Abstract
Aim:
To determine the diagnostic reliability of 18F-FDOPA, 13N-Ammonia and 18F-FDG PET/CT in primary brain tumors and comparison with magnetic resonance imaging (MRI).
Materials and Methods:
A total of 23 patients, 8 preoperative and 15 postoperative, undergoing evaluation for primary brain tumors were included in this study. Of them, 9/15 were operated for high grade gliomas (7/9 astrocytomas and 2/9 oligodendrogliomas) and 6/15 for low grade gliomas (5/6 astrocytomas and 1/6 oligodendroglioma). After PET study, 2 of 8 preoperative cases were histopathologically proven to be of benign etiology. 3 low grade and 2 high grade postoperative cases were disease free on 6 months follow-up. Tracer uptake was quantified by standardized uptake values (SUVmax) and the SUV max ratio of tumor to normal symmetrical area of contra lateral hemisphere (T/N). 18F-FDOPA uptake was also quantified by SUVmax ratio of tumor to striatum (T/S). Conventional MR studies were done in all patients.
Results:
Both high-grade and low-grade tumors were well visualized with 18F-FDOPA PET. Sensitivity of 18F-FDOPA PET was substantially higher (6/6 preoperative, 3/3 low grade postoperative, 7/7 high grade postoperative) than with 18F-FDG (3/6 preoperative, 1/3 low grade postoperative, 3/7 high grade postoperative) and 13N-Ammonia PET (2/6 preoperative, 1/3 low grade postoperative, 1/7 high grade postoperative). FDOPA was equally specific as FDG and Ammonia PET in operated cases but was falsely positive in two preoperative cases. Sensitivity of FDOPA (16/16) was more than MRI (13/16).
Conclusion:
18F-FDG uptake correlates with tumor grade. Though 18F-FDOPA PET cannot distinguish between tumor grade, it is more reliable than 18F-FDG and 13N-Ammonia PET for evaluating brain tumors. 18F-FDOPA PET may prove to be superior to MRI in evaluating recurrence and residual tumor tissue. 13N-Ammonia PET did not show any encouraging results.
Keywords: 13N-Ammonia, 18F-FDG, 18F-FDOPA, brain tumors, MRI, PET, sensitivity
INTRODUCTION
Treatment of brain tumors consists of a combination of surgery, radiotherapy and chemotherapy. Changes occurring in the treated tissue result in a residual necrotic mass. Major challenges faced in brain tumor management are to identify residual tumor or recurrence post treatment, to differentiate this from radiation necrosis and to check for anaplastic transformation in stable low grade tumors. Distinguishing viable residual or recurrent tumor from radiation necrosis has important implications on the patient's management as viable tumor needs early intervention while necrotic tissue is managed symptomatically. CT and MRI may not differentiate necrotic tissue from recurrence/ residual tumor tissue.[1–4] Stereotactic biopsy and concurrent chemo radiotherapy (CCRT) further question MRI reliability. Nuclear medicine techniques can identify residual/ recurrent tumor based on differential metabolism of active tumor. 18F-2Fluoro, 2Deoxy d-Glucose (18F-FDG) has been the conventionally used tracer. FDG PET has a good prognostic value but comes with diagnostic limitations in detection and differentiation of low-grade brain tumors and radiation induced changes.[5] After initial report by Heiss et al, amino acid PET tracers are coming up as an attractive alternative for brain tumor imaging because of the high uptake in tumor and low uptake in normal brain tissue. The best studied amino acid tracer is 11C-methionine. Because of the short half-life of 11C (20 min), 18F-labeled aromatic amino acid analogs have been developed for tumor imaging. Tumor uptake of 3, 4-dihydroxy-6-18F-fluoro-L-phenylalanine (18F-FDOPA) has been reported to be similar to that of 11C-methionine.[6] 13N-NH3 was labeled as a promising tracer for distinguishing radiation necrosis from recurrence in a study by Xiangsong et al.[7] We initiated a study comparing 18F-FDG PET with two such tracers: 13N- Ammonia and 18F-6 Fluoro-L-DOPA (18F-FDOPA) PET in brain tumors.
