Abstract
Background:
Integrated Management of Childhood Illness (IMCI) is a cost- effective strategy that improves the quality of care through the use of evidence- based management protocols for the most common causes of childhood death and illness. Evidence- based clinical guidelines are critical to promoting rational use of medicines. Despite the large number of studies that assessed process and outcome of care delivered to children utilizing IMCI protocol, there is a scarcity of studies that assessed the effect of adopting IMCI on the drug use.
Aims:
To examine the impact of adopting IMCI guidelines on drug use at one of the primary health care (PHC) centers, Alexandria, Egypt.
Settings and Design:
Retrospective cohort study, conducted in clinic “A” not adopting IMCI guidelines and clinic “B” adopting IMCI guidelines at one of the PHC centers in Alexandria, Egypt for the period from January-- end of June 2010.
Materials and Methods:
A data collection sheet was designed to collect the required variables (based on WHO/ INRUD selected drug use indicators) from the medical records of children under five years.
Statistical Analysis Used:
SPSS version 16 was used. Percentages, means, and standard deviations were measured. Chi square, t, and Fisher's exact tests were applied.
Results:
Correct drug choice, dose, dosage form, route of administration were significantly higher in the clinic adopting IMCI {clinic B} (89.3%, 87.3%, 91.3%, and 91.3%, respectively) than in the clinic not adopting it {clinic A} (78% each). Non pharmacological remedies prescribed were significantly higher in clinic B than A (64.7% vs 4.6%). Average no of drugs/ encounter was lower in clinic B than A (0.93± 0.2 vs 1.37 ± 0.6) and the difference between clinics was statistically significant. Difference between clinics regarding percentages of drugs prescribed by generic name, antibiotics prescribed, drugs prescribed from essential drug list, and drugs prescribed out of stock was significant.
Conclusion:
Adopting IMCI strategy improved prescribing performance and treatment regimen.
Keywords: IMCI, improving drug use, prescribing indicators, technical quality of treatment regimen
INTRODUCTION
The Integrated Management of Childhood Illnesses (IMCI) is a cost -effective strategy that improves the quality of care through the use of evidence-based management protocols for the most common causes of childhood death and illness. Standard case management discourages inappropriate care such as the overuse of antibiotics and other drugs. IMCI contributes to approaches to improve the basic functions of drug management-selection, procurement, distribution and use-leading to sustainable systems that makes essential drugs available at all levels.[1] Evidence presented at the International Conference on Irrational Use of Medicine (ICIUM) 2004 suggests that this has led to an increased proportion of children needing antibiotics being prescribed them correctly, a large increase in the proportion of caregivers advised how to administer oral antibiotics and reduction in misuse of antibiotics.[2]
Rational drug use is well recognized as an important part of health policy. The term rational drug use is in this overview limited to the medical therapeutic view accepted at the WHO conference of 1985 in Nairobi: Rational use of drugs requires that patients receive medications appropriate to their clinical needs, in doses that meet their own requirements, for an adequate period of time, and at the lowest cost to them and their community.[3]
Treatment with medicines is one of the most cost- effective interventions known, and the proportion of national health budget spent on medicines ranges between 10% and 20% in developed countries, and between 20% and 40% in developing countries.[4,5]
Three out of 8 Millennium Development Goals (MDGs), 8 of 16 MDGs targets and 18 of 48 MDGs indicators are health related. Most health targets cannot be reached without better access to safe, effective and affordable medicines.[6]
The aim of the WHO's medicines strategy for 2004--2007 was that people everywhere have access to essential medicines they need; that the medicines are safe, effective and of good quality; and that the medicines are prescribed and used rationally.[7] Evidence presented in the ICIUM 2004 made it clear that misuse of medicines continues to be widespread and has serious health and economic implications especially in resource poor settings.[8] The WHO estimates that more than half of all medicines are prescribed, dispensed or sold inappropriately, and that half of all patients fail to take them correctly, resulting in wastage of scarce resources and widespread health hazards.[9]
Among WHO recommendations on how to promote rational use of medicines, it encourages formulating and using evidence based clinical guidelines for training, supervision and supporting critical decision making about medicines. The guidelines take an evidence based, syndromic approach to case management that supports rational, effective, and affordable use of drugs and diagnostic tools.[10]
Treatment of childhood illnesses, especially infectious diseases, constitutes a large share of medicines use. Many problems in pediatric use of medicines have been identified.[11]
In Egypt, the Ministry of Health and Population adopted the IMCI strategy in 1997 in the context of its efforts to integrate vertical program activities. In March 1998, the IMCI working group developed a detailed national plan of activities addressing all components of the IMCI strategy. IMCI has been included in the basic package of services to be delivered at the Primary Health Care (PHC) facilities, an initiative supported by WHO, UNICEF, USAID, and the World Bank.[1]
To assess the impact of interventions targeting drug management and use, standardized indicators for monitoring and evaluating the quality of drug management and use have been recommended in the ICIUM 2004 for use in PHC.[11]
In an attempt to assess the impact of adopting IMCI on drug use, the following study has been carried out in one PHC center in Alexandria (Egypt), utilizing WHO/INRUD prescribing indicators for PHC facilities.[12]
MATERIALS AND METHODS
Study design: Retrospective cohort study between two clinics (one adopting IMCI guidelines and the second not) at one PHC center in Alexandria, Egypt.
