Abstract
The transition of a society from traditional to market-based diets (termed the nutrition transition) has been associated with profound changes in culture and health. We are developing biomarkers to track the nutrition transition in the Yup’ik Eskimo population of Southwest Alaska based on naturally occurring variations in the relative abundances of carbon and nitrogen stable isotopes (δ15N and δ13C values). Here, we provide three pieces of evidence toward the validation of these biomarkers. First, we analyzed the δ15N and δ13C values of a comprehensive sample of Yup’ik foods. We found that δ15N values were elevated in fish and marine mammals and that δ13C values were elevated in market foods containing corn or sugar cane carbon. Second, we evaluated the associations between RBC δ15N and δ13C values and self-reported measures of traditional and market food intake (n = 230). RBC δ15N values were correlated with intake of fish and marine mammals (r = 0.52; P < 0.0001). RBC δ13C values were correlated with intake of market foods made from corn and sugar cane (r = 0.46; P < 0.0001) and total market food intake (r = 0.46; P < 0.0001). Finally, we assessed whether stable isotope ratios captured population-level patterns of traditional and market intake (n = 1003). Isotopic biomarkers of traditional and market intake were associated with age, community location, sex, and cultural identity. Self-report methods showed variations by age and cultural identity only. Thus, stable isotopes show potential as biomarkers for monitoring dietary change in indigenous circumpolar populations.
Introduction
The transition of a society from traditional to market-based diets (termed the nutrition transition) has been associated with profound changes in culture and health (1–4). Many indigenous circumpolar populations are undergoing this transition (5–7), which is associated with increased rates of chronic disease (6, 8). Dietary change can be difficult to monitor due in part to the lack of baseline data and in part to the limitations of existing methods for dietary assessment. Self-report methods that are feasible to collect from large populations (e.g., FFQ) are subject to large error and bias (9, 10), whereas more reliable methods (e.g., repeated 24-h recall) can be prohibitively expensive (11, 12). Dietary biomarkers provide a promising alternative to self-report methods, because they are unbiased, more reliable, and can be measured from archived samples (13–16). We are developing biomarkers of traditional and market intake for the Yup’ik Eskimo population of Southwest Alaska based on the relative abundances of naturally occurring carbon and nitrogen stable isotopes (17, 18). Isotopic markers have been widely used as markers of diet in ecological and anthropological studies (19–22). Furthermore, they are inexpensive to measure, precise, and can be measured in multiple tissue types, including serum, RBC, and hair (16–18, 23, 24).
Stable isotope biomarkers are informative in the Yup’ik population, because many commonly consumed traditional and market foods are isotopically distinct (25–29). The nitrogen stable isotope ratio (δ15N) indicates consumption of marine mammals and fish (25, 28), which are a large component of the traditional Yup’ik diet (30–32). This biomarker has been recently validated for Yup’ik Eskimos based on comparisons with the marine fatty acids EPA and DHA (17, 18). Thus, we propose that δ15N will indicate consumption of traditional marine foods in this population. The carbon isotope ratio (δ13C) is elevated in plants using the C4 (Hatch-Slack) photosynthetic pathway relative to those using the more common C3 (Calvin) photosynthetic pathway (33). The most common representatives of these plants in the U.S. agricultural system are corn and sugar cane, which are widely present in the market diet as sweeteners (29), as ingredients in processed foods, and indirectly via domestic animals raised on corn (34, 35). The carbon isotope ratio has shown moderate associations with reported C4-based sweetener and sweetened beverage intake in the U.S. population (23, 24, 36). Here, we propose that δ13C will provide an independent biomarker of market food intake for the Yup’ik Eskimo population.
The overall objective of this study was to evaluate isotopic biomarkers of market and traditional food intake in a Yup’ik Eskimo study population. Developing reliable and accurate markers of dietary change for this population could help to predict increases in disease incidence and develop appropriate dietary interventions. The specific aims of this study were 3-fold. First, we determined expected relationships between dietary intake and RBC isotope ratios by completing a comprehensive analysis of δ15N and δ13C values in traditional and market foods important to the Yup’ik population. Second, we evaluated the association between RBC δ15N and reported fish and marine mammal intake, and RBC δ13C and reported market food intake based on 4 d of diet records from 230 Yup’ik Eskimos. Finally, we evaluated whether variations in dietary intake by age, community location, and cultural identity that were previously reported for this population based on self-report were also seen using isotopic biomarkers (30, 37–39). The extensive nature of previous dietary assessment in this population provides an ideal framework with which to evaluate the efficacy of these proposed biomarkers.
Methodology
Participant recruitment and procedures.
Data are from the Center for Alaska Native Health Research study, a cross-sectional, community-based, participatory research study of genetic, nutritional, and psychosocial risk factors affecting obesity and related disease in Yup’ik Eskimos (40, 41). The Center for Alaska Native Health Research study was approved by the University of Alaska Fairbanks Institutional Review Board, the National and Area Indian Health Service Institutional Review Boards, and the Yukon-Kuskokwim Health Corporation Human Studies Committee.
Between 2003 and 2005, 1003 participants aged 14–94 y were recruited from 10 communities in southwest Alaska as described in detail elsewhere (41). We categorized these communities as either coastal (<5 miles from the coast) or inland. At entry into the study, participants completed extensive interviewer-administered demographic questionnaires. A subset of 315 participants from the first 7 communities completed a single interviewer-administered 24-h dietary recall as well as a 3-d food record. A subset of 767 participants from all 10 communities completed a wellness questionnaire. We used the responses to two questions about cultural identification, which asked how much an individual felt they followed a Yup’ik or Kass’aq (non-native) way of life. Responses to these questions were coded numerically (1 = follows the lifestyle a lot, 2 = some, 3 = not at all) and were not mutually exclusive (e.g., a person could respond “follows the lifestyle a lot” for both ways of life).