MATERIALS AND METHODS
Patients
Preoperative and postoperative cases of brain tumors admitted to our Centre were included in the study. Inclusion criteria for operated cases were after three months of surgery and after four months of completion of radiotherapy. Twenty three patients with male to female ratio of 14:8 and mean age of 43.25 ±14.9 years were included in this analysis. Lesions lower than or equal to WHO grade II were low grade and higher than WHO grade II were high-grade tumors. 8/23 were preoperative cases and 15/23 were postoperative cases with suspected recurrence or residual tumor tissue. Of the 15 postoperative cases, 9 were operated for high grade gliomas (7/9 were astrocytomas and 2/9 were oligodendrogliomas) and 6 were operated for low grade gliomas (5/6 were astrocytomas and 1/6 was oligodendroglioma). 5 of 8 preoperative cases were histopathologically proven as astrocytomas (2 low grade and 3 high grade) after PET study. 1 of 8 preoperative was proven to be meningioma. Histopathology of remaining 2 turned out to be of benign etiology (neurocysticercosis and pyogenic abscess); hence these 2 were included in disease free category. 3 of the 6 low grade cases and 2 of the 9 high grade cases were disease free on clinicoradiological follow up. Thus, 7 of 23 cases had no tumor tissue. Ethical Committee clearance was obtained and informed consent taken from the patients. 13N-Ammonia PET, 18F-FDG PET, 18F-FDOPA PET and conventional MR studies were obtained on separate days in all patients. PET/CT studies were performed within 2 weeks of MRI studies.
PET/CT imaging
All patients were fasting for at least 4 hours before the study and advised adequate hydration. This reduced radiation exposure by rapid tracer excretion. The study was performed on a SIEMENS BIOGRAPH II PET/CT scanner. 296-370 MBq F-18 FDG was injected intravenously and serial static scanning was performed for 10 minutes, 1 h (54–72 min) after the injection with the patient supine and head immobilized in a headrest. Similarly, PET scan with 555–740 MBq of 13N-Ammonia and 185-296 MBq of 18F-FDOPA was done on separate days. 10 minute single bed image acquisition was done, 5 minutes after 13N-Ammonia injection and 15 minutes after 18F-FDOPA injection. An initial scout of the head with localizer positioning was followed by the low dose CT acquisition at 110 mA and 120 KV for attenuation correction. PET emission data corrected for photon attenuation, photon scatter, and random coincidences were reconstructed by use of iterative reconstruction with ordered-subset expectation maximization and reformatted into transaxial, coronal and sagittal views. A 3D image and fusion images of PET and CT were obtained.
MRI
MRI sequences were acquired on a 1.5 T scanner. Sequences obtained generally included sagittal T1-weighted (T1W; TR 400-550, TE14, slice thickness 5 mm), axial T1W (TR 400, TE 15, slice thickness 3 mm), T2-weighted (T2W) fast spin-echo (TR 4000, TE 126-130, slice thickness 3 mm) and contrast-enhanced (Gd-diethylenetriaminepentaacetic acid [Gd-DTPA]; 0.1 mmol/kg body weight), enhanced axial, sagittal and coronal T1W images (TR 400, TE 15, slice thickness 3 mm), with a field of view of 24 cm and a matrix size of 256×256. All scans contained at least T1 pre and post-contrast and T2W images.
Image analysis
Standard visual image interpretation was performed independently by 2 nuclear medicine physicians for PET studies and 2 neuroradiologists for MRI. Clinical information was available to the interpreting physicians. For PET, any tracer activity above background levels was considered abnormal. Background was defined as the normal symmetrical area in contralateral hemisphere. For quantitative image analyses, counts in the region of interest (ROI) were normalized to injected dose per patient's body weight by calculation of SUVs. The SUVmax was determined in the tumor (T) ROI drawn over 3 consecutive transaxial slices and then averaged, and T/N ratio was determined by dividing the tumor SUVmax by the SUVmax of the contralateral normal tissue (N). In addition, FDOPA uptake was quantified by T/S ratio by dividing the tumor SUVmax by the SUVmax of the striatum (S). A standard circular ROI of diameter 8 mm was used in each case. T/N ≥ 1.0 was used as cut off for viable tumor tissue for FDG and Ammonia PET. T/N ≥ 1.3 and T/S ≥1.0 were used as cut off for viable tumor tissue for FDOPA PET.[8] PET/CT findings were further validated with histological findings and clinicoradiological follow up.
Statistical analysis
Gold standard for statistical analysis was the assessment of disease status by the clinician based on histological correlation wherever possible and clinicoradiological follow up.
RESULTS
Twenty three lesions in 23 patients were analyzed. Both high-grade and low-grade tumors were well visualized on 18F-FDOPA PET. Comparing with gold standard, 16/23 cases had tumor and 7/23 were disease free. FDOPA detected all the 16 confirmed tumor cases with T/N- 2.39 ± 0.78 and T/S -1.4 ± 0.4. FDG detected 7/16 with T/N-1.71 ± 0.31. Ammonia detected 4/ 16 with T/N-1.7 ± 0.3. Sensitivity of MRI was 13/16 [Table 1]. 5 of 7 disease free cases had postoperative gliosis and were negative on both FDG and FDOPA PET. 1 (postoperative low grade) of these 5 was false positive on Ammonia. Histopathology in 2 preoperative cases was neurocysticercosis and pyogenic abscess. These 2 were included in disease free category. FDOPA was false positive in both and FDG was false positive in pyogenic abscess. Thus, specificities of FDOPA, FDG, Ammonia and MRI were 5/7, 6/7, 6/7 and 3/7. 6 of 8 preoperative cases were proven to be astrocytomas on postoperative histopathology (3 low grade and 3 high grade). All were positive on FDOPA PET, but it could not differentiate between tumor grade. FDG and Ammonia PET were positive only in high grade cases. FDOPA PET/CT delineated the extent of disease better than MRI.