Study setting: Two clinics (clinic “A” not adopting IMCI guidelines and clinic “B” adopting IMCI guidelines) at one PHC center in Alexandria, Egypt.
Study subjects: Medical records of children under five years who attended the clinics for the period from January to end of June 2010 were investigated. Medical records were reviewed to collect patient's demographic data as well as the drug use data of the last encounter. Medicines prescribed at the last encounter were thoroughly investigated to obtain the required data on prescribing indicators and treatment regimen (drug choice, dose, dosage form, route of administration, and documentation of health education instructions and follow- up). Data collection at both clinics was carried out after implementation of IMCI protocol at clinic B.
Sample size: Assuming that after implementation of IMCI the drug choice was correct in 77.5% of children's records while among other groups (those not adopting IMCI), the drug choice was correct in 62.3% of the records.[13] To be able to reject the null hypothesis of equal proportion with power 80% and α = 0.05, the minimum required sample size for each group is 142. The number of medical records for each group was increased to 150. Selection of Zambia data was used due to lack of data available from Egypt. Medical records were selected using systematic random sampling technique.
Inclusion criteria
Last encounter for each child and its prescription were considered.
Children under five years.
Medical records that include prescription of the last encounter.
Data collection tool and technique
A data collection sheet was designed to collect the required variables (based on WHO selected drug use indicators).[12] Data was extracted from the medical records by a physician. A peer reviewer, who is a physician, IMCI trainer and certified from IMCI training center affiliated to Egyptian Ministry of Health, was asked to evaluate the adequacy of drug choice, dose, dosage form, and route of administration for all cases. For determining rational drug use, treatment regimen and prescribing performance of the last encounter were assessed. Treatment regimen was determined through assessment of correct drug choice, dose, dosage form, route of administration; and documentation of health education instructions and follow-up visits. For determining the prescribing performance, prescribing indicators were calculated as follows:
Average number of drugs per encounter: Calculated by dividing the total number of different drug products prescribed, by the number of encounters surveyed. It is not relevant whether the patient actually received the drugs.
Percentage of drugs prescribed by generic name: Calculated by dividing the number of drugs prescribed by generic name by the total number of drugs prescribed, multiplied by 100.
Percentage of antibiotics prescribed.
-
Percentage of injections prescribed.
Percentages, calculated by dividing the number of antibiotics or injections prescribed, by the total number of drugs prescribed, multiplied by 100.
Percentage of drugs prescribed from essential drugs list or formulary: Calculated by dividing the number of products prescribed which are listed on the essential drugs list or local formulary by the total number of products prescribed, multiplied by 100.
Percentage of drugs prescribed out of stock: Calculated by dividing the number of drugs prescribed out of stock, by the total number of drugs prescribed, multiplied by 100.
Percentage of antibiotics prescribed for cough or cold: Calculated by dividing the number of antibiotics prescribed for cough or cold, by the total number of antibiotics prescribed, multiplied by 100.
Percentage of non- pharmacological remedies prescribed: Calculated by dividing the number of non-pharmacological remedies prescribed, by the total number of drugs prescribed, multiplied by 100.
