001. Scale bars are 20 µm (A, C), and 10 µm (F), 5 µm (G). See also Supplementary Figure S5C–H and S6A–E.
To extend these observations and to test whether α-catenin could be an important negative regulator of Yap1 in vivo, we examined Yap1 localization in α-catenin mutant tissues. We first analyzed Yap1 localization in epidermis of E18.5 mice with a K14-Cre driven deletion of α-catenin. Skin of control mice displayed the typical heterogeneous sub-cellular localization pattern where only a few basal cells display predominant nuclear Yap1 protein (Fig 6F). This pattern was drastically changed in α-catenin mutant epidermis where basal cells were, in their great majority, positive for nuclear Yap1 and showed substantially enhanced nuclear staining (Fig. 6F, S5G). Additionally, in some regions, we observed nuclear Yap1 staining extending into the suprabasal cell compartment. Nuclear Yap1 was never seen suprabasally in control tissue (Fig. 6F). We next evaluated the localization of Yap1 in a panel of 19 human skin SCCs that expressed varying levels of α-catenin (Fig. S5H). As shown in Fig. S5H, no or low expression of α-catenin clearly correlated with enhanced nuclear Yap1 localization in these tumors.
Having established that α-catenin is crucial for the inactivation of Yap1 in keratinocytes in vitro and in vivo, we tested whether ectopic expression of α-catenin could result in the recruitment of Yap1 to AJs. Remarkably, enforced expression of α-catenin in low-density cultures led to the re-localization of Yap1 to membrane regions containing AJ components (Fig. 6G, S6A). To test whether a different AJ protein could mediate this recruitment of Yap1 to the membrane, we performed similar experiments with E-cadherin. Cells that over-expressed E-cadherin, however, did not show localization of Yap1 to membrane regions (Fig. S6B). These observations support the idea that α-catenin plays a direct role in the recruitment of Yap1 to AJs. This result, together with the siRNA depletion data (Fig. 6B), speaks to the unique ability of α-catenin, and not E/P-cadherin or β-catenin, to regulate Yap1 localization and activity. These findings also argue that Yap1 hyperactivation is not due simply to loss of AJ-mediated cellular adhesion.
Genetic ablation of α-catenin in murine epidermis leads to keratinocyte hyperproliferation and squamous cell carcinoma (Kobielak and Fuchs, 2006; Vasioukhin et al., 2001), observations highly reminiscent of the Yap1 gain-of-function phenotypes described here. Altogether, our data suggest that the growth-suppressive effects of α-catenin in the epidermis might be mediated through inactivation of Yap1. We tested this prediction experimentally and found that reduction of Yap1 levels in a α-catenin KD HaCaT cell line rescued its hyperproliferative phenotype to the level of control keratinocytes (Fig. 6H, S6C–E). Conversely, ectopic expression of α-catenin is suppressed keratinocytes proliferation (Fig. 6I), but expression of Yap1-S127A was able to rescue these inhibitory effects (Fig. 6I).
α-catenin binding prevents Yap1 dephosphorylation and activation
Knockdown of α-catenin results in decreased Yap1 S127 phosphorylation, which is likely the cause of increased Yap1 nuclear localization and activity. To begin understanding the potential mechanisms linking α-catenin levels and Yap1 phosphorylation, we performed a Yap1 co-IP followed by MS in α-catenin KD cells. These experiments revealed a selective interaction of Yap1 with the catalytic subunit of the protein phosphatase 2A (PP2Ac) in cells with reduced α-catenin levels (data not shown). Co-IP and immunoblotting experiments confirmed this observation (Fig. 7A). These results suggested that loss of α-catenin could result in a more efficient association of Yap1 and PP2Ac, which could potentially dephosphorylate Yap1-S127 triggering its activation. PP2A is a highly promiscuous enzyme that catalyzes the dephosphorylation of a wide range of substrates. Purified PP2Ac, in fact, is able to efficiently dephosphorylate Yap1 S127 in vitro in an okadaic acid-dependent manner (Fig. 7B). Importantly, reduction of PP2Ac levels in α-catenin KD cells, suppresses the increase in TBS-reporter activity and S127A phosphorylation observed after depletion of α-catenin (Fig 7C, S7). Thus, PP2Ac acts downstream or in parallel of α-catenin, and the increased Yap1 activity in α-catenin KD cells is dependent on PP2Ac activity.