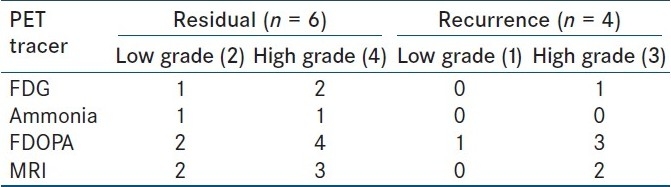
Table 1.
Sensitivity and specificity of FDG, ammonia, FDOPA and MRI

Comparison of PET tracers and MRI in cases of residual tumor and recurrence
There were 6 cases (2 low grade and 4 high grade) with residual tumor. All were FDOPA positive, 1/2 low grade and 1/4 high grade were Ammonia avid, while 1/2 low grade and 2/4 high grade were FDG avid. MRI detected residual in both the low grade cases and 3/4 high grade cases. 4 cases had recurrence (1 low grade and 3 high grade). All were FDOPA positive, FDG was positive in only 1/3 high grade while none was positive on Ammonia PET. MRI failed to detect recurrence in low grade and 1 of 3 high grade cases. Hence, FDOPA PET was most sensitive for detecting residual tumor and recurrence in both low grade and high grade tumors [Table 2].
Table 2.
Comparison of FDG, ammonia, FDOPA PET and MRI in residual tumor and recurrence
DISCUSSION
To our knowledge, this is the first clinical study to systematically compare MRI with 18F-FDOPA, 18F-FDG and 13N-Ammonia PET in brain tumors and to evaluate their diagnostic accuracies with pathologic and clinicoradiological follow-up findings. Our results suggest the superiority of 18F-FDOPA PET over 18F-FDG PET, 13N-Ammonia PET and MRI for visualizing low-grade tumors and evaluating recurrent and residual tumors.18F-FDOPA PET of gliomas demonstrated lower SUVs than did 18F-FDG PET. However, the contrast between tumor and normal tissue was higher [Figure 1] with 18F-FDOPA (T/N- 2.39 ± 0.78) than with 18F-FDG (T/N-1.7 ± 0.3) and 13N-Ammonia (T/N-1.7 ±0.3) because of the low normal brain tissue uptake of 18F-FDOPA. This property proved useful in detecting low-grade tumors as well as recurrent tumors. For example, 3 of 4 patients with recurrent tumors had negative 18F-FDG PET results but positive 18F-FDOPA PET. All 4 cases were negative on 13N-Ammonia PET. 2 of 3 low grade tumors with residual/recurrent tissue were negative on FDG and 13N-Ammonia PET [Table 2]. All 3 were FDOPA PET positive. 18F-FDOPA PET therefore may help to detect low-grade and recurrent tumors with greater sensitivity than 18F-FDG PET and 13N-Ammonia PET. 3 postoperative cases with residual/recurrent tumor tissue (2 high grade and 1 low grade) were missed on MRI [Figure 1]. One of these 3 patients expired 6 months after the PET study. Another symptomatic, high grade postoperative case with minimal peripheral enhancement around the postoperative site on MRI had extensive recurrence delineated by FDOPA PET. FDG and Ammonia PET were negative in this patient and he succumbed to his illness 2 months after the PET study [Figure 2]. Thus, FDOPA PET seems to detect residual tumor and recurrence with higher sensitivity than MRI. Specificity of all PET tracers was higher than MRI. The results suggest that FDOPA may be an ideal tracer for brain tumor imaging.
Figure 1.

A case of operated high grade astrocytoma, with intermittent headache post radiotherapy. Serial MRI showed hematoma at postoperative site. All PET tracers were positive, with best contrast seen on FDOPA PET. This patient expired six months after PET study
Figure 2.
