Abstract
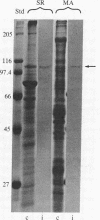
Monospecific antibodies to the calcium transport enzyme (alpha-Ca pump) inhibit mitosis when microinjected into sand dollar embryos. Immunoglobulins were raised against the calcium transport enzyme (Ca pump) of sarcoplasmic reticulum (SR) from rat skeletal muscle and guinea pig ileum smooth muscle. Specific antibodies were further isolated from IgG fractions by using electrophoretically purified SR Ca-pump protein as the immobilized ligand for immunoaffinity chromatography. ELISA demonstrated that common antigenic determinants are shared by SR, SR Ca pump (of rat skeletal and guinea pig ileum smooth muscle), and isolated membrane containing "native" mitotic apparatus (MA). Preimmune sera gave negative results in identical control assays. Triton X-100 extraction of MA removes the Ca-pump antigen. SR Ca pump and the MA Ca pump have nearly identical molecular masses as determined by NaDodSO4/PAGE. These alpha-SR Ca-pump IgGs inhibit ATP-dependent Ca2+ sequestration by purified SR and MA membranes. Indirect immunofluorescence of isolated native MA demonstrated coincident localization of the MA Ca pump, sequestered calcium, and membrane vesicles. Fluorescent foci were regionally concentrated within the volumes of the asters and spindle. Microinjection of the anti-Ca-pump IgGs into one of two sister blastomeres at second metaphase resulted in mitotic arrest of the injected cell accompanied by a rapid loss of spindle birefringence. Karyomeres formed and fused to form nuclei either at the site of the metaphase plate or at the position the chromosomes occupied during anaphase A. The cleavage furrow did not develop in the injected cell, while the sister and neighbor cells continued normal mitotic cycling. Injection later in mitosis yielded cells with two nuclei whose cleavage furrow relaxed completely. Routine control injections of boiled immune IgG, preimmune IgG, Wesson oil, buffer, or goat anti-rabbit IgG did not affect mitosis, birefringence of the MA, or cleavage furrow activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Control of cell division: a unifying hypothesis. J Cyclic Nucleotide Res. 1975;1(5):305–320. [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J. The different roles of serum and calcium in the control of proliferation of BALB/c 3T3 mouse cells. In Vitro. 1976 Feb;12(2):120–123. doi: 10.1007/BF02796358. [DOI] [PubMed] [Google Scholar]
- Chaturvedi A. K., Fox S., Rama Sastry B. V., Landon E. J. Uptake and release of calcium by microsomes of nonvascular smooth muscle. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1132–1139. doi: 10.1016/0006-291x(79)91526-2. [DOI] [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Characterization of an integral membrane glycoprotein associated with the microfilaments of pig intestinal microvilli. EMBO J. 1983;2(3):469–475. doi: 10.1002/j.1460-2075.1983.tb01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. The cytoskeleton of intestinal microvilli contains two polypeptides immunologically related to proteins of striated muscle. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):881–892. doi: 10.1101/sqb.1982.046.01.082. [DOI] [PubMed] [Google Scholar]
- EBASHI S. A granule-bound relaxation factor in skeletal muscle. Arch Biochem Biophys. 1958 Aug;76(2):410–423. doi: 10.1016/0003-9861(58)90166-8. [DOI] [PubMed] [Google Scholar]
- HARRIS P. Electron microscope study of mitosis in sea urchin blastomeres. J Biophys Biochem Cytol. 1961 Nov;11:419–431. doi: 10.1083/jcb.11.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS P. Some structural and functional aspects of the mitotic apparatus in sea urchin embryos. J Cell Biol. 1962 Sep;14:475–487. doi: 10.1083/jcb.14.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. The double array of filaments in cross-striated muscle. J Biophys Biochem Cytol. 1957 Sep 25;3(5):631–648. doi: 10.1083/jcb.3.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. The role of membranes in the ogranization of the mitotic apparatus. Exp Cell Res. 1975 Sep;94(2):409–425. doi: 10.1016/0014-4827(75)90507-8. [DOI] [PubMed] [Google Scholar]
- Hepler P. K. Membranes in the mitotic apparatus of barley cells. J Cell Biol. 1980 Aug;86(2):490–499. doi: 10.1083/jcb.86.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto Y. A method of microinjection. Exp Cell Res. 1974 Aug;87(2):403–406. doi: 10.1016/0014-4827(74)90503-5. [DOI] [PubMed] [Google Scholar]
- Inoué S. Cell division and the mitotic spindle. J Cell Biol. 1981 Dec;91(3 Pt 2):131s–147s. doi: 10.1083/jcb.91.3.131s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Timmen U., Dermietzel R., Frixen U., Leibstein A., Traub O., Willecke K. Immunocytochemical localization of the gap junction 26 K protein in mouse liver plasma membranes. EMBO J. 1983;2(3):295–302. doi: 10.1002/j.1460-2075.1983.tb01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart D. P. Microinjection of echinoderm eggs: apparatus and procedures. Methods Cell Biol. 1982;25(Pt B):13–31. doi: 10.1016/s0091-679x(08)61418-1. [DOI] [PubMed] [Google Scholar]