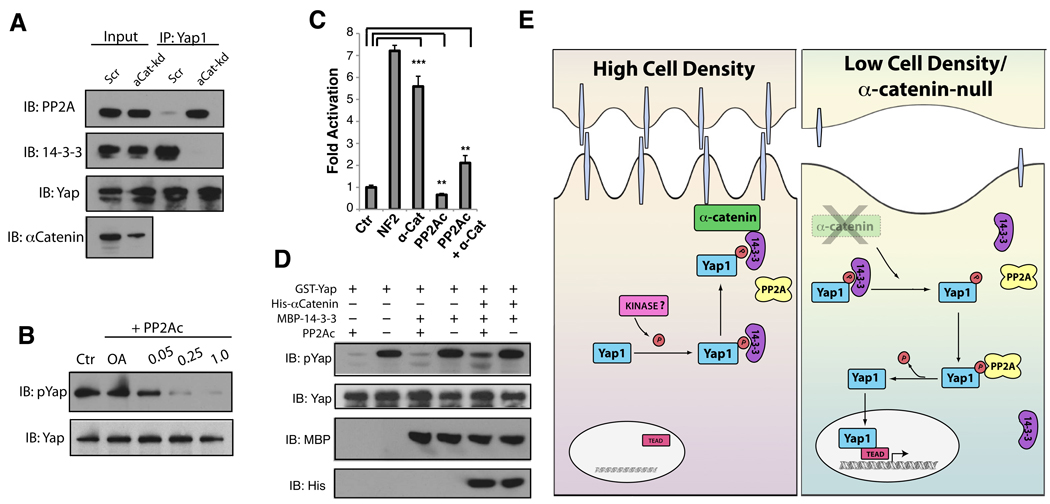
Figure 7. α-Catenin binding prevents Yap1 dephosphorylation and activation.
A, PP2Ac associates with Yap1 in α-catenin-depleted HaCaT cells. The association of Yap1 and 14-3-3 is diminished in the absence of α-catenin. B, Yap1 is dephosphorylated by PP2Ac in vitro. Different concentrations of purified PP2A was incubated with Sf9-purified recombinant Yap1 for 30 minutes. C, siRNA KD of α-catenin increases TBS-reporter activation and the dephosphorylation of Yap1. Simultanious KD of α-catenin and PP2Ac suppresses TBS-reporter transcriptional activity and and increase in Yap1 phosphorylation. D, Western blot analysis of phospo-Yap levels following an in vitro dephosphorylation competition assay of Yap1 by PP2Ac in the presence of recombinant 14-3-3 and α-catenin. E, Schematic model of density-dependent and α-catenin-mediated Yap1 activation. Data are presented as mean ± standard deviation (error bars), **p < 0.01, ***p < 0.001. See also Supplementary Figure S7.
An antagonistic and competitive interaction has been reported between PP2A and 14-3-3 for different phospho-substrates (Chiang et al., 2003; Martin et al., 2008). Hence, we tested whether KD of α-catenin would result not only in increased association of Yap1 with PP2Ac but also in reduced binding to 14-3-3. In effect, Yap1 does not seem to be bound to 14-3-3 in α-catenin KD keratinocytes (Fig. 7A). To determine if a Yap1, 14-3-3 and α-catenin complex could be protective against PP2Ac-mediated dephosphorylation, an in vitro dephosphorylation assay was performed in the presence and absence of these complex-forming proteins. Though the presence of 14-3-3 alone was not able to mitigate phosphatase activity, addition of α-catenin to the complex resulted in diminished phosphorylation (Fig 7D).
One of the many PP2A complexes has been previously implicated in the regulation of Hippo signaling by controlling phosphorylation of the Hpo kinase in Drosophila (Ribeiro et al., 2010). Our experiments in mammalian cells extend the role of PP2Ac as a regulator of mammalian Hippo signaling and suggest that α-catenin might regulate Yap1 activity by modulating its ability to be dephosphorylated at S127.
