Abstract
Background & objectives:
There is no published literature on the extent of vitamin B12 deficiency in elderly Indians as determined by plasma vitamin B12 levels and methylmalonic acid (MMA) levels. Vitamin B12 deficiency is expected to be higher in elderly Indians due to vegetarianism, varied socio-economic strata and high prevalence of Helicobacter pylori infection. We therefore, studied the dietary habits of south Indian urban elderly population and measured vitamin B12, MMA red cell folate and homocysteine (Hcy) levels.
Methods:
Healthy elderly urban subjects (175, >60 yr) were recruited. Detailed history, physical examination and neurological assessment were carried out. Food Frequency Questionnaire (FFQ) for dietary analysis for daily intake of calories, vitamin B12, folate and detailed psychological assessment for cognitive functions was carried out. Blood samples were analyzed for routine haematology and biochemistry, vitamin B12, red cell folate, MMA and Hcy.
Results:
The mean age of the study population was 66.3 yr. Median values for daily dietary intake of vitamin B12 and folate were 2.4 and 349.2 μg/day respectively. Sixty two (35%) participants consumed multivitamin supplements. Plasma vitamin B12 level and the dietary intake of vitamin B12 was significantly correlated (P=0.157). Plasma vitamin B12 and Hcy were inversely correlated (P= -0.509). Red cell folate was inversely correlated with Hcy (P= -0.550). Significant negative correlation was observed between plasma vitamin B12 and MMA in the entire study population (P= -0.220). Subjects consuming vitamin supplements (n=62) had significantly higher plasma vitamin B12 levels, lower MMA levels and lower Hcy levels. There was no significant correlation between plasma vitamin B12, MMA, Hcy and red cell folate and any of the 10 cognitive tests including Hindi Mental Status Examination (HMSE).
Interpretation & conclusions:
Our study is indicative of higher vitamin B12 (2.4 μg/day) intakes in urban south Indian population. Thirty five per cent of the study population consumed multivitamin supplements and therefore, low plasma vitamin B12 levels were seen only in 16 per cent of the study subjects. However, MMA was elevated in 55 per cent and Hcy in 13 per cent of the subjects.
Keywords: Cognitive assessment, geriatric, methylmalonic acid, nutrition, vitamin B12 deficiency
Over the last few decades, many investigators have reported a high prevalence of vitamin B12 deficiency in elderly population1–3; although a small minority has disputed this incidence4–6. The published prevalence of subnormal vitamin B12 concentration in elderly varies from 3-40.5 per cent depending on the cut-off used for defining deficiency of cobalmine level in serum1. The unique value of blood methylmalonic acid (MMA) as a specific marker of functional vitamin B12 is also established. It has been demonstrated that elderly population has higher MMA levels which exponentially increases with age. Blood MMA has proved to be a reliable indicator of functional vitamin B12 status rather than serum vitamin B12 levels in elderly population3,7,8.
There is no information on the extent of vitamin B12 deficiency in elderly Indians as determined by plasma vitamin B12 levels. More importantly, there is no data on functional vitamin B12 status in elderly Indians as determined by blood MMA levels. We hypothesize that vitamin B12 deficiency may be higher in elderly Indians due to vegetarianism, varied socio-economic strata and high prevalence of Helicobacter pylori infection9. We undertook this study to collect data on the dietary habits of urban south Indian elderly subjects, measure their plasma vitamin B12 levels, MMA levels, red cell folate and homocysteine (Hcy) levels. In addition, as deficiency of vitamin B12 and elevated Hcy levels have been shown to reduce cognitive performance in elderly10,11, cognitive functions in this urban south Indian elderly population were also studied.
Material & Methods
Subjects: One hundred and seventy five healthy men and women (>60 yr) were recruited between April and December 2009 from various Senior Citizen Associations in Bangalore (84%) and outpatient department at St. John's Medical College Hospital (14%), Bangalore. The subjects were recruited based on convenience sampling technique. Sample size was estimated based on expected prevalence of vitamin B12 deficiency of 30 per cent with a relative precision of 25 per cent. This was estimated to be 150. The purpose of the study was explained and a written consent was obtained from each of the participants. Demographic, socio-economic and lifestyle information was obtained using a questionnaire. Education was recorded as completed years of formal education and monthly income was categorized.
