Abstract
During colon inflammation, Th17 cells and immunosuppressive regulatory T cells (Treg) are thought to play promotive and preventative roles, respectively. Dietary (n-3) PUFA favorably modulate intestinal inflammation in part by downregulating T-cell activation and functionality. We used the Fat-1 mouse, a genetic model that synthesizes long-chain (n-3) PUFA de novo, to test the hypothesis that (n-3) PUFA protect against colonic inflammation by modulating the polarization of Treg and Th17 cells during colitis. Male and female wild-type (WT) and Fat-1 mice were administered dextran sodium sulfate (DSS) in the drinking water (2.5%) to induce acute (5 d DSS) or chronic (3 cycles DSS) colitis and the percentage of Treg and Th17 cells residing locally [colonic lamina propria (cLP)] and systemically (spleen) was determined by flow cytometry. The percentage of Treg in either tissue site was unaffected by genotype (P > 0.05); however, during chronic colitis, the percentage of Th17 cells residing in both the spleen and cLP was lower in Fat-1 mice compared to WT mice (P < 0.05). Colonic mucosal mRNA expression of critical Th17 cell cytokines and chemokine receptors (IL-17F, IL-21, and CCR6) were lower, whereas expression of the Th17 cell suppressive cytokine, IL-27, was greater in Fat-1 mice compared to WT mice during chronic colitis (P < 0.05). Moreover, colon histological scores were improved in Fat-1 mice (P < 0.05). Collectively, these results demonstrate for the first time, to our knowledge, that (n-3) PUFA can modulate the colonic mucosal microenvironment to suppress Th17 cell accumulation and inflammatory damage following the induction of chronic colitis.
Introduction
Approximately 50% of people with IBD9 use self-prescribed, oral, complementary, alternative medicines/diets, such as FO (1). Long-chain (n-3) PUFA found in FO, specifically EPA and DHA, elicit potent anti-inflammatory effects and have been demonstrated to enhance remission of chronic intestinal inflammation (2); however, the mechanisms underlying this effect have not been rigorously examined to date. In IBD, activation of two inflammatory mucosal CD4+ T cell subsets, Th1 and Th17 cells, play a central role in both the induction and persistence of chronic inflammation in part by producing proinflammatory cytokines (3–6). The inflammatory Th1 subset is inhibited by (n-3) PUFA (ref 7,8); however, the impact on the Th17 cell subset remains unknown. Moreover, with respect to T cell function, (n-3) PUFA have been shown to alter plasma membrane micro-organization (lipid rafts) at the immunological synapse, ultimately suppressing signal transduction and nuclear translocation/activation of transcription factors (9–12). Interestingly, reduced membrane levels of glycosphingolipids, a major component of lipid rafts, resulted in decreased Th17 cell differentiation, whereas other effector T cell subsets were unaffected (13). This indicates that any mediator capable of modulating lipid rafts, such as (n-3) PUFA, may play a role in attenuating T cell signaling and Th17 cell differentiation. In contrast, dietary (n-6) PUFA and their inflammatory eicosanoid products (i.e., PGE2) enhance ulcerative colitis (14) and induce IL-23 production by DC, thereby promoting Th17 differentiation (15, 16). Therefore, any agent that is capable of downregulating PGE2 levels, such as (n-3) PUFA, would be expected to suppress Th17 differentiation (17, 18). Additionally, we have demonstrated that (n-3) PUFA suppress colonic STAT3 activation (19), which is noteworthy, because STAT3 is a key regulator of Th17 differentiation (20). Therefore, we hypothesized that (n-3) PUFA are capable of altering T cell polarization to modulate chronic inflammatory sequelae in the intestine. To date, no studies to our knowledge have examined the effect of (n-3) PUFA on mucosal effector T cell subsets.
The role of the IL-23/Th17 cell pathway in the pathogenesis of IBD has been documented in humans with active disease (21, 22) and in mouse IBD models, wherein disease severity is reduced by blockade or deficiency of IL-23 and/or IL-17 (23, 24). In contrast, immunosuppressive CD4+ FOXP3+ Tregs limit the severity of colitis by suppressing inflammatory cytokine production and reducing colonic tissue damage (25, 26). Furthermore, reduced number and/or activity of Tregs is associated with the development and/or progression of various inflammatory diseases (27). Human data pertaining to the influence of Tregs in IBD are limited; however, IBD patients have increased FOXP3+ CD4+ T cells residing in mucosal lymphoid tissues, which retain their suppressor activity ex vivo (28, 29). Although the severity of colonic inflammation may be limited by Tregs, a change in the proportion of Tregs alone is unable to counterbalance chronic mucosal inflammation. Interestingly, mucosal biopsies from IBD patients had increased mRNA expression of both FOXP3 and IL-17A; however, the ratio of blood Tregs (CD4+ CD25bright FOXP3+):Th17 cells was decreased (21). Therefore, development of innocuous antiinflammatory strategies to favorably modulate T cell subsets (Th17, Th1, and Treg) may prove to be beneficial in the treatment of IBD.
Fat-1 transgenic mice express the Fat-1 gene that encodes an (n-3) fatty acid desaturase enzyme, which converts (n-6) PUFA to (n-3) PUFA by introducing a double bond into fatty acyl chains, thereby providing a means to investigate the biological properties of (n-3) PUFA independently of dietary manipulation (17, 30). Biochemically, splenic CD4+ T cells isolated from Fat-1 mice (genetic model) and mice consuming a 4% FO diet (dietary model) have the same fatty acid composition and degree of (n-3) PUFA phospholipid enrichment (31). Importantly, the dietary and genetic models do not differ in the immunosuppressive functionality of (n-3) PUFA (7, 31). Thus, the Fat-1 mouse model recapitulates the critical features of dietary FO intervention and represents a relevant model for assessing the mechanistic aspects of (n-3) PUFA biology.