DISCUSSION
The present findings provide a mechanistic paradigm for the regulation of SC proliferation and tissue expansion in response to extracellular cues. In this molecular circuitry, cellular ‘neighborhood’ information is provided by density-dependent cell-cell junctions (Fig. 7E). A critical and unique component of these junctions, α-catenin, acts as a signaling molecule to translate this context-dependent information into SC proliferation and tissue expansion. α-catenin achieves this through modulating the activity of Yap1, the transcriptional co-activator of the size-restricting Hippo signaling pathway.
Yap1 as a critical regulator of epidermal SCs
Relatively little is known about the transcriptional networks controlling epidermal SC maintenance. Arguably, only p63 and Tcf3/Tcf4 have been implicated as essential factors for the long-term proliferative capacity of epidermal SCs in vivo ((Nguyen et al., 2009); Senoo et al., 2007). Our work here provides loss- and gain-of-function genetic evidence indicating that Yap1 and the TEAD proteins are critical endogenous regulators of epidermal SC and progenitor proliferation. While this manuscript was under review, Zhang et al. reported that transgenic activation of Yap1 in the skin led to an expansion of basal epidermal progenitors and inhibition of terminal differentiation (Zhang et al., 2011). These authors also find that nuclear Yap progressively declines with age and that it correlates with the proliferative potential of epidermal progenitors. Our work corroborates and extends these observations and together they highlight the emerging role of Yap1 and TEAD factors in skin homeostasis. In the future, it will be important to identify the transcriptional targets of this complex that mediate its function.
Our results also extend previous observations linking Yap1/TEAD activity to SC function. In the intestine, Yap1 activation leads to the expansion of an undifferentiated progenitor population (Camargo et al., 2007), whereas its deletion causes no obvious defects during development or homeostasis, albeit it impairs regeneration (Cai et al., 2010). Loss of Yap1 in the liver leads to defective biliary development but unimpaired regeneration after partial hepatectomy (Zhang et al., 2010). Thus, it seems that the requirement for endogenous Yap1 in tissue-specific SCs varies significantly according to the cellular context. The intrinsic need for Yap1/TEAD activity in epidermal SCs during development is more akin to the role that this complex plays in human embryonic SCs where it is required for their self-renewal and suppression of differentiation (Lian et al., 2010).
α-catenin as a regulator of Yap1 activity
Our findings provide additional insight into the role of α-catenin in growth and tumor suppression. Previously, α-catenin was considered to be a simple structural linker between the cadherin/catenin complex and the actin cytoskeleton. Over the past several years, it has become evident that α-catenin also functions as a signaling molecule in a variety of cellular processes in addition to its role in cellular adhesion (Stepniak et al., 2009). Interestingly, ablation of epidermal E- and P-cadherins leads to AJ disruption, defects in intracellular adhesion and epidermal integrity, but no significant increase in cellular proliferation (Tinkle et al., 2008). These findings, together with our data (Fig. 6B, S5D) demonstrate that hyperproliferation and Yap1 activation in the absence of α-catenin are not simply due to loss of cell adhesion and epithelial integrity. Additionally, in human cancers, loss of α-catenin is usually a stronger prognostic factor than loss of E-cadherin (Benjamin and Nelson, 2008). Thus, while it seems that AJ components function coordinately in mediating keratinocyte adhesion, they differ in their ability to influence proliferative responses. Particularly, it was shown that aberrant activation of the Ras-MAPK pathway was partially responsible for the hyperproliferative phenotypes observed in α-catenin mutant skin (Vasioukhin et al., 2001). It is still unclear to what extent Yap1, Ras-MAPK, or other signaling pathways contribute to growth suppression mediated by α-catenin in the skin. Whether Yap1 mediates all or only part of α-catenin tumor suppressive activity awaits the results of epistatic experiments in vivo.
14-3-3 and Yap1 phosphorylation in the skin
Our data would suggest that 14-3-3 proteins are also critical growth suppressors in the epidermis. The 14-3-3 proteins are a large group of highly conserved and homologous dimeric proteins. A particular isoform, 14-3-3σ, is unique among the family in that it is selectively expressed in stratified epithelia. Interestingly, 14-3-3σ is barely detectable in basal cells but is abundantly expressed in the suprabasal layer of human epidermis (Dellambra et al., 2000). Downregulation of 14-3-3σ in primary keratinocytes results in the expansion and immortalization of cells expressing p63 (Dellambra et al., 2000; Pellegrini et al., 2001). Additionally, the epidermis of a mouse mutant carrying a dominant-negative mutation in 14-3-3σ displays hyperplasia, expansion of K14 and K5-expressing progenitor cells, and enhanced proliferative capacity in vitro (Herron et al., 2005; Li et al., 2005). These phenotypes are reminiscent of Yap1 overexpression and α-catenin deletion in the epidermis and further indicate that 14-3-3σ is part of a critical molecular circuitry controlling epidermal progenitor expansion.