Detailed history, physical examination and neurological assessment were carried out by a qualified physician. Medical history that included gastrointestinal (GI) symptoms, neurologic and psychiatric symptoms, underlying medical and surgical conditions, was obtained using a structured questionnaire. Subjects with underlying heart disease, renal failure, stroke and major surgery within the last year were excluded. Subjects with previous neurologic or psychiatric symptoms were also excluded. This ensured inclusion of only healthy elderly subjects in this study. Subjects with well controlled diabetes and hypertension were included. The Institutional Ethical Review Board of St. John's Medical College approved the research protocol.
Nutritional assessment: Habitual dietary intake for the preceding six months was assessed using a food frequency questionnaire12 that was interviewer administered by a trained nutritionist. This questionnaire was adapted from the one developed for the urban middle class residing in south India. It has a food list of 108 items, derived from a food database developed over a period of many years from studies at the Division of Nutrition, St John's Medical College, and has four frequency categories (daily, weekly, monthly and yearly)12. Nutrient composition of the food item was calculated using standard food conversion tables for the ingredients12. Wherever available, Indian data were used. However, for some nutrients, for which Indian data were not available, USDA data in the public domain were used13. A replica sheet of the questionnaire was made in Microsoft Excel and the information was entered. The program computed nutrient scores by multiplying the relative frequency of consumption of each food item by its nutrient content of the standard portion size. Nutrient information was obtained on total caloric intake, daily folate and vitamin B12 intake and many other macro- and micro-nutrients. Information on dietary supplements being consumed was separately recorded to arrive at the total daily intake of vitamin B12.
Neuropsychological assessment: The cognitive measures consisted of a series of neuropsychological tests applicable for use in elderly population. The cognitive battery included neuropsychological tests specially adapted from the Consortium to Establish a Registry for Alzheimer's disease (CERAD)14 and the Indo-U.S. Cross National Dementia Epidemiology Study15. This battery consisted of 10 sub-tests, which measured various domains of cognitive abilities and was administered by a trained clinical psychologist. The cognitive domains that were assessed included verbal reproduction and language function, language capacity and impairment of expressive language or speech, mathematical abilities, attention and concentration, visual perception and motor execution and various aspects of memory such as immediate memory, delayed recall and delayed recognition. In addition, subjects were also assessed on an Indian adaptation of "Mini Mental Status Examination" (MMSE) - a widely used screening instrument for the detection of cognitive deficits16. The Indian adaptation of the “Mini Mental Status Examination” is known as the “Hindi Mental Status Examination” (HMSE)17. The Kannada version of this instrument was used to suit the local population; this was done using the translation-back translation procedure18. Depression, which can confound assessment of cognitive abilities in elderly, was measured on “Geriatric Depression Scale”19. Finally, the functional status of the subjects was assessed using “everyday ability scale”20 adapted for Indian population.
Biochemical measurements: Approx. 4 ml of blood was collected from each subject and routine haematological work up, including haemoglobin, total leucocyte count, platelet count, haematocrit, red cell indices, blood smear evaluation, neutrophil lobe count, blood sugars and serum creatinine was done on all the subjects using standard haematology and biochemical techniques. Whole blood was treated with ascorbic acid and stored for red cell folate analysis. The plasma was separated and stored at -80°C until analysis for vitamin B12, Hcy and MMA. Red cell folate and vitamin B12 was measured by the electrochemiluminescence method (Elecsys 2010, Roche Diagnostics Mannheim, USA). The intra- assay coefficient of variation (CV) for the trilevel level controls for red cell folate and vitamin B12 were 2.6, 2.4, 1.7; 4.6, 4.2, 2.8; and inter-assay were 6.4,5.1,5.8; 6.8, 5.3, 3.2; respectively.