Exposure to multiple cycles of DSS represents a wounding model of IBD that induces mucosal injury and subsequent inflammation via the recruitment and activation of inflammatory cells and mediators, ultimately leading to the development of colitis (32). Thus, in this study, Fat-1 and WT mice were exposed to acute or chronic rounds of DSS treatment to determine whether the endogenous production of (n-3) PUFA protects against chronic injury/inflammation by influencing local (cLP) and systemic (spleen) Th17 and FOXP3+ Treg cell populations. Additionally, we assessed how (n-3) PUFA influence the local mucosal cytokine microenvironment to support changes in the resident T cell phenotype during chronic colitis. Lastly, we assessed the influence of (n-3) PUFA on the ex vivo ability of splenic DC to produce key cytokines that could potentially influence effector T cell differentiation and/or function.
Materials and Methods
Mice and diet.
Specific pathogen-free male and female Fat-1 transgenic mice on a C57BL/6 background were genotyped, phenotyped, and housed as previously described (17, 30). Mice were maintained under barrier conditions and consumed ad libitum a 10% safflower oil diet (Research Diets, D03092902R) that was adequate in all nutrients and met the NRC nutrition requirements (33, 34). The diet contained (g/kg diet) 401 sucrose, 200 casein, 150 corn starch, 3 dl-methionine, 35 AIN 76 salt mix, 10 AIN 76 mineral mix (33), 2 choline bitartrate, 50 fiber (cellulose), and 100 safflower oil. GC fatty acid analysis of the diet identified trace levels of (n-3) PUFA (0.17% α-linolenic acid). This study was approved by and followed guidelines set by the Public Health Service Policy and the Institutional Animal Care and Use Committee at Texas A&M University.
Experimental design, colitis induction, and histological scoring.
To induce colonic inflammation, 2.5% DSS (molecular weight, 36,000–50,000; MP Biomedicals) was administered in the drinking water. Mice were either untreated (no DSS) or exposed to DSS for 5 d (acute colitis) and either killed immediately (acute DSS exposure, no recovery time) or following a recovery period lasting 3 d, 1 wk, or 2 wk, wherein fresh tap water was provided (Supplemental Fig. 1). Chronic colitis was induced by administering three consecutive cycles of DSS treatment, wherein one cycle was composed of 5 d of DSS exposure followed by 16 d of fresh tap water consumption.
Changes in lymphocyte populations over time following acute DSS exposure were made in the temporal analysis, whereas a comparison between lymphocyte populations in acute compared to chronic colitis were made separately. Mice were killed by CO2 asphyxiation and the spleen (n = 5–8 mice/genotype) and colon (n = 6–10 mice/genotype) were aseptically removed for lymphocyte isolation. In a confirmatory experiment, colons were removed from chronically DSS-treated mice (WT, n = 12; Fat-1, n = 15) and the degree of colon inflammation and injury were graded (score 0–3) by a board-certified pathologist (B.W.) who was unaware of the treatments, as previously described (17).
Isolation of splenic and cLP lymphocytes.
Splenic lymphocytes were isolated as previously described (35). cLP lymphocytes were isolated as previously described (36) with the minor modification that Ca2+ and Mg2+-free HBSS medium (Sigma Aldrich) supplemented with 1 mmol/L glutamine, 0.1% BSA, 5 mmol/L DTT, and 30 mmol/L EDTA containing 20 kU/L of both type II and type IV collagenase (Worthington) and 5 kU/L DNase (Worthington) was used as the digesting solution.
Flow cytometry analysis of Treg and Th17 cells.
One million viable splenic and cLP cells were obtained from each mouse and, following FcγR blocking, cells underwent surface (CD4 and CD25) and intracellular (FOXP3 and IL-17A) staining. Staining protocol details and representative histograms are shown in Supplemental Figure 2. Antibodies used included FITC-anti-CD4 (RM 4–5), APC-anti-CD25 (PC61.5), PE-anti-FOXP3 (FJK-16s, eBioscience), and PE-anti-IL-17 (TC11–18H10, BD Pharmingen). Flow cytometric analysis was conducted using an Accuri C6 flow cytometer (Accuri Cytometers).
RNA isolation and measurement of mRNA expression.
Following three cycles of DSS (chronic) exposure, RNA was isolated from scraped mucosal samples from a subset of mice (Fat-1 n = 8, WT n = 5) using RNAqueous Total RNA kit (Ambion). RT of 1 μg of sample RNA was performed using M-MLV Reverse Transcriptase (Invitrogen). qRT-PCR was used to quantify mRNA expression and amplification was performed using the Taqman Universal PCR master mix (Applied Biosystems). Taqman gene expression kits (Applied Biosystems) were used for each gene of interest: murine Il6 (Mm00446190_m1), Il17a (Mm00439618_m1), Il17f (Mm00521423_m1), Il21(Mm00517640_m1), Il23(Mm00518984_m1), Il27(Mm004611664_m1), Ifng (Mm01168134_m1), Il23r (Mm00519943_m1), Ccl20 (Mm01268754_m1), and Ccr6 (Mm99999114_s1). Amplification of mRNA (fluorescence) was recorded over 40 cycles and the corresponding Ct was used to calculate mRNA expression according to the calculation: 2(ΔCt). Target gene expression was normalized to ribosomal 18 S expression.
Enrichment and culture of splenic CD11c+ DC.