As predicted from work in Drosophila, Mst1/2 and Lats1/2 are critical for Yap1 S127 phosphorylation in a wide array of cell lines (Dong et al., 2007; Oh and Irvine, 2008; Zhao et al., 2007). Our data here demonstrate that Mst1/2, and potentially Lats1/2, are dispensable for Yap1 phosphorylation and activity in the epidermis. Our results parallel those of Zhao et al. and identify an unexpected diversity in the mammalian kinase components necessary to phosphorylate Yap1 in relation to what is predicted from work in Drosophila. For instance, in mouse liver, Mst1/2 signal through an unknown intermediary kinase distinct from Lats1/2 to control Yap1 phosphorylation. On the other hand, mouse embryonic fibroblasts do not require Mst1/2 but do depend on Lats1/2 to phosphorylate Yap1 (Zhao et al., 2007). We have tested whether pharmacological manipulation of MAP kinase and insulin signaling, pathways previously shown to be involved in α-catenin signaling (Vasioukhin et al., 2001), had effects on Yap1 phoshporylation. Inhibition of IGFR, JNK and Src kinases resulted in no relative change in Yap1-S127 phosphorylation (data not shown). Thus, the identity of the kinases responsible for Yap1 inactivation in the epidermis remains unknown.
Several examples exist in the literature that demonstrate that competition between 14-3-3 proteins and PP2A complexes for a substrate is critical for regulation (Chiang et al., 2003; Martin et al., 2008). Our data suggest that binding of the Yap1/14-3-3 complex to α-catenin would stabilize this complex and/or inhibit access of PP2Ac (Fig. 7). When binding to α-catenin is lost, PP2Ac could then more efficiently compete for binding to S127 in Yap1 and dephosphorylate it, allowing Yap1 to translocate into the nucleus, bind to TEADs, and activate a pro-proliferative gene expression program (Fig 7). Recently, α-catenin has been found to interact with NF2, another upstream component of the Hippo pathway (Gladden et al., 2010). Interestingly, it was reported that NF2 could link α-catenin to Par3, thus providing a bridge between AJs and polarity complexes. Together with the recent identification of the apical polarity protein Crumbs as a functional regulator of the Hippo pathway (Chen et al., 2010; Grzeschik et al., 2010; Ling et al., 2010; Robinson et al., 2010; Varelas et al., 2010), these findings raise the intriguing possibility that a signaling complex of α-catenin, NF2, and other cell polarity proteins associated with the cell membrane could make up an important signaling hub that may allow cells to sense cues such as density and positioning. These complexes then could relay those signals to transcriptional effectors such as Yap1 (see below). The LIM protein Ajuba, might provide an additional avenue for crosstalk between AJs and Hippo signaling. Ajuba can interact with α-catenin and has been recently shown to inhibit Yap1 phosphorylation via interaction with Lats (Das Thakur et al., 2010). Further studies are needed to determine whether and to what extent α-catenin, NF2, and polarity complexes in epidermal cells control proliferation through Yap1.