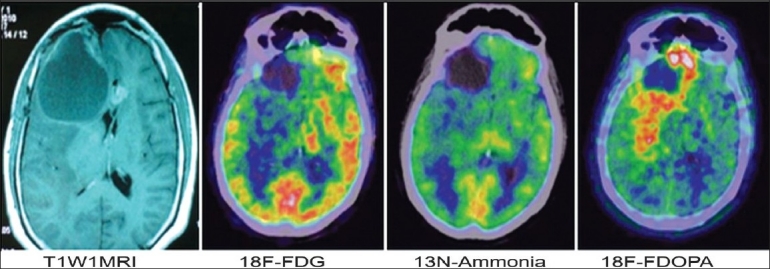
A case of high grade astrocytoma with complains of multiple episodes of seizures, post CCRT. Post contrast T1W1 shows mild peripheral enhancement around postoperative gliosis in tight frontal lobe. FDG and ammonia PET are negative. FDOPA shows extensive recurrence with extension in parietal region and pericallosal spread into left frontal lobe. Patient expired two months after PET study
FDOPA, a phenyl alanine analog, is not metabolized in tumor tissue. Its uptake is dependent on the manifold up regulation of neutral amino acid transporters in both low grade and high grade tumors, as compared to normal brain tissue.[9] This gives the good contrast with FDOPA PET in both low grade and high grade tumors. There is no such up regulation in areas of treatment induced changes like radiation necrosis, making FDOPA highly specific. In Corpus Striatum, FDOPA is metabolically trapped in the form of 6 Fluoro Dopamine. As per study done by Chen et al, peak striatum uptake occurs 37 minutes after injection, hence it does not interfere with tumor detection.[9]
13N-Ammonia extraction by brain tissues increases non-linearly with CBF, and depends primarily upon CBF, capillary permeability- surface product (PS) and integrity of the glutamate-glutamine synthetase reaction. The glutamine synthetase reaction is the major route for metabolic trapping of 13N-ammonia in brain tissues.[10] The uptake of 13N-ammonia in brain tumors is probably governed by two main factors: the capillary blood flow in the tumor and the metabolic properties of the tumor.[10]
Sample size of this study is small to draw any statistically significant results. However, the results suggest superiority of 18F-FDOPA PET for imaging of residual/recurrent brain tumors. The data needs to be validated further with a larger study including more number of patients.
CONCLUSION
18F-FDOPA PET gives excellent visualization of both high-grade and low-grade tumors with good contrast. It is a sensitive tool with good specificity to evaluate residual tumor and recurrence in both low grade and high grade tumors. It seems to be more dependable than MRI in evaluation of residual tumor and recurrence. Potential future prospect can be FDOPA PET guided biopsy in heterogeneous gliomas. FDG uptake correlates well with tumor grade. 13N-Ammonia PET did not show any encouraging results.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Dooms GC, Hecht S, Brant-Zawadzki M, Berthiaume Y, Norman D, Newton TH. Brain radiation lesions: MR imaging. Radiology. 1986;15:149–55. doi: 10.1148/radiology.158.1.3940373. [DOI] [PubMed] [Google Scholar]
- 2.Van Dellen JR. Danziger A Failure of computerized tomography to differentiate between radiation necrosis and cerebral tumour. S Afr Med J. 1978;53:171–2. [PubMed] [Google Scholar]
- 3.Nelson DR, Yuh WT, Wen BC, Ryals TJ, Cornell SH. Cerebral necrosis simulating an intraparenchymal tumor. AJNR Am J Neuroradiol. 1990;11:211–2. [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson SJ, Huhn S, Vigneron DB, Day MR, Wald LL, Prados M, et al. Volume MRI and MRSI techniques for the quantitation of treatment response in brain tumors: presentation of a detailed case study. J Magn Reson Imaging. 1997;7:1146–52. doi: 10.1002/jmri.1880070630. [DOI] [PubMed] [Google Scholar]
- 5.Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP. Differentiaing recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol. 1998;19:407–13. [PMC free article] [PubMed] [Google Scholar]
- 6.Becherer A, Karanikas G, Szabó M, Zettinig G, Asenbaum S, Marosi C, et al. Brain tumour imaging with PET: a comparison between [18F]fluorodopa and [11C]methionine. Eur J Nucl Med Mol Imaging. 2003;30:1561–7. doi: 10.1007/s00259-003-1259-1. [DOI] [PubMed] [Google Scholar]
- 7.Xiangsong Z, Weian C. Differentiation of recurrent astrocytoma from radiation necrosis: a pilot study with 13N-NH3 PET. J Neurooncol. 2007;82:305–11. doi: 10.1007/s11060-006-9286-y. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Silverman DH, Delaloye S, Czernin J, Kamdar N, Pope W, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47:904–11. [PubMed] [Google Scholar]
- 9.Schiepers C, Chen W, Cloughesy T, Dahlbom M, Huang SC. 18F-FDOPA kinetics in brain tumors. J Nucl Med. 2007;48:1651–61. doi: 10.2967/jnumed.106.039321. [DOI] [PubMed] [Google Scholar]
- 10.Schelstraete K, Simons M, Deman J, Vermeulen FL, Slegers G, Vandecasteele C, et al. Uptake of 13N-ammonia by human tumours as studied by positron emission tomography. Br J Radiol. 1982;55:797–804. doi: 10.1259/0007-1285-55-659-797. [DOI] [PubMed] [Google Scholar]