Spleens were pooled from 4–5 mice (equal numbers of males and females) to obtain final samples sizes comprising 3 and 4 pooled samples in the WT and Fat-1 groups, respectively. The DC population was isolated as described (37) with 1 g/L of collagenase D (Roche Diagnostics) dissolved in complete RPMI 1640 medium with 25 mmol/L HEPES (Irvine Scientific), supplemented with 5% FBS (Irvine Scientific), 2 mmol/L GlutaMAX (Gibco), 100 kU/L penicillin, and 0.1 kg/L streptomycin (Gibco) (henceforth “complete medium”), followed by discontinuous centrifugation. The DC population was enriched using CD11c magnetic beads (Miltenyi Biotec) and cells were sorted on a BD FACS Aria II flow cytometer based on expression of CD11chi (Supplemental Fig. 3). Surface staining for CD11c was conducted using PE-anti-mouse CD11c (1 mg/L) (HL3, BD Pharmingen) utilizing a different clone relative to the CD11c microbeads. Purity of the sorted CD11chi DC population was 96% in both the WT and Fat-1 mice and the average cell viability (by Trypan blue exclusion) exceeded 96%. In 96-well, flat-bottom Falcon plates, 2 × 105 viable CD11chi DC/well were cultured for 24 h at 37°C in complete RPMI and the resultant supernatant was stored at −80°C. LPS (E. coli 055:B5, Sigma Aldrich) was used as an inflammatory stimulus and select cultures were exposed to 10 mg/L LPS, whereas unstimulated cultures received an equivalent volume of complete RPMI.
Cytokine analysis of culture supernatants.
In vitro cytokine concentrations of IL-1β, IL-6, IL-10, IL-12p40, IL-17, IFNγ, and TNFα were simultaneously measured utilizing the Bio-Plex Pro Mouse Cytokine Group I multiplex kit (BioRad) on a Bio-Plex 200 System and accompanying software package, Bio-Plex Manager 6.0 (BioRad). Milliplex MAP kits (Millipore) were used to individually measure IL-23 (MPXMCYP3–74K), IL12p70 (MPXMCYTO-70K), and TGF-β1 (TGFB-64K-01). Samples were acid activated per the manufacturers’ instructions for analysis of TGFβ1. For all cytokine assessments, samples were analyzed in duplicate.
Statistics.
Normally distributed data were analyzed by two-way ANOVA with main effects genotype and treatment (i.e., acute or chronic DSS exposure, Fig. 2, or LPS exposure, Fig. 3). In the temporal analysis assessing lymphocyte populations during the recovery phase postacute DSS exposure, wherein the main effects were genotype and time. Least squares means was used for post hoc comparisons. Differences were considered significant at P < 0.05. Normally distributed data sets are presented as means ± SEM. Nonparametric data were analyzed using the Kruskal-Wallis test (α = 0.05). Data are presented as medians and the Wilcoxon rank sums are shown in the figure legend. All analyses were conducted using the SAS system (SAS Institute) for Windows (version 9.0).
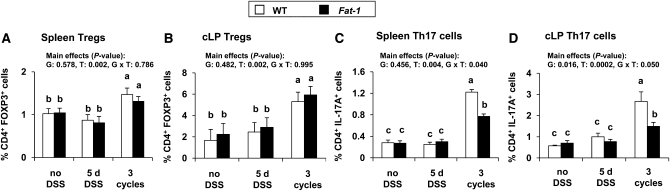
FIGURE 2.
Percentage of splenic Treg (A), cLP Treg (B), splenic Th17 cells (C), and cLP Th17 cells (D) in WT and Fat-1 mice untreated (no DSS), exposed to 5 d DSS (acute colitis), or 3 cycles DSS (chronic colitis). P values for effects of G, T, and G × T are shown. Bars represent mean ± SEM, n = 5–8 (spleen) or 6–10 (colon). Means without a common letter differ, P < 0.05. cLP, colon lamina propria; DSS, dextran sodium sulfate; G, genotype; G × T, interaction of genotype and treatment; T, treatment; Tregs, regulatory T cells.
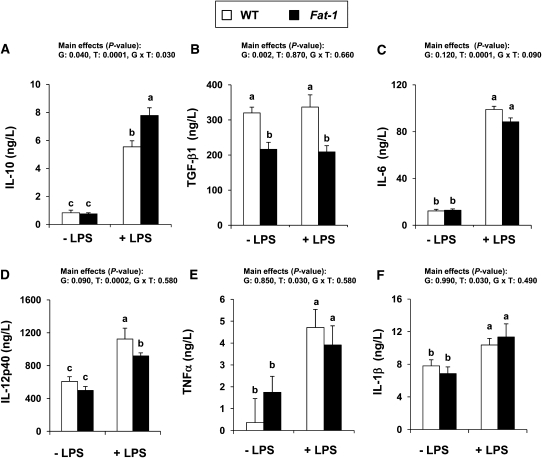
FIGURE 3.
Ex vivo production of IL-10 (A), TGFβ1 (B), IL-6 (C), IL-12p40 (D), TNFα (E), and IL-1β (F) by splenic CD11chi DC from WT and Fat-1 mice exposed to 3 cycles DSS and cultured for 24 h with or without 10 mg/L LPS. Bars represent mean ± SEM, n = 3 (WT) or 4 (Fat-1) pooled samples from 4–5 mice. P values for effects of G, T, and G × T are shown. Means without a common letter differ, P < 0.05. DC, dendritic cell; DSS, dextran sodium sulfate; G, genotype; G × T, interaction of genotype and treatment; T, treatment; WT, wild type.
Results
Fat-1 mice are more resistant to DSS-induced chronic inflammation.
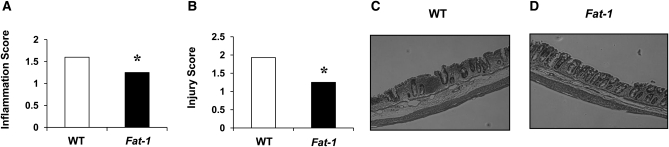
The effect of (n-3) PUFA on colon histological changes following acute DSS exposure were not measured but have been documented elsewhere (19). Therefore, as a confirmatory experiment, histological evaluation was conducted on mice exposed to 3 cycles of DSS (chronic colitis) (Fig. 1). Fat-1 mice had lower colonic inflammation (P = 0.04) and injury (P = 0.03) scores compared to WT mice.