Crowd control and the Hippo pathway
An important long-standing question in the Hippo signaling pathway is the identity of extracellular signals that modulate its activity. It has been speculated that this pathway could be involved in interpreting morphogen gradients to regulate tissue size, and therefore the existence of a secreted or membrane ligand has been postulated (Harvey and Tapon, 2007). Our data here suggest that in the epidermis this regulation is, at least partly, mediated by α-catenin and AJs. Based on the massive overgrowth phenotypes obtained by deletion of α-catenin in the skin and the developing brain, Vasioukhin et al. postulated that AJs could act as molecular biosensors of cell density and positioning (Lien et al., 2006a; Lien et al., 2006b). Our data here support and extend this notion and indicate that Yap1, through its interaction with α-catenin is a critical mediator of this ‘crowd control’ molecular circuitry in the epidermis (Fig. 7). In this model, increased cellular density or differentiation, which is sensed by an increased number of AJs, higher calcium concentration (Menon and Elias, 1991), and selective expression of 14-3-3 in the suprabasal layers, limits SC expansion by inactivating Yap1. Low basal cell density, as in a growing embryo or after wounding, would translate into nuclear Yap1 and proliferation. When this molecular network is defective, e.g., by deletion of α-catenin, inactivation of 14-3-3 or activation of Yap1, hyperproliferation and tumors can arise. Further elucidation of the transcriptional program controlled by Yap1 and identification of modulators of its interaction with α-catenin, including the kinase that mediates Yap1 inactivation in keratinocytes, will likely provide insights into the mechanisms that underlie SC maintenance, tissue expansion, and tumorigenesis.
EXPERIMENTAL PROCEDURES
Generation of gene targeted mice
For Generation of cKO mice, a targeting strategy was designed to allow Cre-mediated deletion of exon 1 and 2 of Yap1. Progeny carrying the targeted Yap1 allele were bred to K14-Cre mice. To generate the mutated Yap1S79A allele a point mutation was introduced by site-directed PCR mutagenesis in the mYap1 targeting construct substituting a serine at amino acid position 79 (TCC) for an alanine (GCG). Mice were generated in the same way as described for the Yap1fl/fl mice. Mice carrying the Yap1S79A allele were bred to K14-Cre Yap1fl/+ mice to generate K14 Cre Yap1S79A/fl mice.
Cell isolation, in vitro culture and clonogenic assays
Primary keratinocytes isolated from the epidermis of E18.5 control and transgenic mice were cultured on mitomycin-C-treated 3T3 feeder cells in FAD medium. For clonogenic assays colonies of primary mouse keratinocytes were, fixed and stained with 1% rhodamine B (Sigma) nine days after initial plating.
Skin engraftments, and barrier function assay
Full thickness skins of E18.5 K14-Cre Yap1fl/fl and littermate Ctr mice were removed from torsos and spread on a sterile petri dish and briefly stored at 4°C. During this time, each skin graft recipient site was prepared by removal of a patch of full thickness skin on the back of an anesthetized Foxn1nu/Foxn1nu mouse. For skin barrier assays newborn mice were rinsed in PBS and successively dehydrated in methanol for 1 min each. Mice were then rehydrated with PBS and stained in 0.1% toluidine blue in PBS, destained with PBS for 20 min, and then photographed.
Mass Spectrometry and Proteomic Analysis
Approximately 4 mg of pre-cleared high density HaCaT cells were used for Yap1 immunoprecipitation. Immunoprecipitates were loaded onto SDS-PAGE and stained with colloidal coomassie. Mass spectrometry analysis was performed at the proteomics facility at Children’s Hospital Boston.
Dephosphorylation Assay
Catalytically active recombinant PP2A (PP2Ac) was purchased from Millipore. Recombinant proteins were incubated in the presence of varying concentrations of PP2Ac. For PP2Ac and 14-3-3 competition assays, Yap1, α-catenin and 14-3-3 were pre-incubated in 50 mM Tris (pH 7.4), 150 mM NaCl, 5 mM MgCl2, 0.02% Triton X-100 for 1 hour prior to PP2Ac addition.