Statistical analysis
SPSS version 16 was used for data entry and analysis. Percentages, means, and standard deviations (SDs) were calculated. Difference between the two clinics was measured using t, Chi square, and Fisher's exact tests. Level of significance at 5% was used.
Ethical consideration: Formal approval from the Ministry of Health was taken before conducting the research. Confidentiality of the data collected from medical records was considered.
RESULTS
The results of the study are organized in the following categories:
Table 1 illustrates distribution of the study sample according to average number of drugs prescribed / last encounter in two clinics at one PHC Center, Alexandria, Egypt (2010): Only 6% of children attending clinic A had no drugs prescribed at all, while this category constituted 36.7% of children attending clinic B with a statistically significant difference (P = 0.000. Children with three drugs prescriptions constituted a minority in both clinics (1.3% in both). The average number of drugs in clinic B was lower than that in clinic A (0.93± 0.2 vs 1.37 ± 0.6, respectively).
Table 1.
Distribution of the study sample according to average number of drugs prescribed/ last encounter in two clinics at one PHC center, Alexandria, Egypt (2010)
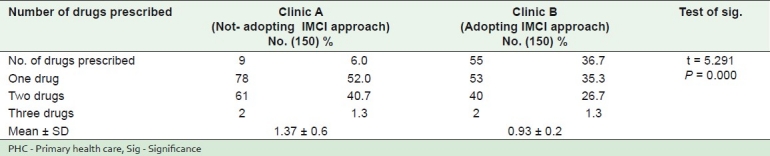
Table 2 shows distribution of the study sample according to treatment regimen provided in two clinics at one PHC Center, Alexandria, Egypt (2010): All the items assessing the treatment regimen provided were significantly better in clinic B than A.
Table 2.
Distribution of the study sample according to the treatment regimen provided in two clinics at one PHC center, Alexandria, Egypt (2010)
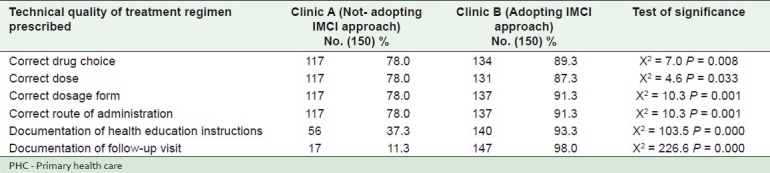
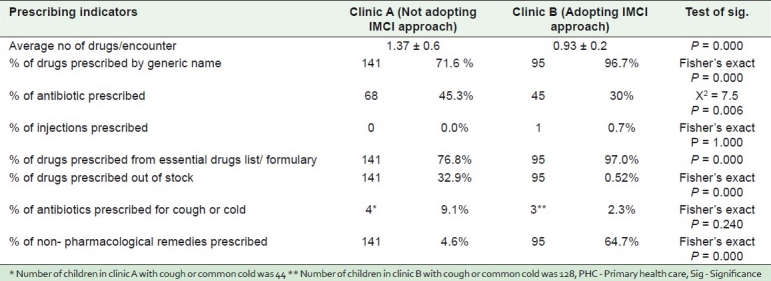
Table 3 reveals distribution of the study sample according to prescribing indicators in two clinics at one PHC Center, Alexandria, Egypt (2010): All prescribing indicators were significantly better in clinic B than A except the percentage of injections and percentage of antibiotics prescribed for cough or common cold.
Table 3.
Distribution of the study sample according to prescribing indicators in two clinics at one PHC center, Alexandria, Egypt (2010)
DISCUSSION
The first step to improving medicines use is to measure how medicines are used and this forms the basis of advocacy for change.[14] The present study utilized WHO/INRUD prescribing indicators to measure the difference between two clinics: One not adopting IMCI (clinic A), and the other adopting it (clinic B), in an attempt to demonstrate the positive effect of adopting IMCI evidence based guidelines on improving drug use.