FIGURE 1.
Colon inflammation (A), injury (B) scores, and representative images for WT (C) and Fat-1 mice (D) exposed to 3 cycles of DSS. Values are medians and the Wilcoxon rank sums for WT and Fat-1, respectively, were: 169 and 84 (A) and 213 and 88 (B), n = 12 (WT) or 15 (Fat-1). *Different from WT, P < 0.05 (Kruskal-Wallis test). Representative images are at 100× magnification. WT, wild type.
No effect of (n-3) PUFA on Treg and Th17 cell numbers following acute DSS exposure.
A temporal analysis was conducted to assess the kinetic response of Treg and Th17 cells in the spleen and cLP during the recovery period following acute (5 d) DSS exposure (Table 1). WT and Fat-1 mice had a similar response to acute DSS exposure, since there was no effect of genotype on changes in lymphocyte populations (Treg and Th17 cells) in either tissue site (spleen and cLP) at each post-acute DSS recovery time point investigated. Therefore, when the genotypes were combined, the proportion of splenic Tregs was greater 3 d after acute DSS exposure compared to untreated mice (P < 0.001) and continued to increase as recovery time progressed, whereas in the cLP, the Treg kinetic response was delayed in DSS-treated mice and differed from untreated mice only at the 2-wk recovery time point post-acute DSS exposure (P = 0.03) (Table 1). Compared to untreated mice, the kinetic response of Th17 cells to acute DSS exposure was greater in the cLP (locally) and the spleen (systemically) at 3 d postacute exposure (P < 0.001 in both tissue sites) (Table 1). Furthermore, as recovery time progressed, in both tissue sites the percentage of Th17 cells continued to rise and differed from the both the percentage detected at 3 d recovery post-acute DSS exposure (spleen, P < 0.0001 and cLP, P = 0.004) and mice not permitted time to recover (5 d DSS) (P = 0.0001 in the spleen and cLP).
TABLE 1.
Percentage of Tregs and Th17 cells in the spleen and cLP of WT and Fat-1 mice that were untreated (no DSS), exposed to DSS for 5 d (acute colitis), or allowed to recover post-acute DSS exposure for 3d, 1 wk, or 2 wk1
| Spleen |
cLP |
|||
| Treatment | % Treg | % Th17 | % Treg | % Th17 |
| No DSS | 1.03 ± 0.10b | 0.28 ± 0.02c | 1.95 ± 0.69b | 0.60 ± 0.06c |
| 5 d DSS (acute colitis, no recovery) | 0.74 ± 0.05b | 0.27 ± 0.02c | 3.06 ± 0.26b | 0.87 ± 0.10c |
| 5 d DSS + 3 d recovery | 1.45 ± 0.08a | 0.38 ± 0.02b | 4.11 ± 0.87b | 1.47 ± 0.20b |
| 5 d DSS + 1 wk recovery | 1.45 ± 0.10a | 0.66 ± 0.06a | 3.61 ± 0.51b | 1.41 ± 0.20b |
| 5 d DSS + 2 wk recovery | 1.33 ± 0.15a | 0.73 ± 0.04a | 5.88 ± 0.86a | 2.74 ± 0.48a |
Values are means ± SEM, = 10–14. Because there was no effect of genotype, data from WT and Fat-1 mice were combined. Means in a column without a common letter differ, P < 0.05. cLP, colon lamina propria; DSS, dextran sodium sulfate; Tregs, regulatory T cells; WT, wild type.
During chronic DSS exposure (n-3) PUFA sustain Tregs and lower Th17 cells in the mucosa.
The effects of acute and chronic DSS exposure on the proportion of Treg and Th17 cells in the cLP and spleen of Fat-1 and WT mice were subsequently determined. The acute colitis phase (5 d DSS) had no effect on the percentage of Treg or Th17 cells residing in the cLP and spleen in either genotype compared to untreated mice (Fig. 2). In contrast, following the induction of chronic colitis (3 cycles of DSS exposure), both Fat-1 and WT mice had a greater proportion of Tregs and Th17 cells residing in both the cLP and spleen compared to the untreated and acute colitis groups ( ). During chronic colitis, there was no difference between Fat-1 and WT mice in the percentage of Tregs residing in either tissue site (Fig. 2A,B); however, Fat-1 mice had a lower percentage of Th17 cells in both the spleen (P = 0.005) and cLP (P = 0.01) compared to WT mice (Fig. 2C,D). Collectively, these results demonstrate that (n-3) PUFA can favorably alter the ratio between Treg and Th17 cell populations following chronic DSS exposure by sustaining Treg and lowering Th17 cell numbers, respectively.
The colonic mucosal cytokine microenvironment is modulated by (n-3) PUFA in a manner consistent with decreased Th17 cell maintenance.
Because (n-3) PUFA lowered the percentage of Th17 cells in the cLP following the induction of chronic inflammation in the colon (Fig. 2), we measured mRNA expression levels of key cytokines and cytokine receptors involved in the differentiation, trafficking, and/or long-term maintenance of the Th17 cell population (Table 2). In Fat-1 mice, colonic mucosal mRNA expression of IL-17F was lower (P = 0.03) compared to WT, whereas there was no difference between genotypes for IL-17A. Additionally, colonic mucosal IL-21 expression was lower (P = 0.02) in Fat-1 mice compared to WT, consistent with a role of (n-3) PUFA in affecting cLP Th17 cell polarization and activation. There were no differences in the mRNA expression levels for IL-6 and IL-23 between genotypes. Signaling via the IL-23R is critical for maintaining the Th17 cell phenotype; however, there was no difference in the mucosal expression of IL-23R. Interestingly, colonic mRNA expression of IL-27, a cytokine that elicits suppressive effects on Th17 cells (38, 39), was greater in Fat-1 mice (P = 0.04) relative to WT mice. Colonic mucosal expression of IFNγ was lower (P = 0.04) in Fat-1 mice, consistent with the inhibitory effect of (n-3) PUFA on Th1-mediated effects (7, 8). Lastly, mRNA expression of CCR6, a chemokine receptor expressed on Th17 cells involved in the trafficking of Th17 cells to the colon (40), was lower (P = 0.03) in Fat-1 mice, whereas expression of its ligand, CCL20, did not differ between genotypes.