- Kiehart D. P. Studies on the in vivo sensitivity of spindle microtubules to calcium ions and evidence for a vesicular calcium-sequestering system. J Cell Biol. 1981 Mar;88(3):604–617. doi: 10.1083/jcb.88.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Luckasen J. R., White J. G., Kersey J. H. Mitogenic properties of a calcium ionophore, A23187. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5088–5090. doi: 10.1073/pnas.71.12.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSCATELLO U., ANDERSSON-CEDERGREN E., AZZONE G. F., von der DECKEN The sarcotubular system of frog skeletal muscle. A morphological and biochemical study. J Biophys Biochem Cytol. 1961 Aug;10(4):201–218. doi: 10.1083/jcb.10.4.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D. H. Isolation of proteins of the sarcoplasmic reticulum. Methods Enzymol. 1974;32:291–302. doi: 10.1016/0076-6879(74)32030-7. [DOI] [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- Martonosi A., Halpin R. A. Sarcoplasmic reticulum. X. The protein composition of sarcoplasmic reticulum membranes. Arch Biochem Biophys. 1971 May;144(1):66–77. doi: 10.1016/0003-9861(71)90455-3. [DOI] [PubMed] [Google Scholar]
- McFarland B. H., Inesi G. Solubilization of sarcoplasmic reticulum with Triton X-100. Arch Biochem Biophys. 1971 Aug;145(2):456–464. doi: 10.1016/s0003-9861(71)80005-x. [DOI] [PubMed] [Google Scholar]
- Meissner G., Conner G. E., Fleischer S. Isolation of sarcoplasmic reticulum by zonal centrifugation and purification of Ca 2+ -pump and Ca 2+ -binding proteins. Biochim Biophys Acta. 1973 Mar 16;298(2):246–269. doi: 10.1016/0005-2736(73)90355-6. [DOI] [PubMed] [Google Scholar]
- Meissner G. Isolation and characterization of two types of sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1975 Apr 21;389(1):51–68. doi: 10.1016/0005-2736(75)90385-5. [DOI] [PubMed] [Google Scholar]
- PORTER K. R., MACHADO R. D. Studies on the endoplasmic reticulum. IV. Its form and distribution during mitosis in cells of onion root tip. J Biophys Biochem Cytol. 1960 Feb;7:167–180. doi: 10.1083/jcb.7.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER K. R., PALADE G. E. Studies on the endoplasmic reticulum. III. Its form and distribution in striated muscle cells. J Biophys Biochem Cytol. 1957 Mar 25;3(2):269–300. doi: 10.1083/jcb.3.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H. Die Bindung des Erschlaffungsfaktors von Marsh an die Muskelgrana. Biochim Biophys Acta. 1957 Nov;26(2):373–377. doi: 10.1016/0006-3002(57)90019-7. [DOI] [PubMed] [Google Scholar]
- Paul D., Ristow H. J. Cell cycle control by Ca++-ions in mouse 3T3 cells and in transformed 3T3 cells. J Cell Physiol. 1979 Jan;98(1):31–39. doi: 10.1002/jcp.1040980105. [DOI] [PubMed] [Google Scholar]
- Rixon R. H., Whitfield J. F. The control of liver regeneration by parathyroid hormone and calcium. J Cell Physiol. 1975 Dec;87(2):147–155. doi: 10.1002/jcp.1040870203. [DOI] [PubMed] [Google Scholar]
- Salmon E. D., Segall R. R. Calcium-labile mitotic spindles isolated from sea urchin eggs (Lytechinus variegatus). J Cell Biol. 1980 Aug;86(2):355–365. doi: 10.1083/jcb.86.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A. Measurements of cation transport with metallochromic indicators. Methods Enzymol. 1979;56:301–338. doi: 10.1016/0076-6879(79)56030-3. [DOI] [PubMed] [Google Scholar]
- Silver R. B., Cole R. D., Cande W. Z. Isolation of mitotic apparatus containing vesicles with calcium sequestration activity. Cell. 1980 Feb;19(2):505–516. doi: 10.1016/0092-8674(80)90525-5. [DOI] [PubMed] [Google Scholar]
- Silver R. B., Saft M. S., Taylor A. R., Cole R. D. Identification of nonmitochondrial creatine kinase enzymatic activity in isolated sea urchin mitotic apparatus. J Biol Chem. 1983 Nov 10;258(21):13287–13291. [PubMed] [Google Scholar]
- Swierenga S. H., MacManus J. P., Whitfield J. F. Regulation by calcium of the proliferation of heart cells from young adult rats. In Vitro. 1976 Jan;12(1):31–36. doi: 10.1007/BF02832790. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Dedman J. R., Brinkley B. R., Means A. R. Calcium-dependent regulator protein: localization in mitotic apparatus of eukaryotic cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1867–1871. doi: 10.1073/pnas.75.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J. F., Boynton A. L., MacManus J. P., Sikorska M., Tsang B. K. The regulation of cell proliferation by calcium and cyclic AMP. Mol Cell Biochem. 1979 Nov 1;27(3):155–179. doi: 10.1007/BF00215364. [DOI] [PubMed] [Google Scholar]
- Wick S. M., Hepler P. K. Localization of Ca++-containing antimonate precipitates during mitosis. J Cell Biol. 1980 Aug;86(2):500–513. doi: 10.1083/jcb.86.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]