Biological sample collection.
Blood was collected into EDTA tubes and processed in rural communities using a portable centrifuge. Serum, lymphocytes, and RBC were stored in aliquots at −20°C in a portable freezer. Within 6 d, samples were shipped to the University of Alaska Fairbanks and stored at −80°C. Aliquots of RBC were removed for stable isotope analysis, as described below. RBC were chosen for analyses, because they reflect dietary intake over a period of 1–3 mo (42–44) and thus provide a more stable estimate of dietary intake than serum (45). RBC aliquots were autoclaved for 20 min at 121°C to destroy blood-borne pathogens, apportioned into tin capsules (3.5 × 3.75 mm), and oven dried at 60°C to a final weight of 0.2–0.4 mg. Neither autoclaving nor the use of EDTA-treated tubes affects RBC carbon or nitrogen isotope ratios (28).
Food sample collection.
We sampled a broad range of traditional and market foods that were known to contribute to this study population’s diet (n = 254). Foods were defined based on their NDS-R (software version 4.06; University of Minnesota) food codes. Traditional foods were harvested from the local environment and samples were donated by residents from 3 Yup’ik Eskimo communities. Market foods were purchased from community grocery stores or grocery stores in Fairbanks, Alaska. We sampled 3 or more representatives of foods contributing >5% of energy (based on dietary self-report data), and one or more representatives from foods contributing 1–5% of energy. We sampled more rarely consumed traditional food items (contributing <1% energy) when donated. Marine mammal samples were collected under permit number 932–1905–00IMA-009526 issued by the National Marine Fisheries Service and the U.S. Fish and Wildlife Service, under the authority of the Marine Mammal Protection Act and Endangered Species Act. A list of foods sampled and their sampling frequencies is given in Supplemental Table 1.
Food samples were grouped into traditional or market-based food groups. Traditional foods were divided into four groups: aquatic (marine mammals, fish, and seal oil), terrestrial animals (birds and mammals), terrestrial plants (berries and wild plants), and waterfowl (ducks, geese, and swans). We defined waterfowl separately, because these species forage in both marine and terrestrial habitats. Furthermore, we note that the “aquatic” category contains both marine and freshwater fish species. Although freshwater and marine fish are expected to differ slightly in δ15N and δ13C values, their values are likely more similar to each other than to other classes of foods. Market foods were divided into five groups: market grains and vegetables (foods from C3 plants, including pasta, wheat, rice, nuts, fruits, and vegetables), corn and cane sugar (C4-based foods, including beverages sweetened with high fructose corn syrup, cane sugar, candy, corn), meat (chicken, turkey, beef, eggs), dairy (milk, cheese), or mixed (containing ingredients from more than one group). For isotope analysis, food samples were oven dried at 60°C for at least 48 h, ground to a fine powder, and weighed into tin capsules to a final weight of 0.2–0.4 mg.
Stable isotope analysis.
Samples were analyzed at the Alaska Stable Isotope Facility by continuous-flow isotope ratio MS using a Costech ECS4010 Elemental Analyzer (Costech Scientific) interfaced to a Finnigan Delta Plus XP isotope ratio mass spectrometer via the Conflo III interface (Thermo-Finnigan). The conventional means of expressing natural abundance isotope ratios is as δ values in permil (‰) relative to international standards, defined as δX = (Rsample – Rstandard)/(Rstandard) · 1000‰ (46). Here, R is the ratio of heavy to light isotope (15N:14N or 13C:12C) and the standards are atmospheric N for nitrogen and PeeDee Belemnite for carbon. Because the foods and RBC samples from this study had a lower 13C:12C than the standard, the values of δ13C were negative. We concurrently prepared and ran multiple peptone working standards (δ15N = 7.0, δ13C = −15.8‰, n = 128) to assess analytical accuracy and precision, measured as the SD of these analyses. Accuracy was within 0.1‰ and precision was within 0.2‰ for both isotopes.
Assessment of dietary intake.
Dietary intake was estimated using an interviewer-administered 24-h dietary recall and a 3-d food record. Data from these instruments were combined to achieve a stable estimate of dietary intake. The 24-h dietary recall was collected from each participant by certified interviewers using a computer-assisted recall (NDS-R version 4.06). Participants were asked to recall all food and beverages consumed over the 24-h period covering the day prior to the interview using a multiple pass approach to minimize recall bias. Although most participants were bilingual, a native Yup’ik speaker trained in the use of NDS-R was available for non-English speakers.
When completing the 3-d food record, participants were instructed to maintain their usual eating habits. A research team member reviewed all records for completeness, which were then entered into the NDS-R software package by certified coders. A second researcher reviewed all entries for accuracy. Records were considered unreliable and excluded from analysis if daily energy intake was >5000 or <500 kcal (38 participants had 1 d excluded, 4 had 2 d excluded). Individuals who had >2 d considered unreliable (n = 2) or whose 3-d food record or 24-h recall was incomplete (n = 83) were excluded from self-report analyses; 230 individuals remained.
Dietary analysis.
The contributions of traditional and market foods to an individual’s diet were assessed as follows: all food items were assigned to traditional and market food groups (as defined above and given in Supplemental Table 1) based on their food codes from the NDS-R Food and Nutrient Database 33 (July 2003). A few Alaska Native foods were missing from the database; these were substituted for similar food items where appropriate or the foods were added to the database. We then summed total energy consumed for 3 categories of foods: traditional aquatic, market, and C4-based market foods and used these totals for analyses. Foods assigned to traditional and market food groups were mutually exclusive.