TABLE 2.
Colonic mucosal mRNA expression in WT and Fat-1 mice exposed to 3 cycles of DSS (chronic colitis)1
| Gene | WT | Fat-1 |
| relative expression2 | ||
| Il17a | 3.25 ± 0.45 | 2.55 ± 0.77 |
| Il17f | 2.93 ± 1.03 | 1.44 ± 0.19* |
| Il21 | 2.28 ± 0.60 | 0.76 ± 0.40* |
| Il27 | 0.70 ± 0.26 | 3.59 ± 0.88* |
| Ifng | 3.19 ± 0.53 | 1.05 ± 0.17* |
| Il6 | 4.61 ± 1.80 | 2.33 ± 0.51 |
| Il23 | 3.08 ± 0.52 | 4.10 ± 0.46 |
| Il23r | 5.40 ± 0.72 | 5.20 ± 0.30 |
| Ccr6 | 15.47 ± 1.36 | 7.80 ± 2.02* |
| Ccl20 | 12.20 ± 3.90 | 7.43 ± 2.80 |
Values are means ± SEM, = 5 (WT) or 8 (Fat-1). *Different from WT, P < 0.05. DSS, dextran sodium sulfate; WT, wild type.
Data were normalized to ribosomal 18 S.
Splenic CD11c+ DC cytokine production is altered by (n-3) PUFA.
Cytokines produced by APC can influence the differentiation, proliferation, and/or maintenance of the Th17 cell population and, moreover, this cellular compartment itself may also be directly affected by IBD and/or (n-3) PUFA. Therefore, cytokine secretion by splenic purified CD11chi DC from both Fat-1 and WT mice exposed to three cycles of DSS was examined. Although CD11chi DC were not isolated from the colon (target tissue), outcomes from the spleen are relevant, because the percentage of Th17 cells was lower in this tissue in Fat-1 mice compared to WT. Following LPS stimulation, DC isolated from Fat-1 mice produced higher levels of IL-10 protein compared to WT mice (Fig. 3A), whereas TGFβ1 levels were suppressed in Fat-1 cultures (Fig. 3B). DC from Fat-1 mice produced less IL-12p40 compared to WT mice (Fig. 3D). The genotypes did not differ in the levels of IL-6, TNFα, and IL-1β (Fig. 3C,E,F), whereas IL-12p70 and IL-23p19 were below detectable levels in both groups. Collectively, these data indicate that (n-3) PUFA alter DC cytokine production, and thus their influence on the local cytokine microenvironment, in a manner consistent with the suppression of inflammation and Th17 cell polarization.
Discussion
In the present investigation, we confirmed (17) that Fat-1 mice have an enhanced ability to resolve chronic DSS-induced colitis (Fig. 1), which is consistent with the effect of dietary (n-3) PUFA on long-term resolution of colon inflammation and mucosal repair (17, 18). Following acute DSS exposure, (n-3) PUFA had no influence on the proportion of Treg and Th17 cells residing in the spleen and cLP (Fig. 2). Conversely, during chronic colitis, the proportion of Th17 cells in both the spleen and cLP was lower in Fat-1 mice, whereas the proportion of Treg was unaffected in either tissue site (Fig. 2). Importantly, the magnitude of the rise in the percentage of Th17 cells in WT mice was similar to changes reported in humans with IBD (21), thereby demonstrating the ability of the chronic DSS model to recapitulate this critical aspect of IBD pathology. A subpopulation of T cells coexpressing Th17 and FOXP3 has been identified (41, 42), and although their contribution cannot be definitively excluded from our assessment of Th17 cells, the size of this subpopulation has been reported to be low (41). Previously, dietary (n-3) PUFA have been shown to increase FOXP3 expression in murine T cells (43, 44); however, these cells did not retain suppressive function in vitro (44). Similarly, in human IBD, an increase in Treg numbers alone is insufficient to overcome the inflammatory effects of other upregulated effector T cell populations (i.e., Th17 and Th1) within the mucosa (21, 25, 26, 28, 29). Thus, during chronic colitis, (n-3) PUFA improve the clinical phenotype and favorably modulate the balance between Treg and Th17 cell populations residing locally (cLP) and systemically (spleen) by lowering the proportion of Th17 cells, which are strongly implicated in the pathogenesis of IBD (21–24) and modulate the mucosal cytokine microenvironment in a manner consistent with reduced Th17 cell function (Table 2).
To probe some possible mechanisms underlying the (n-3) PUFA-dependent reduction in cLP Th17 cells, we considered the expression of genes involved in several pathways affecting the mucosal cytokine microenvironment. Although mRNA and protein expression levels do not necessarily correlate, documenting (n-3) PUFA-induced changes in gene expression can provide insight into the underlying mechanisms driving the reduction in Th17 cell numbers in Fat-1 mice. Additional studies are required to distinguish between individual (n-3) PUFA effects on Th17 cell polarization, expansion, maintenance, and/or trafficking of Th17 cells to the inflamed mucosa.