The combined measurement of Hcy and MMA was performed by gas chromatography-mass spectrometry (GC-MS) method. An aliquote of 500 μl of plasma was lyophilized in a freeze dryer (Labconco, Kansas, MO, USA) to concentrate the analytes. The lyophilized mass was reconstituted with 200 μl of MiliQ water. The plasma was treated with D,L-dithioerythritol containing deuterated homocysteine (d8-Hcy), to cleave disulphide bonds in the deuterated homocysteine, protein and cysteine bound homocysteine and dimeric form of homocysteine. Plasma was deproteinized by ethanol containing deuterated methyl malonic acid (d3-MMA). The supernatant containing Hcy, MMA and their deuterated standards were derivatized with methycholorformate and extracted into toluene. The N(S)-methoxycarbonyl ethyl ester derivatives in the extract were injected onto the GC-MS (Varian 3800, Palo Alto, CA, USA) and separated on a CP sil 24-CB low-bleed/MS capillary column (15m X 0.25 mm (i.d); film thickness, 0.25 μm) from Varian, USA. The molecules were analyzed in the selective ion monitoring mode of the MS. The ion pairs of m/z 174/177 for MMA/d3 -MMA and 233/237 for Hcy/d4-Hcy were quantified. The concentrations of Hcy and MMA were computed from calibration curves drawn using area ratios of analyte and deuterated analyte, against known concentration of the analyte. The intra- and inter-assay CV for Hcy and MMA were 4.6, 6.2 and 9.3, 10.1 respectively21.
Statistical analysis: Data recorded on a pre-designed proforma were entered on an excel spreadsheet and entries double checked for any errors. Normality was checked using Kolmogorov-Smirnov (KS) test. Vitamin B12 intake and plasma levels of MMA and Vitamin B12 were not normally distributed, and therefore, non-parametric statistical tests were used for vitamin B12 and its metabolites. Categorical data were presented using number and percentage and continuous data as median and quartiles. Since plasma vitamin B12 levels were not normally distributed, Spearman's correlation was performed between vitamin B12 and the metabolites to assess the extent of their correlation. The data were divided into two groups based on supplement intakes and were compared using Mann-Whitney U test. Categorical variables were compared using Chi-square test. The cognitive parameters were compared between the study groups using Mann-Whitney U test. P<0.05 was considered significant. Data were analyzed using SPSS version 11.5.
Results
Anthropometric and demographic characteristics: One hundred and seventy five elderly subjects voluntarily participated in this study. The mean BMI was 24.4 ± 3.8 kg/m2 and the mean weight and height were 63.1 ± 10.2 kg and 161.1 ± 9.4 cm, respectively. The mean age of the study population was 66.3± 6.8 yr and 22 per cent (n=38) of the participants were older than 70 yr. The number of men and women were almost equal and 68 per cent of subjects reported an educational qualification of graduation and above. All the subjects belonged to urban areas of Bangalore city and 75 per cent (n=131) belonged to upper middle socio-economic strata19. Three-fourth of the subjects were walking at least once a week for 30 min or more. All were healthy on physical examination. Well-controlled diabetes and/or hypertension was encountered in 45.6 per cent of the subjects (Table I).
Table I.