Because market foods included food groups that were partially C4 based (mixed foods, meat, and dairy) as well as entirely C4 based (corn and cane sugar), we defined C4-based market food intake by weighting energy derived from market-based food groups based on their fractional C4 carbon content and summing this weighted energy (kcal). This fractional content (fC4) was calculated using the mean δ13C of food groups and an isotopic mixing model, as follows:
where fC4 is the fraction of the food that is C4 based, δ13CC4 is the mean carbon isotope ratio of C4-based plant foods (corn and cane sugar) (Table 1), and δ13CC3 is the carbon isotope ratio of C3-based plant foods [grains and vegetables (47)]. We note that this is a highly simplified mixing model that does not take into account differences in macronutrient composition among foods (48) and uses a mean δ13C for food classes rather than adjusting foods individually. However, the purpose of the calculation is to get a broad estimate of how much of the market diet is derived from C4 sources rather than to present a highly precise measure.
TABLE 1.
Nitrogen and carbon isotope ratios of market and traditional foods1
| Food group | δ15N (‰‰) | δ13C2 (‰‰) |
| Subsistence foods | ||
| Aquatic3 | 14.2 ± 3.2a | −21.1 ± 3.4b,c |
| Waterfowl | 7.3 ± 2.1b | −23.8 ± 3.6c,d |
| Terrestrial animals | 3.7 ± 4.1b,c | −24.5 ± 4.2c,d |
| Terrestrial plants | −0.3 ± 2.2c | −27.4 ± 1.8d |
| Market foods | ||
| Corn and cane sugar | 4.0 ± 0.6b,c | −12.4 ± 1.3a |
| Meat | 3.7 ± 1.4b,c | −17.2 ± 1.2b |
| Mixed | 1.9 ± 2.3c | −21.2 ± 2.2b,c |
| Dairy | 4.5 ± 0.5b,c | −21.4 ± 1.3b,c,d |
| Grains and vegetables | 2.1 ± 1.9c | −26.6 ± 2.0d |
Values are mean ± SD. Means in a column without a common letter differ, < 0.05. Means were calculated using the mean isotope ratio for each food within the group, given in Supplemental Table 1.
Carbon isotope ratios are negative, because all samples had less 13C than the standard against which they were measured.
Includes marine mammals and marine and freshwater fish species.
Correcting RBC δ13C values for the influence of fish and marine mammal intake.
Our aim was to use δ13C as an index of C4-based market food intake; however, both C4 and aquatic foods have elevated δ13C values relative to C3-based market foods (27, 29). Therefore, we used δ15N values to adjust for the influence of aquatic foods on RBC δ13C as follows:
where δ13CA = adjusted δ13C value, δ13C and δ15N are measured RBC isotope ratios, and δ15Nno aquatic = mean δ15N value for all members of the population reporting no traditional aquatic food intake (n = 42). This was measured to be 7.8 ‰. Δδ13/Δδ15Naquatic foods = the increase in δ13C for each unit increase in δ15N across all fish and marine mammal samples. This was measured to be 0.91 (see Results).
We tested the accuracy of this approach by assessing the agreement between prediction of C4-based market foods based on δ13CA values and a multiple linear regression model including δ13C and δ15N as independent variables (49). Agreement between the two methods was good (mean difference: 0.0 ± 1.7% of total energy). Although both of these methods account for the influence of aquatic food intake on δ13C values, we chose to adjust RBC δ13C values in this study to generate a single, independent variable that can be used in multiple analyses.
Statistical analyses.
All statistical analyses were performed using JMP version 8 (SAS Institute). We evaluated differences in the sex and age distributions between the complete sample of participants and those who completed dietary interviews or cultural identity questions using a chi-square test. We used ANOVA to compare the isotope ratios of food samples in each market and traditional food group and compared means using Tukey-Kramer’s honest significant difference. We assessed the associations between isotope ratios and dietary intake data, as well as between dietary intake variables, using linear regression.
We evaluated the effects of demographic variables on intake variables (isotope and self-report) using ANCOVA models, where relationships were linear and met assumptions for parametric statistical tests. Demographic variables (age, sex, community location) were the independent variables in these models. ANOVA was used to assess the effects of cultural identity on dietary intake. Cultural identity variables were the independent variables in these models. The effects of demographic and cultural identity variables on intake were assessed separately, because cultural identity questions were only completed by a subset of the population.
In all analyses that compared dietary self-report data to isotope, demographic, or cultural identity information, intake was expressed as the percentage of total energy represented by each food group. Where dietary self-report information was compared to other dietary self-report information, intake was expressed as total energy reported (kcal). Normality was confirmed using normal probability plots; all percentage intake variables were log-transformed to normalize data and results were back transformed for ease of interpretation (50). Outliers were identified by using Mahalobnis distance >3 and excluded from analyses (n = 3 for aquatic food intake, n = 6 for C4 intake, n = 3 for total traditional food intake, and n = 13 for total market intake). Data are means ± SD. A significance level of 0.05 was used throughout the analyses.
Results
Stable isotope ratios of food.