A considerable difference was found between the two clinics concerning the number of drugs prescribed / the last encounter, where more than one third of prescriptions in clinic B had no drugs prescribed at all, in comparison to 6% only in clinic A [Table 1]. This may be explained by the fact that many health problems require no medication but physicians prescribe medicine to inappropriately satisfy patients. Rational prescribing is advocated to avoid wastage of medicines and avoid possible/ adverse drug effects in patients. Moreover unnecessary medication to patients has cost implications for national health services. After adopting IMCI strategy, there was a norm established by the Egyptian health services for maximum number of drugs/ patient from a PHC center to be three drugs. This could explain lower average number of drugs in clinic B (adopting IMCI) than clinic A (not adopting IMCI), and the difference is significant [Table 3]. Similar results were reported in Iran, after an outcome based education on rational prescribing, the involved General Practitioners significantly reduced the total number of prescribed drugs.[15] Choosing the correct drug, giving the correct dose and dosage form, and prescribing the correct route of administration are other indicators on the treatment that were significantly better in clinic B than clinic A [Table 2]. This may suggest the benefit of using IMCI guidelines. More or less typical and comparable results were reported in Zambia, where prescribers (after a continuing education intervention) improved more than in the control health centers in choosing the correct drug, with the correct dose.[13]
Providing health education to parents or caregivers is an important element of IMCI.[10] In our study, percentage of documentation of health education instructions was higher in clinic B than clinic A [Table 2]. This may be attributed to role of IMCI in improving health education given to parents coming for curative care of their child. Parents are very receptive to advice from health workers at this stage to prevent future health problems in their children. A critical example for the need of educating caregivers, in Africa, where approximately 80% of childhood deaths occur at home, before the child has any contact with a health facility.[16]
It is also interesting to note that percentage of antibiotics prescribed in clinic A was higher than that prescribed in clinic B [Table 3]. Over-use of antibiotics results in increase of antibiotic resistance which is one of the problems under the irrational use of medicine.[17] However, it is difficult to judge in such a situation whether antibiotics were prescribed irrationally as this may be due to differences in patient population in terms of diseases. Experience of Oman showed that prescriptions containing antibiotics were reduced from 60% in 1995 to 38% in 2006 (after adopting a national approach on rational use of drugs).[18]
Injections were prescribed in only 0.7% of cases in clinic B [Table 3]. This may be a benefit of adopting IMCI because the cost of injection therapy is always higher than that of oral therapy. Also, blood-borne diseases such as hepatitis, HIV/AIDS can be transmitted by the use of non-sterile injections.[19]
In the present study, a significant difference in favor of adopting IMCI was also shown in the percentage of drugs prescribed by generic name, drugs prescribed from essential drugs lists, and drugs prescribed out of stock [Table 3]. Prescribing drugs by generic name is a safety precaution for patients as it gives clear identification, and enables easy information exchange and better communication between health care providers; and prescribing from essential drug list issued by WHO means rational prescribing: Drugs from the list are older drugs, already tested in practice, with established clinical use and lower cost than newer drugs.[20] Furthermore, in Ethiopia, the introduction of Essential Drugs List resulted in a significant decrease of non essential prescribing.[21]
Percentage of drugs prescribed out of stock was greatly lower in clinic B than A [Table 3]. This implies the benefit of using IMCI strategy as prescribing out of stock medicines may be due to lack of up to date knowledge of physicians about the essential drugs in the PHC pharmacy, a situation that is not good. It also implies that there is perhaps shortage of drugs from the source or there is over prescribing by physicians not adopting IMCI guidelines which depletes the pharmacy of the needed drugs soon enough. This result is in line with the randomized controlled trial in Zambia, where the proportion of prescribed drugs that were out of stock decreased from 16.4% to 9% after the intervention.[13]
CONCLUSION AND RECOMMENDATION
Drug use is the end of the therapeutic consultation. Health professionals have a responsibility to ensure that the right drug is prescribed, dispensed and taken. Indicators exist to measure drug use and change practices. Accordingly the following is recommended:
PHC physician's training should include how to utilize prescribing indicators in order to ensure rational and economic prescribing.
Formulation and use of evidence based clinical guidelines is crucially recommended for supporting rational utilization of drugs.
Footnotes
Source of Support: Nil,
Conflict of Interest: Nil.