Demographic characteristics of the subjects
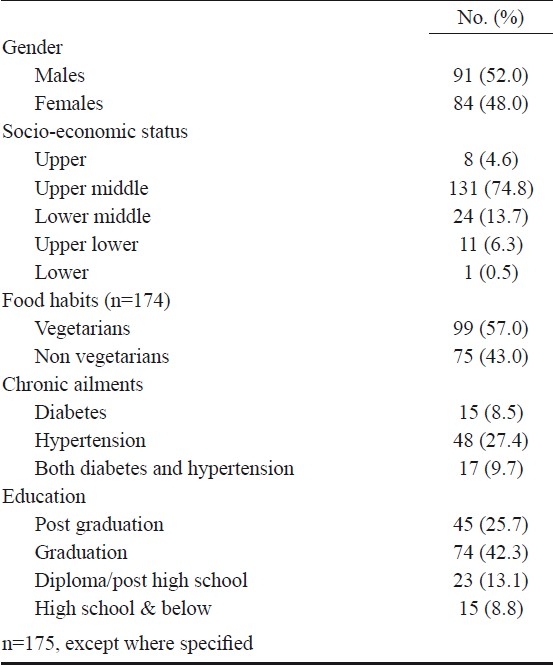
Nutrient consumption and blood levels of nutrients and metabolites: Vegetarianism was observed in 57 per cent (n=99) of the study population. The range and median (lower, upper quartiles) of daily dietary intakes of vitamin B12 and folate and blood levels of the vitamins and their metabolites are described in Table II. The recommended daily intake of vitamin B12 and folate is 2 μg/day and 400 μg/day respectively21,22. Daily vitamin B12 consumption was lesser than recommended daily intake in 25 per cent of the subjects; of these 31 per cent were vegetarians. Fifty one per cent of the study population was consuming less than recommended daily intake of folate. The average (mean ± SD) daily caloric intake of the given population was 1883±467 cals, carbohydrate was 277±68 g, protein was 56±14 g and fat was 61±19 g.
Table II.
Intakes of micronutrients, biochemical status and haematological indices in study subjects
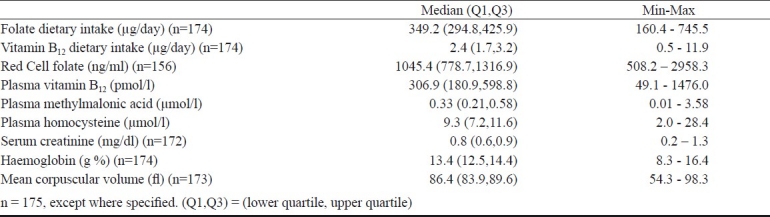
Median haemoglobin (Hb) of the population was 13.4 g/dl (Table II) and low Hb levels were noted in 14 per cent (n=25) of the subjects, two third of whom were men. There was no significant correlation between Hb, mean corpuscular volume (MCV) and plasma vitamin B12 levels. All the subjects had normal serum creatinine levels. Plasma levels of vitamin B12 were subnormal (< 150 pmol/l) in 16 per cent of the study population whereas, elevated MMA (>0.3 μmol/l) was found in 55 per cent of the population. Elevated Hcy levels were found in 13 per cent of the subjects and red cell folate was normal for all the subjects (Table III). Women (90%) were found to have normal vitamin B12 levels (≥150 pmol/l) as compared with men (78%) (P=0.025), similarly most women (96%) had normal Hcy levels (≤15 μmol/l) compared with men (79%) (P=0.001).
Table III.
Deficiency of micronutrients based on intake and biochemical status
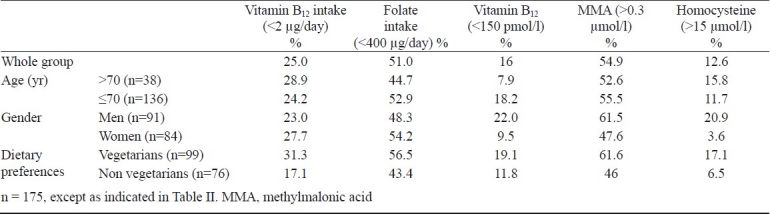
Plasma vitamin B12 level and the dietary intake of vitamin B12 were not significantly correlated. Significant negative correlation was observed between plasma vitamin B12 and MMA for the entire data set (ρr= -0.22). Plasma vitamin B12 and red cell folate were inversely correlated (ρ= -0.509, ρ= -0.550 respectively) with Hcy. Age had no significant correlation with plasma vitamin B12 levels (ρ= -0.03), MMA levels (ρ= -0.05) or Hcy levels (ρ=0.14) (Table IV). Vitamin B12 was divided into quintiles of its distribution and scatter plot of the median for each quintile, for vitamin B12 and MMA and; vitamin B12 and Hcy was plotted (Fig. a & b).