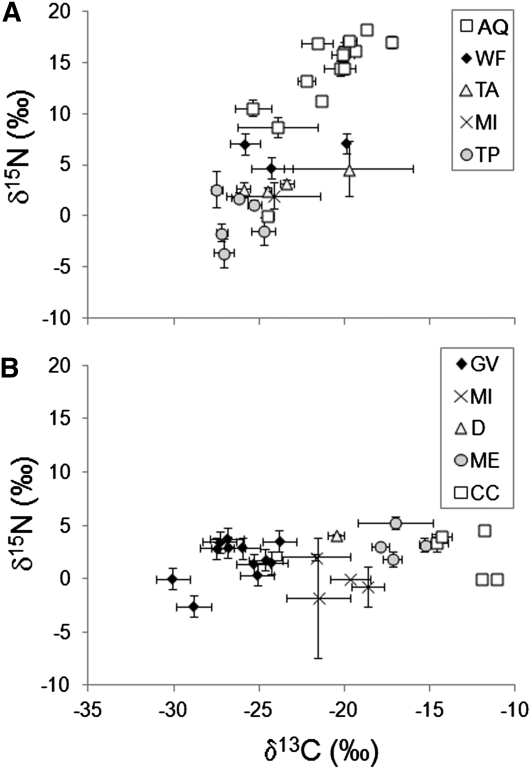
Nitrogen isotope ratios varied among traditional and market food groups (δ15N: P < 0.001) (Table 1; Supplemental Table 1). Traditional aquatic foods had substantially higher δ15N values than any other food group (Table 1). Marine fish species had higher δ15N values than freshwater fish species (P = 0.0013, marine δ15N = 14.3 ± 3.4‰, freshwater δ15N = 11.5 ± 2.7‰); however, their values overlapped and were both significantly higher than all other food groups. Thus, we continued to group these species together as “aquatic foods” for further analyses. Excluding aquatic foods, animal-based food groups had higher mean δ15N values than plant-based food groups (P = 0.0037). We found a strong positive relationship between δ13C and δ15N values of traditional foods (β = 1.86; r = 0.82; P < 0.0001) (Fig. 1A) and aquatic foods only (β = 0.91; r = 0.71; P < 0.0001) but not market foods (β = 0.14; r = 0.38; P = 0.08) (Fig. 1B).
FIGURE 1.
Carbon and nitrogen isotope ratios of a representative sample of traditional (A) and market (B) food items, see Supplemental Table 1 for a complete list. Only foods with n > 2 are represented in this figure. Foods were assigned to groups, which are abbreviated in the legend as follows: AQ, aquatic; WF, waterfowl; TA, terrestrial animals; MI, mixed; TP, terrestrial plants; GV, grains and vegetables; D, dairy; ME, nontraditional meats; CC, corn and cane sugar based.
Carbon isotope ratios differed between food groups (δ13C: P < 0.001) (Fig. 1), with a clear distinction between C3- and C4-based foods. Corn and cane sugar (C4) had elevated δ13C values relative to grains and vegetables (C3) (Table 1). The mean isotopic difference between these groups was almost 13‰ and was consistent with reported δ13C values for C3 and C4 plants generally (51). Market meats, dairy, and mixed foods had δ13C values intermediate between C3- and C4-based market foods, reflecting corn-based feeds or ingredients. Marine fish species had higher δ13C values compared to freshwater species (P < 0.0001; marine δ13C = −20.4 ± 1.8‰, freshwater δ13C = −24.3 ± 3.3).
Sample population.
Females were slightly over-represented relative to males in the whole study population (54%), the subset of participants with dietary self-report data (59%), and the subset of participants reporting cultural identification (56%; all P < 0.01) (Table 2). The age distribution of participants with dietary self-report data differed from the complete study sample (P < 0.01), with reduced participation by those who were >60 y old. The age distribution of participants reporting cultural identification did not differ from the complete study sample.
TABLE 2.
Effect of sex, age, and community location on δ15N, δ13CA values, and self-reported intake of traditional aquatic and C4-based market foods in a community based sample of Yup'ik Eskimos participating in the Center for Alaska Native Health Research study1
| Isotope measures |
Self-report measures |
|||||
| n | δ15N (‰‰) | δ13CA2 (‰‰) | n | Traditional aquatic foods (% total energy) | C4-based market foods (% total energy) | |
| Whole population | ||||||
| Total | 1003 | 9.0 ± 1.5 | −20.8 ± 1.6 | 230 | 13 ± 14 | 33 ± 14 |
| Sex | ||||||
| Male | 460 | 8.8 ± 1.5 | −20.7 ± 1.6 | 95 | 12 ± 14 | 33 ± 15 |
| Female | 543 | 9.1 ± 1.5 | −21.0 ± 1.6 | 135 | 14 ± 14 | 33 ± 13 |
| Age, y | ||||||
| 14–<20 | 200 | 7.8 ± 0.7 | −19.6 ± 0.8 | 59 | 6 ± 9 | 43 ± 13 |
| 21–<40 | 374 | 8.6 ± 1.1 | −20.4 ± 1.2 | 81 | 13 ± 12 | 35 ± 13 |
| 41–<60 | 303 | 9.5 ± 1.4 | −21.4 ± 1.4 | 78 | 18 ± 15 | 25 ± 11 |
| >60 | 126 | 10.7 ± 1.7 | −22.8 ± 1.4 | 12 | 21 ± 20 | 21 ± 10 |
| Location,% | ||||||
| Coastal | 402 | 9.6 ± 1.8 | −21.2 ± 1.7 | 88 | 15 ± 16 | 34 ± 14 |
| Upriver | 601 | 8.6 ± 1.1 | −20.6 ± 1.4 | 142 | 12 ± 12 | 32 ± 14 |
| Cultural identification subset | ||||||
| Total | 767 | 9.0 ± 1.5 | −20.8 ± 1.5 | 216 | 13 ± 14 | 33 ± 14 |
| Yup'ik | ||||||
| High | 349 | 9.3 ± 1.6 | −21.2 ± 1.6 | 92 | 16 ± 17 | 29 ± 13 |
| Medium | 399 | 8.7 ± 1.4 | −20.5 ± 1.4 | 118 | 11 ± 11 | 35 ± 13 |
| Low | 19 | 7.8 ± 0.9 | −19.6 ± 1.3 | 6 | 2 ± 4 | 48 ± 21 |
| Kass'aq | ||||||
| High | 129 | 8.3 ± 1.2 | −20.0 ± 1.3 | 44 | 8 ± 9 | 36 ± 13 |
| Medium | 575 | 9.0 ± 1.5 | −20.9 ± 1.5 | 159 | 14 ± 15 | 33 ± 14 |
| Low | 63 | 9.7 ± 1.8 | −21.5 ± 1.7 | 13 | 18 ± 14 | 30 ± 15 |
Values are mean ± SD.