REFERENCES
- 1.Geneva: WHO; 1999. WHO. IMCI information package. Integrated Management of Childhood Illness and Health Sector Reform. UNICEF; pp. 1–4. WHO/CHS/CAS/98.1L. REV1. [Google Scholar]
- 2.Norris P. Geneva: WHO; 2007. Interventions to improve antimicrobial use: Evidence from ICIUM 2004; p. 1. [Google Scholar]
- 3.West Hartford, Connecticut, USA: Kumarian Press; 1997. Management Sciences for Health. Managing drug supply. [Google Scholar]
- 4.Council of churches. Promoting rational use of medicine. Contact. 2006;183:1–19. [Google Scholar]
- 5.Le Grand A, Hogezeil HV, Haaijer-Ruskamp FM. Intervention research in the rational use of drugs: A review. Health Policy Plan. 1999;14:89–102. doi: 10.1093/heapol/14.2.89. [DOI] [PubMed] [Google Scholar]
- 6.Geneva: WHO; 2009. WHO. Continuity and change. Implementing the third WHO medicines strategy 2008-2013; p. 8. WHO/EMP/2009.1. [Google Scholar]
- 7.WHO. Progress in the rational use of medicines. Report by the secretariat. Sixtieth World Health Assembly. Provisional agenda item 12.17. A16/24. 2007 Mar 22; [Google Scholar]
- 8.Second International Conference on Improving Use of Medicines. Chiang Mai- Thailand: 2004. March, April. ICIUM. Policies and programmes to improve use of medicines. Recommendations from ICIUM 2004. [Google Scholar]
- 9.Geneva: WHO; 2004. WHO. WHO medicines strategy. Countries at the core. 2004-2007; p. 4. WHO/EDM/2004.5. [Google Scholar]
- 10.Geneva: WHO; 2001. WHO. Model chapter for textbooks. IMCI. Integrated Management of Childhood Illness. Department of child and adolescent health and development. UNICEF; p. 2. WHO/FCH/CAH/00.40. [Google Scholar]
- 11.Medicines use in children. ICIUM 2004 theme summary. [Last cited on 2010 Nov 06]. Available from: http://www.icium.org/icium2004/recommendations.asp .
- 12.Geneva: WHO; 1993. WHO. How to investigate drug use in health facilities. Selected drug use indicators; pp. 9–10. WHO/DAP/93.1. [Google Scholar]
- 13.Bexell A, Lwand E, Hofsten BV, Tembo S, Eriksson B, Diwan VK. Improving drug use through continuing education: A randomized controlled trial in Zambia. Clin Epidemiol. 1996;49:355–7. doi: 10.1016/0895-4356(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 14.Geneva: WHO; 2009. WHO. Medicines use in primary care in developing and transitional countries. Fact book summarizing results from studies reported between 1990- 2006. WHO/EMP/MAR/20093. [Google Scholar]
- 15.Esmaily HM, Silver I, Shiva S, Gargani A, Maleki-Dejazi N, Al-Maniri A, et al. Can rational prescribing be improved by an outcome based educational approach.: A randomized trial completed in Iran? J Contin Educ Health Prof. 2010;30:11–8. doi: 10.1002/chp.20051. [DOI] [PubMed] [Google Scholar]
- 16.Oluwole D, Mason E, Costello A. Management of childhood illness in Africa: Early evaluations show promising results. BMJ. 2000;320:594–5. doi: 10.1136/bmj.320.7235.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geneva: WHO; 2005. WHO. Irrational drug use causing rise of anti-microbial resistance. Who report 2005. [Google Scholar]
- 18.Jaffer B. Geneva: World Health Assembly; 2007. National approach to promote rational use of medicines. The Omani experience. WHO/NGO technical briefing seminar. [Google Scholar]
- 19.Geneva: WHO; 2010. [Last cited 2011 June 8]. WHO. Rational use of medicines. Available from: http://www.who.int/mediacentre/factsheets/fs338/en/index.html . [Google Scholar]
- 20.Geneva: WHO; 1997. WHO. The use of essential drugs. Seventh report of the WHO Expert Committee (including the revised model list of essential drugs) p. 74. [Google Scholar]
- 21.Lindtjorn B. Essential drug list in a rural hospital. Does it have any influence on drug prescription? Trop Doct. 1987;17:151–5. doi: 10.1177/004947558701700404. [DOI] [PubMed] [Google Scholar]