Table IV.
Bivariate correlations of nutrient intakes and metabolites
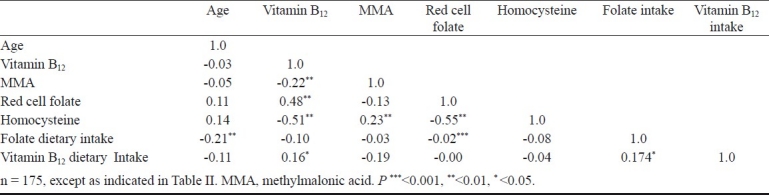
Fig.
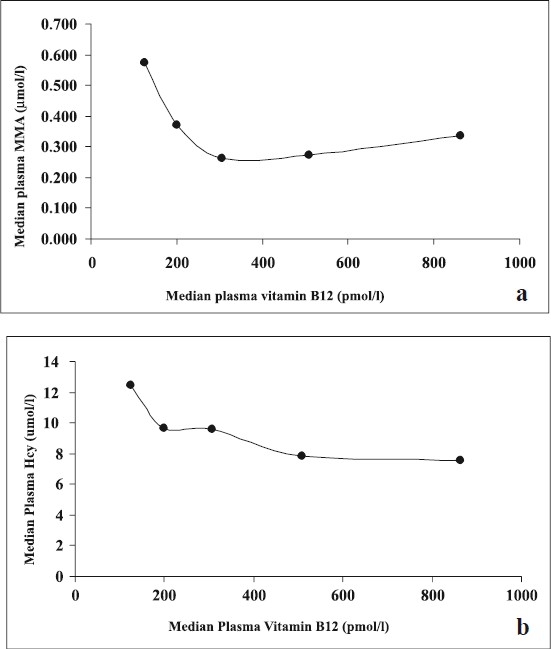
Scatter plot of the median for each quintile: (a) for vitamin B12 and MMA and (b) for vitamin B12 and Hcy.
Analysis of supplemental vitamin intake: Multivitamin supplements were consumed almost on a daily basis by 35 per cent (n=61) of the participants and, therefore, subjects were categorized as supplement consumers and non-consumers. The range and median (lower, upper quartiles) of the dietary intakes and plasma concentration for the two groups are described in Table V. The median (lower, upper quartiles) and range of total intake (diet and supplements) of vitamin B12 and folate for the supplement consumers was 16.9 (10.2, 18.0), 3.31 - 33.64 μg/day and 1757 (501, 1862), 170 - 2141 μg/day respectively. Thirty one per cent of the subjects who were taking supplements had dietary deficiency of vitamin B12 as analyzed by food frequency questionnaire but only 5 per cent have low plasma vitamin B12 levels. In the supplement non-consumer group 39 per cent had dietary deficiency of vitamin B12 and 22 per cent had low plasma vitamin B12 levels.
Table V.
Analysis of supplemental vitamin intake
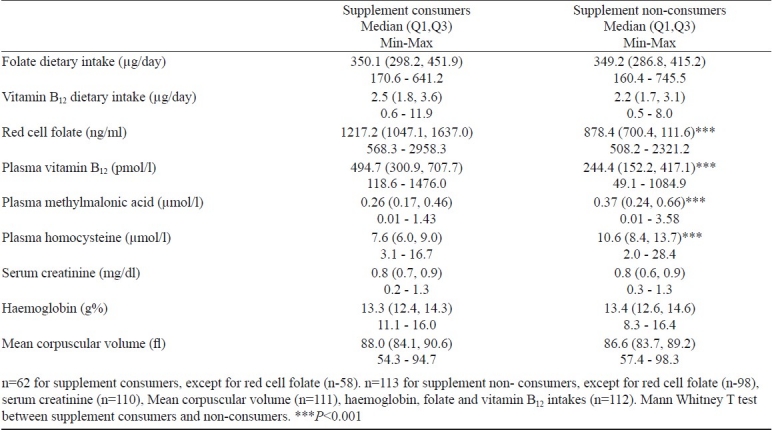
Subjects consuming vitamin supplements (n=62) had significantly higher plasma vitamin B12, lower MMA and Hcy levels compared with subjects who were not taking supplements (P<0.001). Only 2 subjects consuming supplements had higher than normal Hcy levels.