Supplementary Material
ACKNOWLEDGEMENTS
We thank K. Shrestha and S. Zaghlul for their technical assistance; L. Zon and A. von Giese and members of the Camargo lab for critical discussion; and members of the Gregory laboratory for advice on mass-spectrometry experiments. This work was supported by grants from the Stand Up to Cancer-AACR initiative, NIH grant R01 CA131426 and funds from the Whitehead Institute Fellows Program. FDC is a V Foundation Scholar and Pew Scholar in the Biomedical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18:309–316. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Belteki G, Haigh J, Kabacs N, Haigh K, Sison K, Costantini F, Whitsett J, Quaggin SE, Nagy A. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 2005;33:e51. doi: 10.1093/nar/gni051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin JM, Nelson WJ. Bench to bedside and back again: molecular mechanisms of alpha-catenin function and roles in tumorigenesis. Semin Cancer Biol. 2008;18:53–64. doi: 10.1016/j.semcancer.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, Halder G. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci U S A. 2010;107:15810–15815. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CW, Kanies C, Kim KW, Fang WB, Parkhurst C, Xie M, Henry T, Yang E. Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Mol Cell Biol. 2003;23:6350–6362. doi: 10.1128/MCB.23.18.6350-6362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Thakur M, Feng Y, Jagannathan R, Seppa MJ, Skeath JB, Longmore GD. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr Biol. 2010;20:657–662. doi: 10.1016/j.cub.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Dellambra E, Golisano O, Bondanza S, Siviero E, Lacal P, Molinari M, D’Atri S, De Luca M. Downregulation of 14-3-3sigma prevents clonal evolution and leads to immortalization of primary human keratinocytes. J Cell Biol. 2000;149:1117–1130. doi: 10.1083/jcb.149.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depaepe V, Suarez-Gonzalez N, Dufour A, Passante L, Gorski JA, Jones KR, Ledent C, Vanderhaeghen P. Ephrin signalling controls brain size by regulating apoptosis of neural progenitors. Nature. 2005;435:1244–1250. doi: 10.1038/nature03651. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden AB, Hebert AM, Schneeberger EE, McClatchey AI. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell. 2010;19:727–739. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20:573–581. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Hariharan IK. Growth regulation: a beginning for the hippo pathway. Curr Biol. 2006;16:R1037–R1039. doi: 10.1016/j.cub.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- Herron BJ, Liddell RA, Parker A, Grant S, Kinne J, Fisher JK, Siracusa LD. A mutation in stratifin is responsible for the repeated epilation (Er) phenotype in mice. Nat Genet. 2005;37:1210–1212. doi: 10.1038/ng1652. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science. 2005;309:1256–1259. doi: 10.1126/science.1112521. [DOI] [PubMed] [Google Scholar]
- Kobielak A, Fuchs E. Links between alpha-catenin, NF-kappaB, and squamous cell carcinoma in skin. Proc Natl Acad Sci U S A. 2006;103:2322–2327. doi: 10.1073/pnas.0510422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Li Q, Lu Q, Estepa G, Verma IM. Identification of 14-3-3sigma mutation causing cutaneous abnormality in repeated-epilation mutant mouse. Proc Natl Acad Sci U S A. 2005;102:15977–15982. doi: 10.1073/pnas.0508310102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V. alphaE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science. 2006a;311:1609–1612. doi: 10.1126/science.1121449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Klezovitch O, Vasioukhin V. Cadherin-catenin proteins in vertebrate development. Curr Opin Cell Biol. 2006b;18:499–506. doi: 10.1016/j.ceb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, Wu S, Pan D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci U S A. 2010;107:10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Potente M, Janssens V, Vertommen D, Twizere JC, Rider MH, Goris J, Dimmeler S, Kettmann R, Dequiedt F. Protein phosphatase 2A controls the activity of histone deacetylase 7 during T cell apoptosis and angiogenesis. Proc Natl Acad Sci U S A. 2008;105:4727–4732. doi: 10.1073/pnas.0708455105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon GK, Elias PM. Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch Dermatol. 1991;127:57–63. [PubMed] [Google Scholar]
- Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, Pasolli HA, Fuchs E. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat Genet. 2009;41:1068–1075. doi: 10.1038/ng.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–1088. doi: 10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Mazack V, Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP) J Biol Chem. 2008;283:27534–27546. doi: 10.1074/jbc.M804380200. [DOI] [PubMed] [Google Scholar]
- Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro PS, Josue F, Wepf A, Wehr MC, Rinner O, Kelly G, Tapon N, Gstaiger M. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol Cell. 2010;39:521–534. doi: 10.1016/j.molcel.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol. 2010;20:582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo M, Pinto F, Crum CP, McKeon F. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- Stepniak E, Radice GL, Vasioukhin V. Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb Perspect Biol. 2009;1 doi: 10.1101/cshperspect.a002949. a002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle CL, Pasolli HA, Stokes N, Fuchs E. New insights into cadherin function in epidermal sheet formation and maintenance of tissue integrity. Proc Natl Acad Sci U S A. 2008;105:15405–15410. doi: 10.1073/pnas.0807374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, Rossant J, Wrana JL. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19:831–844. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.