Previously, we showed that (n-3) PUFA suppress the activation of STAT3, a critical transcription factor regulating Th17 differentiation (19). In combination with TGFβ, the presence of either IL-6 or IL-21 in the cellular microenvironment drives Th17 cell differentiation (45). Previously (n-3) PUFA have been shown to reduce ex vivo IL-6 production (46); however, colonic expression was unaffected in the present study. Conversely, IL-21 gene expression was lower in Fat-1 mice, which is noteworthy, because IL-21 promotes the induction and/or perpetuation of T-cell–dependent inflammatory processes controlling both Th1 and Th17 cell responses (4). The combination of TGFβ and IL-21 suppresses FOXP3 expression in addition to supporting Th17 cell differentiation (47, 48), thereby modulating the balance between Treg and Th17 cells. In IBD, mucosal expression of IL-21 and its receptor are elevated (49, 50) and blockade of IL-21 signaling results in downregulated expression of IL-17 and IFNγ (50). Thus, IL-21 signaling provides a mechanism through which both pathogenic Th17 and Th1 effector cells can be modulated. Interestingly, we demonstrated that (n-3) PUFA also lower gene expression of IL-17F and IFNγ (Table 2) and therefore disruption of IL-21 signaling may represent one potential mechanism through which (n-3) PUFA suppress expression of not only Th17 cell, but also Th1 cell-mediated inflammation, as previously reported (7, 8). Collectively, these results suggest a combined inhibitory effect of (n-3) PUFA on biological functions of both Th17 and Th1 effector T cell populations during chronic colitis.
In addition to Th17 cells, other innate cellular sources of IL-17 have been defined (51). Thus, a scraped mucosal sample could include all resident IL-17–producing cellular sources, which may help to explain the apparent conflicting findings of a reduced percentage of Th17 cells (CD4+ IL-17A+) in the cLP of Fat-1 mice, but no difference in mucosal gene expression of IL-17A. IL-17A and IL-17F signal through the same receptor complex and exhibit redundant biological activities in the pathogenesis of colitis (52, 53). In this context, IL-17F mRNA expression was lower in Fat-1 mice (Table 2) and therefore may be indicative of (n-3) PUFA-mediated suppression of Th17 cell function. Furthermore, (n-3) PUFA enrichment in the chronically inflamed colonic mucosa resulted in greater IL-27 mRNA expression (Table 2), a cytokine that has been shown to antagonize Th17 cell development (38, 39). Therefore, a complementary mechanism through which (n-3) PUFA modulate colonic Th17 cells could be via the suppressive actions of IL-27. Lastly, (n-3) PUFA suppressed colonic mucosal mRNA expression of the chemokine receptor CCR6 (Table 2), which is characteristically expressed by Th17 cells and is important for their migration and localization to intestinal mucosal tissues (40). It remains plausible that (n-3) PUFA could suppress Th17 cell trafficking to the inflamed colonic mucosa by decreasing expression of a critical homing receptor. In IBD, IL-23 drives colonic inflammation (22, 23) and is involved in expanding Th17 cell responses, including survival (4). Interestingly, (n-3) PUFA had no effect on mucosal IL-23 or IL-23R expression. These findings suggest that the reduced proportion of Th17 cells in the cLP of Fat-1 mice is unlikely a result of reduced maintenance of a differentiated Th17 cell population through IL-23-mediated signaling. Collectively, analysis of mucosal gene expression data indicate that (n-3) PUFA likely affect the colonic microenvironment by a combination of mechanisms centered on Th17 cell differentiation, function, and trafficking.
Splenic Th17 cell numbers were reduced in Fat-1 mice, suggesting that cytokine secretion by discrete cell types also residing in the spleen can influence the local cytokine microenvironment and Th17 cell polarization. Therefore, we determined if (n-3) PUFA altered the splenic DC cytokine profile. Fat-1 mice exhibited greater IL-10 and reduced TGF-β1 ex vivo production, respectively (Fig. 3). This is noteworthy, because despite being conventionally regarded as an anti-inflammatory cytokine (54), TGFβ is an important promoter of Th17 polarization (55), whereas the anti-inflammatory actions of IL-10 suppress colonic inflammation (56). Moreover, Th17 cells express the IL-10 receptor and are directly suppressed via IL-10 signaling (57), indicating that increased IL-10 production by (n-3) PUFA, in any cell type, can ultimately skew the cytokine microenvironment in a manner consistent with suppression of Th17 cells. Previous studies have also shown that APCs can negatively contribute to the regulation of Th17 cell differentiation through an IL-10 mechanism (58). Further, these data are consistent with previous findings indicating that (n-3) PUFA can alter the DC activation state via a lipid raft-dependent mechanism (59, 60). Therefore, as a consequence of action at the cell membrane, (n-3) PUFA may modulate the tolerogenic conditioning of DC and suppress Th17 cell polarization, at least in part by limiting the production of TGFβ and promoting IL-10 production. Collectively, these data indicate that (n-3) PUFA alter DC cytokine production in a manner consistent with the suppression of inflammation and Th17 cell polarization.
In summary, the present study has defined a novel role for (n-3) PUFA in the suppression of inflammatory Th17 cells during chronic-DSS induced colitis, wherein the imbalance between regulatory and inflammatory effector T cell induction contributes to the etiology of IBD (21, 56, 61). Changes in mucosal cytokine gene expression provide insight into some of the preliminary mechanisms through which (n-3) PUFA may modulate Th17 cell function within the inflamed colon. Further studies are required to characterize the molecular targets and elucidate the signaling mechanisms by which (n-3) PUFA suppress local T-cell activation and ameliorate IBD. Collectively, these findings strongly suggest that (n-3) PUFA offer potential as an innocuous anti-inflammatory agent or as an adjunctive therapy with therapeutic drugs designed to reset the balance between Treg and Th17 cells during chronic colonic inflammation, thereby reducing disease severity and improving the clinical outcome.