Neuropsychological assessments: There was no significant correlation between plasma vitamin B12, MMA, Hcy & red cell folate levels and any of the 10 cognitive tests. Age and gender did not influence any of the neuropsychological assessments. Subjects who had above high school education did better in Verbal Fluency for Categories (P=0.01), Naming Test (P=0.037), HMSE (P<0.001) and Calculation (P=0.011) in comparison to individuals with lower than high school education. In addition, Naming test score was significantly higher for those belonging to the upper and upper middle class socio-economic status (P=0.034). It was also observed that none of the subjects scored below the cut-off score of HMSE, which is 1917.
Discussion
This study was carried out prospectively in an urban south Indian healthy elderly population. The findings of our study contradict the expected low plasma vitamin B12 levels and high MMA levels in elderly population because about 80 per cent of the sample belonged to either upper middle or upper income groups. There was a significant correlation between plasma vitamin B12 and MMA levels and a plot of the median vitamin B12 and MMA for each segment of quintiles of vitamin B12 distribution showed a nadir beyond 300 pmol/l of vitamin B12. However, there was a non significant increase in plasma MMA concentration for subjects with vitamin B12 >400 pmol/l, and could be attributed to the non linear relationship of plasma vitamin B12 and MMA. A similar observation has been reported in a subset population of the British National Diet and Nutrition Survey8. Refsum et al10 also observed an increasing serum MMA value from 0.84 μmol/l in young adults with vitamin B12 greater than 200 pmol/l to 2.64 μmol/l for vitamin B12 less than 100 pmol/l, and a plateau of the relationship between vitamin B12 and MMA was observed beyond a vitamin B12 concentration of 250 pmol/l8.
Plasma levels of vitamin B12 were subnormal only in 16 per cent but elevated MMA was found in 55 per cent of the population. The elevated MMA in subjects with normal to high vitamin B12 can be explained by evaluating the determinants of MMA. Age and plasma creatinine contribute to only 17 per cent variability of MMA and data on other determinants are sparse10,22. Therefore, caution needs to be exercised in extrapolating MMA levels to indicate the prevalence of vitamin B12 deficiency.
Our study population was consuming a higher than recommended vitamin B12 in the daily diet. Almost half of the subjects were vegetarians; despite this the daily dietary intake of vitamin B12 was high. After adjusting for the vitamin supplements, the daily total intake of vitamin B12 was 3.5 times higher than the FDA recommendation20. It appeared that supplements had a significant impact on the normalization of the plasma vitamin B12 levels, MMA and Hcy levels. This is likely to have a significant effect on the elderly subjects’ well-being and longevity. The average daily dietary intake of folate almost equals the FDA recommended daily intake of folate22. After considering the supplements, the total folate intake in our subjects was three times the recommended intake. This probably resulted in normal Hcy levels in the supplement-consuming group.
Refsum et al10 have demonstrated vitamin B12 deficiency (<150 pmol/l) in 47 per cent and elevated MMA levels (>0.26 μmol/l) in 73 per cent of their adult (age 35-54 yr) subjects. However, only one fourth of them were healthy, while almost half had evidence of cardiovascular disease. Therefore, these results are not reflective of vitamin B12 deficiency in disease free adult Indian population. Yajnik et al24 have attempted to assess prevalence of vitamin B12 deficiency in middle aged (33-46 yr) rural and urban men. Two third of them had low plasma vitamin B12 levels. In this study MMA levels were not analyzed. Both these studies from India have not used a validated and structured tool to assess dietary intake of vitamin B12.