Carbon isotope ratios are negative, because all samples had less 13C than the standard against which they were measured.
Associations between stable isotope ratios and reported dietary intakes.
Nitrogen isotope ratios were significantly correlated with intake of traditional aquatic foods based on dietary self-report (Table 3). Reported intake of total traditional and traditional aquatic foods was highly correlated (Table 3). Aquatic foods accounted for 77% energy from traditional sources.
TABLE 3.
Relationships between stable isotope biomarkers and self-reported measures of traditional aquatic and C4-based market food intake (n = 230)
| β2 | CI | Intercept | r | P | |
| δ15N1 vs. total aquatic, % | 1.05 | 1.04–1.06 | −0.3 | 0.52 | <0.0001 |
| Total aquatic vs. total traditional, kcal | 1.05 | 1.04–1.06 | 56.7 | 0.93 | <0.0001 |
| δ13CA vs. C4, % | 1.19 | 1.14–1.24 | 2.0 | 0.46 | <0.0001 |
| δ13CA vs. total market, % | 1.08 | 1.06–1.10 | 1.2 | 0.46 | <0.0001 |
| C4 vs. total market, kcal | 1.55 | 1.39–1.71 | 594.6 | 0.78 | <0.0001 |
Independent variables are listed first.
Where the dependent variables have been transformed, estimates of β are back-transformed for ease of interpretation.
Adjusted carbon isotope ratios were positively correlated with C4 market food intake (Table 3) as well as total market food intake. Intake of C4-based market foods was positively associated with total market food intake (Table 3). C4 sources accounted for 40% of total energy from market foods.
Population level patterns of traditional aquatic food intake.
Mean RBC δ15N values reflected a high and variable intake of aquatic foods (Table 2). Aquatic foods were reported by 82% of people with dietary self-report data (n = 188) and mean intake was 210 ± 245 kcal.
We found strong associations between RBC δ15N values and sex, community location, and age (Table 2). Values of RBC δ15N increased with age (β = 0.052; CI = 0.048–0.056; P < 0.0001) and were higher in coastal communities (P < 0.0001) and females (P < 0.0001). There was a community location by age interaction (P < 0.0001), because differences in δ15N values between coastal and inland locations increased with age. In the self-report data, we found an increase in intake of aquatic foods with age (β = 1.003; CI = 1.002–1.004; P < 0.0001) but not community location (P = 0.11) or sex (P = 0.09).
Intake of aquatic foods was positively associated with following a Yup’ik way of life as indicated by nitrogen isotope ratios (P < 0.0001) and self-report data (P = 0.016). Following a Kass’aq way of life was negatively associated with nitrogen isotope ratios (P = 0.045) and self-reported intake of aquatic foods (P = 0.045).
Population level patterns of C4-based market food intake.
Adjusted RBC carbon isotope ratios reflected a mixed diet of C3 and C4 foods (Table 2). All participants for whom we have dietary self-report information reported consuming market foods (n = 230) and mean intake of C4-based market foods was 543 ± 301 kcal.
We found strong associations between sex, age, and community location and RBC δ13CA values (Table 2). Values of RBC δ13CA decreased with age (β = −0.057; CI = −0.062 to −0.053; P < 0.0001), and were higher in upriver communities (P < 0.0001) and males (P = 0.0011). There was a community location by age interaction (P = 0.0223). In the self-report data, we did not find differences in market food intake by sex or community location, although there was a decrease in intake of market foods with age (β = 0.98; P < 0.0001) (Table 2).
RBC δ13CA values were positively associated with following a Kass’aq way of life (P < 0.0001) and negatively associated with following a Yup’ik way of life (P < 0.0001) (Table 2). Self-reported intake of C4-based market foods was negatively associated with a Yup’ik way of life (P = 0.0006) (Table 2) but was not associated with a Kass’aq way of life (P = 0.26).
Discussion
Carbon and nitrogen stable isotope biomarkers capture patterns of traditional aquatic and market food intake in a Yup’ik Eskimo study population. Foods commonly consumed by the Yup’ik population exhibited highly distinct patterns of carbon and nitrogen isotope ratios, with elevated δ15N and δ13C values found in key traditional and market foods, respectively. Self-reported intake of these food groups was associated with RBC δ15N and δ13CA values. These isotope ratios detected demographic and cultural variations in both traditional and market food intake known to exist within this population (30) as well as some that were undetected by self-reported measures. Stable isotope markers have the potential to be useful in assessing the health impacts of dietary change in indigenous circumpolar populations, because they impose low participant burden and can be measured with precise, high throughput, and inexpensive methods.
Traditional aquatic foods had significantly elevated δ15N values relative to all other food groups, causing a 1% increase in RBC δ15N for each 5% increase in energy intake from aquatic foods. RBC δ15N measurements showed that aquatic food consumption increased with age, reflecting trends in total traditional food intake described for this study population (30) and other circumpolar populations (38, 52, 53). RBC δ15N values also captured differences in traditional aquatic food intake between coastal and inland communities, an effect seen primarily in elders, and a slight increase of aquatic food intake in women relative to men. Self-reported intake of traditional aquatic (this study) or total traditional foods (30) did not vary with community location or sex in this population. Thus, stable isotope biomarkers were able to identify patterns of traditional food intake that were not evident in dietary self-report data.