Supplementary Material
Acknowledgments
The authors thank Jane S. Miller for technical assistance. Fat-1 breeder mice were generously provided by Dr. Jing Kang, Harvard School of Medicine. J.M.M., Q.J., R.C.A., D.N.M., and R.S.C. designed the research; J.M.M., Q.J., B.W. conducted the research and analyzed the data; J.M.M., D.N.M., and R.S.C. wrote the paper and had primary responsibility for the final content; E.C. was responsible for animal husbandry. All authors have read and approved the final manuscript.
Footnotes
Supported by NIH grant DK071707, USDA 2008-34402-19195, and by the Vegetable and Fruit Improvement Center. Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship PDF-388466-2010 (J.M.M.).
Supplemental Figures 1–3 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: APC, antigen-presenting cell; cLP, colon lamina propria; DC, dendritic cell; DSS, dextran sodium sulfate; FO, fish oil; FOXP3, forkhead box P3; IBD, inflammatory bowel disease; IL-23R, IL-23 receptor; STAT3, signal transducer and activator of transcription 3; Tregs, regulatory T cells; WT, wild type.
Literature Cited
- 1.Kong SC, Hurlstone DP, Pocock CY, Walkington LA, Farquharson NR, Bramble MG, McAlindon ME, Sanders DS. The incidence of self-prescribed oral complementary and alternative medicine use by patients with gastrointestinal diseases. J Clin Gastroenterol. 2005;39:138–41 [PubMed] [Google Scholar]
- 2.Turner D, Shah PS, Steinhart AH, Zlotkin S, Griffiths AM. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): a systematic review and meta-analyses. Inflamm Bowel Dis. 2011;17:336–45 [DOI] [PubMed] [Google Scholar]
- 3.Liu ZJ, Yadav PK, Su JL, Wang JS, Fei K. Potential role of Th17 cells in the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2009;15:5784–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarra M, Pallone F, Macdonald TT, Monteleone G. IL-23/IL-17 axis in IBD. Inflamm Bowel Dis. 2010;16:1808–13 [DOI] [PubMed] [Google Scholar]
- 5.Shih DQ, Targan SR. Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol. 2008;14:390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zenewicz LA, Antov A, Flavell RA. CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med. 2009;15:199–207 [DOI] [PubMed] [Google Scholar]
- 7.Zhang P, Kim W, Zhou L, Wang N, Ly LH, McMurray DN, Chapkin RS. Dietary fish oil inhibits antigen-specific murine Th1 cell development by suppression of clonal expansion. J Nutr. 2006;136:2391–8 [DOI] [PubMed] [Google Scholar]
- 8.Zhang P, Smith R, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids modulate murine Th1/Th2 balance toward the Th2 pole by suppression of Th1 development. J Nutr. 2005;135:1745–51 [DOI] [PubMed] [Google Scholar]
- 9.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–60 [DOI] [PubMed] [Google Scholar]
- 10.Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J Immunol. 2008;181:6236–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim W, Khan NA, McMurray DN, Prior IA, Wang N, Chapkin RS. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog Lipid Res. 2010;49:250–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yog R, Barhoumi R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress mitochondrial translocation to the immunologic synapse and modulate calcium signaling in T cells. J Immunol. 2010;184:5865–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Y, Gumlaw N, Karman J, Zhao H, Zhang J, Jiang JL, Maniatis P, Edling A, Chuang WL, Siegel C, et al. Lowering glycosphingolipid levels in CD4+ T cells attenuates T cell receptor signaling, cytokine production, and differentiation to the Th17 lineage. J Biol Chem. 2011;286:14787–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tjonneland A, Overvad K, Bergmann MM, Nagel G, Linseisen J, Hallmans G, Palmqvist R, Sjodin H, Hagglund G, Berglund G, et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut. 2009;58:1606–11 [DOI] [PubMed] [Google Scholar]
- 15.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, Tuma R, Ganea D. The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23→IL-17 axis. J Immunol. 2007;178:8138–47 [DOI] [PubMed] [Google Scholar]
- 17.Jia Q, Lupton JR, Smith R, Weeks BR, Callaway E, Davidson LA, Kim W, Fan YY, Yang P, Newman RA, et al. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 2008;68:3985–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieto N, Torres MI, Rios A, Gil A. Dietary polyunsaturated fatty acids improve histological and biochemical alterations in rats with experimental ulcerative colitis. J Nutr. 2002;132:11–9 [DOI] [PubMed] [Google Scholar]
- 19.Jia Q, Ivanov I, Zlatev Z, Alaniz RC, Weeks BR, Callaway E, Goldsby JS, Davidson LA, Fan YY, Zhou L, et al. Fish oil and cucumin combination modulate colonic cytokinetics and gene expression in DSS-treated mice. Br J Nutr. 2011;106:519–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durant L, Watford WT, Ramos HL, Laurence A, Vahedi G, Wei L, Takahashi H, Sun HW, Kanno Y, Powrie F, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–9 [DOI] [PubMed] [Google Scholar]
- 22.Hölttä V, Klemetti P, Sipponen T, Westerholm-Ormio M, Kociubinski G, Salo H, Rasanen L, Kolho KL, Farkkila M, Savilahti E, et al. IL-23/IL-17 immunity as a hallmark of Crohn's disease. Inflamm Bowel Dis. 2008;14:1175–84 [DOI] [PubMed] [Google Scholar]
- 23.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–70 [DOI] [PubMed] [Google Scholar]
- 24.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–43 [DOI] [PubMed] [Google Scholar]
- 26.Powrie F. Immune regulation in the intestine: a balancing act between effector and regulatory T cell responses. Ann N Y Acad Sci. 2004;1029:132–41 [DOI] [PubMed] [Google Scholar]
- 27.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saruta M, Yu QT, Fleshner PR, Mantel PY, Schmidt-Weber CB, Banham AH, Papadakis KA. Characterization of FOXP3+CD4+ regulatory T cells in Crohn's disease. Clin Immunol. 2007;125:281–90 [DOI] [PubMed] [Google Scholar]
- 29.Yu QT, Saruta M, Avanesyan A, Fleshner PR, Banham AH, Papadakis KA. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191–9 [DOI] [PubMed] [Google Scholar]
- 30.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. [DOI] [PubMed] [Google Scholar]
- 31.Fan YY, Kim W, Callaway E, Smith R, Jia Q, Zhou L, McMurray DN, Chapkin RS. fat-1 transgene expression prevents cell culture-induced loss of membrane n-3 fatty acids in activated CD4+ T-cells. Prostaglandins Leukot Essent Fatty Acids. 2008;79:209–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vowinkel T, Kalogeris TJ, Mori M, Krieglstein CF, Granger DN. Impact of dextran sulfate sodium load on the severity of inflammation in experimental colitis. Dig Dis Sci. 2004;49:556–64 [DOI] [PubMed] [Google Scholar]
- 33.Report of the American Institute of Nutrition ad hoc Committee on Standards for Nutritional Studies. J Nutr. 1977;107:1340–8 [DOI] [PubMed] [Google Scholar]
- 34.NRC Subcommittee on Laboratory Animal Nutrition. Nutrient requirements of laboratory animals. 4th rev. ed Washington, DC: National Academy of Sciences; 1995 [Google Scholar]
- 35.Ly LH, Smith R III, Chapkin RS, McMurray DN. Dietary n-3 polyunsaturated fatty acids suppress splenic CD4(+) T cell function in interleukin (IL)-10(−/−) mice. Clin Exp Immunol. 2005;139:202–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sund M, Xu LL, Rahman A, Qian BF, Hammarstrom ML, Danielsson A. Reduced susceptibility to dextran sulphate sodium-induced colitis in the interleukin-2 heterozygous (IL-2) mouse. Immunology. 2005;114:554–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G, Brinster C. Isolation of dendritic cells. Curr Protoc Immunol. 2009;Chapter 3:Unit 3 7 [DOI] [PubMed] [Google Scholar]
- 38.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–36 [DOI] [PubMed] [Google Scholar]
- 39.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–45 [DOI] [PubMed] [Google Scholar]
- 40.Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2009;2:173–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voo KS, Wang YH, Santori FR, Boggiano C, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–9 [DOI] [PubMed] [Google Scholar]
- 43.Woodworth HL, McCaskey SJ, Duriancik DM, Clinthorne JF, Langohr IM, Gardner EM, Fenton JI. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res. 2010;70:7960–9 [DOI] [PubMed] [Google Scholar]
- 44.Yessoufou A, Ple A, Moutairou K, Hichami A, Khan NA. Docosahexaenoic acid reduces suppressive and migratory functions of CD4+CD25+ regulatory T-cells. J Lipid Res. 2009;50:2377–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abraham C, Cho JH. IL-23 and autoimmunity: new insights into the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2009;60:97–110 [DOI] [PubMed] [Google Scholar]
- 46.Damsgaard CT, Lauritzen L, Calder PC, Kjaer TR, Frokiaer H. Reduced ex vivo interleukin-6 production by dietary fish oil is not modified by linoleic acid intake in healthy men. J Nutr. 2009;139:1410–4 [DOI] [PubMed] [Google Scholar]
- 47.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–3 [DOI] [PubMed] [Google Scholar]
- 49.Liu Z, Yang L, Cui Y, Wang X, Guo C, Huang Z, Kan Q, Liu Y. Il-21 enhances NK cell activation and cytolytic activity and induces Th17 cell differentiation in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1133–44 [DOI] [PubMed] [Google Scholar]
- 50.Monteleone G, Monteleone I, Fina D, Vavassori P, Del Vecchio Blanco G, Caruso R, Tersigni R, Alessandroni L, Biancone L, Naccari GC, et al. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn's disease. Gastroenterology. 2005;128:687–94 [DOI] [PubMed] [Google Scholar]
- 51.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89 [DOI] [PubMed] [Google Scholar]
- 52.Leppkes M, Becker C, Ivanov II, Hirth S, Wirtz S, Neufert C, Pouly S, Murphy AJ, Valenzuela DM, Yancopoulos GD, et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–67 [DOI] [PubMed] [Google Scholar]
- 53.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–9 [DOI] [PubMed] [Google Scholar]
- 54.Veldhoen M, Stockinger B. TGFbeta1, a "Jack of all trades": the link with pro-inflammatory IL-17-producing T cells. Trends Immunol. 2006;27:358–61 [DOI] [PubMed] [Google Scholar]
- 55.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4 [DOI] [PubMed] [Google Scholar]
- 56.Kelsall BL. Innate and adaptive mechanisms to control [corrected] pathological intestinal inflammation. J Pathol. 2008;214:242–59 [DOI] [PubMed] [Google Scholar]
- 57.Huber S, Gagliani N, Esplugues E, O'Connor W, Jr, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3 and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu Y, Yang J, Ouyang X, Liu W, Li H, Bromberg J, Chen SH, Mayer L, Unkeless JC, Xiong H. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008;38:1807–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong SW, Kwon MJ, Choi AM, Kim HP, Nakahira K, Hwang DH. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeyda M, Saemann MD, Stuhlmeier KM, Mascher DG, Nowotny PN, Zlabinger GJ, Waldhausl W, Stulnig TM. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-kappaB activation. J Biol Chem. 2005;280:14293–301 [DOI] [PubMed] [Google Scholar]
- 61.Rubin DT, Kavitt RT. Surveillance for cancer and dysplasia in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:581–604 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.