Corn and cane sugar-based market foods exhibited uniquely high δ13C values relative to all other foods. Animal-based market foods (beef, pork, poultry, eggs, and dairy) also had elevated δ13C values, reflecting corn in the diet of commercially raised animals (34, 35). We refer collectively to these foods as “C4-based market foods.” RBC δ13CA values were associated with intake of C4-based market foods as well as total market food intake with each 8% increase in energy from market foods causing a 1‰ increase in δ13CA. The carbon isotope ratio has been moderately associated with sweetener intake in other U.S. populations (23, 24, 36); however, the development of this marker is complicated by its concurrent association with animal protein intake (24, 54). In our study, the fact that multiple market foods are influenced by corn likely strengthens the association between δ13C and market food intake.
Although market foods are known to be a significant source of energy to all age groups in this study population (39), both self-report and isotope data showed that consumption of market foods decreased with age. Adjusted δ13C values also showed that coastal communities consumed slightly less market foods than those inland and men consumed slightly more market foods than women. Both RBC δ13CA and self-reported measures of market food intake were positively associated with a non-native (Kass’aq) way of life. As expected, these patterns are the reverse of those found for traditional food intake. However, the use of δ13CA as an independent biomarker for market food intake based on sugar cane and corn provides an alternative way to assess the nutrition transition that, unlike δ15N, does not require traditional food intake to be aquatic. Such a biomarker could be particularly useful for assessing dietary change in Alaska Native populations relying more heavily on traditional foods such as moose and caribou (55, 56), which are not distinct from market foods in δ15N.
The primary limitation of this study was that we compared stable isotope ratios to self-reported estimates of traditional and market food intake, which are subject to error and biases associated with age, sex, and other individual characteristics. These errors may have obscured the true relationships between isotopic markers and diet in our study population. For example, relationships between δ15N and EPA were very strong for this population [r > 0.8; (17,18)] compared with the relationship between δ15N and reported aquatic food intake presented here (r = 0.52). However, this study had several unique strengths. The Yup’ik Eskimo population of Southwest Alaska is culturally and linguistically among the most intact of Alaska Native peoples and traditional food use is still common (41); therefore, this is an ideal population in which to test markers of both traditional and market foods. Furthermore, the extensive nature of dietary research in this population provided an ideal opportunity to identify relevant foods for isotopic sampling and test stable isotope biomarkers within the context of known dietary patterns.
In summary, we demonstrated that stable nitrogen and carbon isotope ratios in RBC indicate the use of traditional and market foods in a Yup’ik Eskimo study population in Southwest Alaska. Isotopic biomarkers have great potential due to their affordability, reliability, and ability to be measured noninvasively and with low burden (17, 18). Furthermore, because these markers can be measured in stored specimens, they have the potential to provide baseline data for studies of dietary change over time (16). The development of reliable biomarkers of traditional and market-based food intake will help in the evaluation of the overall health impacts of dietary change in circumpolar populations
Supplementary Material
Acknowledgments
The authors thank Tim Howe and Norma Haubenstock at the Alaska Stable isotope facility for their assistance with isotope analysis and Dr. Carlotta Fok for statistical advice. We thank Dr. Todd O’Hara for assistance with marine mammal sample collection. This manuscript was improved by comments from Trixie Lee, Caroline van Hemert, and Dr. Kyungcheol Choy. G.V.M., B.B.B., and D.M.O. designed research; S.H.N., A.B., R.S.C., R.L.P., S.E.H., B.R.L., B.B.B., G.V.M., and D.M.O. conducted research; S.H.N. and D.M.O. conducted the analyses; S.H.N., D.M.O., A.R.K., A.B., and B.B.B. wrote the manuscript; and S.H.N. had primary responsibility for final content. All authors have read and approved the final manuscript.
Footnotes
Supported by a Center of Biomedical Research Excellence grant from the NIH National Center for Research Resources (P20 RR16430-10) and NIH National Institute of Diabetes and Digestive and Kidney Diseases R01DK07442. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources or the NIH.
Supplemental Table 1 is available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Literature Cited
- 1.Kuhnlein HV, Receveur O. Dietary change and traditional food systems of indigenous peoples. Annu Rev Nutr. 1996;16:417–42 [DOI] [PubMed] [Google Scholar]
- 2.Astrup A, Dyerberg J, Selleck M, Stender S. Nutrition transition and its relationship to the development of obesity and related chronic diseases. Obes Rev. 2008;9:48–52 [DOI] [PubMed] [Google Scholar]
- 3.Compher C. The nutrition transition in American Indians. J Transcult Nurs. 2006;17:217–23 [DOI] [PubMed] [Google Scholar]
- 4.Popkin B. The nutrition transition in the developing world. Dev Policy Rev. 2003;21:581–97 [Google Scholar]
- 5.Bjerregaard P, Young T, Dewailly E, Ebbesson S. Indigenous health in the Arctic: an overview of the circumpolar Inuit population. Scand J Public Health. 2004;32:390–5 [DOI] [PubMed] [Google Scholar]
- 6.Haman F, Fontaine-Bisson B, Batal M, Imbeault P, Blais JM, Robidoux MA. Obesity and type 2 diabetes in Northern Canada's remote First Nations communities: the dietary dilemma. Int J Obes (Lond). 2010;34 Suppl 2:S24–31 [DOI] [PubMed] [Google Scholar]
- 7.Deutch B. Recent dietary studies in the Arctic. AMAP Assessment. Oslo: AMAP; 2002 [Google Scholar]
- 8.Murphy NJ, Schraer C, Thiele M, Boyko E, Bulkow L, Doty B, Lanier A. Dietary change and obesity associated with glucose intolerance in Alaska Natives. J Am Diet Assoc. 1995;95:676–82 [DOI] [PubMed] [Google Scholar]
- 9.Byers T. Food frequency dietary assessment: how bad is good enough? Am J Epidemiol. 2001;154:1087–8 [DOI] [PubMed] [Google Scholar]
- 10.Kristal AR, Peters U, Potter J. Is it time to abandon the food frequency questionnaire? Cancer Epidemiol Biomarkers Prev. 2005;14:2826–8 [DOI] [PubMed] [Google Scholar]
- 11.Thompson F, Subar AF. Dietary assessment methodology. : Coulston A, Rock CR, Monsen E, Nutrition and the prevention and treatment of disease. Burlington, MA: Elsevier Academic Press; 2001 [Google Scholar]
- 12.Subar AF, Thompson FE, Potischman N, Forsyth BH, Buday R, Richards D, McNutt S, Hull SG, Guenther PM, Schatzkin A, et al. Formative research of a quick list for an automated self-administered 24-hour dietary recall. J Am Diet Assoc. 2007;107:1002–7 [DOI] [PubMed] [Google Scholar]
- 13.Bingham SA. Biomarkers in nutritional epidemiology. Public Health Nutr. 2002;5:821–7 [DOI] [PubMed] [Google Scholar]
- 14.Jenab M, Slimani N, Bictash M, Ferrari P, Bingham S. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet. 2009;125:507–25 [DOI] [PubMed] [Google Scholar]
- 15.Marshall JR. Methodologic and statistical considerations regarding use of biomarkers of nutritional exposure in epidemiology. J Nutr. 2003;133:S881–7 [DOI] [PubMed] [Google Scholar]
- 16.Kraft RA, Jahren A, Saudek C. Clinical scale investigation of stable isotopes in human blood: δ13C and δ15N from 406 patients at the Johns Hopkins Medical Institutions. Rapid Commun Mass Spectrom. 2008;22:3683–92 [DOI] [PubMed] [Google Scholar]
- 17.O'Brien DM, Kristal AR, Jeannet MA, Wilkinson MJ, Bersamin A, Luick B. Red blood cell 15N: a novel biomarker of dietary eicosapentaenoic acid and docosahexaenoic acid intake. Am J Clin Nutr. 2009;89:913–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nash SH, Kristal AR, Boyer BB, King IB, Metzgar JS, O'Brien DM. Relation between stable isotope ratios in human red blood cells and hair: implications for using the nitrogen isotope ratio of hair as a biomarker of eicosapentaenoic acid and docosahexaenoic acid. Am J Clin Nutr. 2009;90:1642–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gannes LZ, del Rio CM, Koch P. Natural abundance variations in stable isotopes and their potential uses in animal physiological ecology. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:725–37 [DOI] [PubMed] [Google Scholar]
- 20.Hobson KA. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia. 1999;120:314–26 [DOI] [PubMed] [Google Scholar]
- 21.Evershed R. Exploiting molecular and isotopic signals at the Mesolithic-Neolithic transition. Proc Brit Acad. 2007;144:141–64 [Google Scholar]
- 22.Vogel J, van der Merwe N. Isotopic evidence for early maize cultivation in New York state. Am Antiq. 1977;42:238–42 [Google Scholar]
- 23.Davy BM, Jahren AH, Hedrick VE, Comber DL. Association of delta(13)C in fingerstick blood with added-sugar and sugar-sweetened beverage intake. J Am Diet Assoc. 2011;111:874–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung EH, Saudek C, Jahren A, Kao W, Islas M, Kraft R, Coresh J, Anderson C. Evaluation of a novel isotope biomarker for dietary consumption of sweets. Am J Epidemiol. 2010;172:1045–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchardt B, Bunch V, Helin P. Fingernails and diet: stable isotope signatures of a marine hunting community from modem Uummannaq, North Greenland. Chem Geol. 2007;244:316–29 [Google Scholar]
- 26.O'Connell TC, Hedges R. Investigations into the effect of diet on modern human hair isotopic values. Am J Phys Anthropol. 1999;108:409–25 [DOI] [PubMed] [Google Scholar]
- 27.Chisholm BS, Nelson DE, Schwarcz HP. Stable-carbon isotope ratios as a measure of marine versus terrestrial protein in ancient diets. Science. 1982;216:1131–2 [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson MJ, Yai Y, O'Brien D. Age-related variation in red blood cell stable isotope ratios (delta^13C and delta^15N) from two Yupik villages in southwest Alaska: a pilot study. Int J Circumpolar Health. 2007;66:31–41 [DOI] [PubMed] [Google Scholar]
- 29.Jahren AH, Saudek C, Yeung E, Kao W, Kraft R, Caballero B. An isotopic method for quantifying sweeteners derived from corn and sugar cane. Am J Clin Nutr. 2006;84:1380–4 [DOI] [PubMed] [Google Scholar]
- 30.Bersamin A, Zidenberg-Cherr S, Stern J, Luick B. Nutrient intakes are associated with adherence to a traditional diet among Yup'ik Eskimos living in remote Alaska Native communities: The CANHR study. Int J Circumpolar Health. 2007;66:62–70 [DOI] [PubMed] [Google Scholar]
- 31.Johnson JS, Nobmann E, Asay E, Lanier A. Dietary intake of Alaska native people in two regions and implications for health: the Alaska Native Dietary and Subsistence Food Assessment Project. Int J Circumpolar Health. 2009;68:109–22 [DOI] [PubMed] [Google Scholar]
- 32.Nobmann ED, Byers T, Lanier A, Hankin J, Jackson M. The diet of Alaska Native adults: 1987–1988. Am J Clin Nutr. 1992;55:1024–32 [DOI] [PubMed] [Google Scholar]
- 33.Farquhar G, Ehleringer J, Hubick K. Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:503–37 [Google Scholar]
- 34.Chesson LA, Podlesak D, Thompson A, Cerling T, Ehleringer J. Variation of hydrogen, carbon, nitrogen, and oxygen stable isotope ratios in an American diet: fast food meals. J Agric Food Chem. 2008;56:4084–91 [DOI] [PubMed] [Google Scholar]
- 35.Jahren AH, Kraft R. Carbon and nitrogen stable isotopes in fast food: signatures of corn and confinement. Proc Natl Acad Sci USA. 2008;105:17855–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cook CM, Alvig A, Liu Y, Schoeller D. The natural 13C abundance of plasma glucose is a useful biomarker of recent dietary caloric sweetener intake. J Nutr. 2010;140:333–7 [DOI] [PubMed] [Google Scholar]
- 37.Hopkins SE, Kwachka P, Lardon C, Mohatt G. Keeping busy: a Yup'ik/Cup'ik perspective on health and aging. Int J Circumpolar Health. 2007;66:42–50 [DOI] [PubMed] [Google Scholar]
- 38.Redwood DG, Ferucci E, Schumacher M, Johnson J, Lanier A, Helzer L, Tom-Orme L, Murtaugh M, Slattery M. Traditional foods and physical activity patterns and associations with cultural factors in a diverse Alaska Native population. Int J Circumpolar Health. 2008;67:335–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bersamin A, Luick B, Ruppert E, Stern J, Zidenberg-Cherr S. Diet quality among Yup'ik Eskimos living in rural communities is low: The Center for Alaska Native Health Research pilot study. J Am Diet Assoc. 2006;106:1055–63 [DOI] [PubMed] [Google Scholar]
- 40.Boyer BB, Mohatt GV, Lardon C, Plaetke R, Luick BR, Hutchison SH, de Mayolo GA, Ruppert E, Bersamin A. Building a community-based participatory research center to investigate obesity and diabetes in Alaska Natives. Int J Circumpolar Health. 2005;64:281–90 [DOI] [PubMed] [Google Scholar]
- 41.Mohatt GV, Plaetke R, Klejka J, Luick B, Lardon C, Bersamin A, Hopkins S, Dondanville M, Herron J, Boyer B. The center for Alaska Native Health Research study: a community-based participatory research study of obesity and chronic disease-related protective and risk factors. Int J Circumpolar Health. 2007;66:8–18 [DOI] [PubMed] [Google Scholar]
- 42.Berlin NI, Waldmann T, Weissman S. Life span of red blood cell. Physiol Rev. 1959;39:577–616 [DOI] [PubMed] [Google Scholar]
- 43.Cohen RM, Franco R, Khera P, Smith E, Lindsell C, Ciraolo P, Palascak M, Joiner C. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112:4284–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eadie GS, Brown I. The potential life span and ultimate survival of fresh red blood cells in normal healthy recipients as studied by simultaneous Cr51 tagging and differential hemolysis. J Clin Invest. 1955;34:629–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Busch R, Kim YK, Neese RA, Schade-Serin V, Collins M, Awada M, Gardner JL, Beysen C, Marino ME, et al. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta. 2006;1760:730–44 [DOI] [PubMed] [Google Scholar]
- 46.Fry B. Stable isotope ecology. New York, NY: Springer; 2006 [Google Scholar]
- 47.Phillips D. Mixing models in analyses of diet using multiple stable isotopes: a critique. Oecologia. 2001;127:166–70 [DOI] [PubMed] [Google Scholar]
- 48.Phillips D, Koch P. Incorporating concentration dependence in stable isotope mixing models. Oecologia. 2002;130:114–25 [DOI] [PubMed] [Google Scholar]
- 49.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10 [PubMed] [Google Scholar]
- 50.Flanders WD, DerSimonian R, Freedman DS. Interpretation of linear regression models that include transformations or interaction terms. Ann Epidemiol. 1992;2:735–44 [DOI] [PubMed] [Google Scholar]
- 51.O'Leary M. Carbon isotopes in photosynthesis. Bioscience. 1988;38:328–36 [Google Scholar]
- 52.Kuhnlein HV, Receveur O, Soueida R. Arctic indigenous peoples experience the nutrition transition with changing dietary patterns and obesity. J Nutr. 2004;134:1447–53 [DOI] [PubMed] [Google Scholar]
- 53.Nobmann ED, Ponce R, Mattil C, Devereux R, Dyke B, Ebbesson S, Laston S, MacCluer J, Robbins D, Romenesko T, et al. Dietary intakes vary with age among Eskimo adults of northwest Alaska in the GOCADAN study, 2000–2003. J Nutr. 2005;135:856–62 [DOI] [PubMed] [Google Scholar]
- 54.Petzke KJ, Boeing H, Klaus S, Metges CC. Carbon and nitrogen stable isotopic composition of hair protein and amino acids can be used as biomarkers for animal-derived protein intake in humans. J Nutr. 2005;135:1515–20 [DOI] [PubMed] [Google Scholar]
- 55.Kofinas G, Chapin F. BurnSilver S, Schmidt J, Fresco N, Kielland K, Martin S, Springsteen A, Rupp T. Resilience of Athabascan subsistence systems to interior Alaska’s changing climate. Can J For Res. 2010;40:1347–59 [Google Scholar]
- 56.Titus K, Haynes T, Paragi T. The importance of moose, caribou, deer and small game in the diet of Alaskans. : Watson R, Fuller M, Pokras M, Hunt W, Ingestion of lead from spent ammunition: implications for wildlife and humans. Boise (ID): The Peregrine Fund; 2009 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.