Surprisingly, the age related variations in plasma vitamin B12 levels and MMA were not observed in our study subjects in contrast to published literature3,7,8. This may be attributed to inclusion of only healthy subjects in a narrow age band. This study population was devoid of any cardiac, gastrointestinal, cerebrovascular diseases or psychiatric disorders. In addition, this population appeared to be very health conscious as indicated by their mean BMI, frequency of exercise and ‘Off the counter’ use of multivitamin supplements by one third of them. A few other investigators have also refuted age associated decline of vitamin B12 levels4,6,25–27.
A lack of significant association between plasma vitamin B12 and MMA levels with cognitive measures could be due to a number of reasons. Firstly, low vitamin B12 levels were seen in only 16 per cent of study population. Secondly, the cognitive tests were chosen from a battery of neuropsychological tests that were standardized to detect dementia in a rural Indian population and may not be sensitive enough to detect subtle dysfunctions in cognitive abilities associated with nutritional deficiencies. Thirdly, excluding subjects who reported memory difficulties at the time of recruitment might have biased the sample. Finally, majority of the subjects were in the “young old” category with a few older than 70 yr of age.
This study contradicts the previous studies10,27 which reported a high prevalence of clinical and subclinical vitamin B12 deficiency in urban middle class Indians. This also proves that contrary to perception, food vitamin B12 malabsorption may not be as common as was thought earlier. However, before any generalizations are made based on these observations about deficiency of vitamin B12 in elderly Indians especially vegetarians, studies on the prevalence of vitamin B12 deficiency in any subset of population should use a validated tool to quantify dietary intake of vitamin B12. These observations, then, need to be corroborated with plasma vitamin B12, Hcy and MMA levels. Additionally, normal values for MMA and vitamin B12 need to be established in Indian population. As per the 2001 census, 72.2 per cent of the Indian population29 lives in about 6,38,000 villages30 suggesting the need for similar studies on a larger scale in the rural and the underprivileged and other populations.
Acknowledgments
Authors thank Dr Vidya Sathyanarayanan (Department of Psychiatry) for her guidance to conduct the neuropsychological assessments, Drs Arvind Kasturi and Preethesh (Department of Community Medicine) and Shri Jayaprakash (Dignity f0 oundation) for helping us recruit the subjects. Drs Divya Bhargavi, Lincy Fernandes and Monica Reddy carried out the physical examination. Authors also thank Dr Tinku Thomas for providing statistical assistance and Dr Karuna Rameshkumar for haematological analyses.
References
- 1.Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr. 1994;60:2–11. doi: 10.1093/ajcn/60.1.2. [DOI] [PubMed] [Google Scholar]
- 2.Gaffney GW, Horonick A, Okuda K, Meier P, Chow BF, Shock NW. Vitamin B12 serum concentrations in 528 apparently healthy human subjects of ages 12-94. J Gerontol. 1957;12:32–8. doi: 10.1093/geronj/12.1.32. [DOI] [PubMed] [Google Scholar]
- 3.Joosten E, van den Berg A, Riezler R, Naurath HJ, Lindenbaum J, Stabler SP, et al. Metabolic evidence that deficiencies of vitamin B-12 (cobalamin), folate, and vitamin B-6 occur commonly in elderly people. Am J Clin Nutr. 1993;58:468–76. doi: 10.1093/ajcn/58.4.468. [DOI] [PubMed] [Google Scholar]
- 4.Hitzhusen JC, Taplin ME, Stephenson WP, Ansell JE. Vitamin B12 levels and age. Am J Clin Pathol. 1986;85:32–6. doi: 10.1093/ajcp/85.1.32. [DOI] [PubMed] [Google Scholar]
- 5.Elwood PC, Shinton NK, Wilson CID, Sweetnam P, Frazer AC. Haemoglobin, vitamin B12 and folate levels in the elderly. Br J Haematol. 1971;21:557–63. doi: 10.1111/j.1365-2141.1971.tb02717.x. [DOI] [PubMed] [Google Scholar]
- 6.Garry PJ, Goodwin JS, Hunt WC. Folate and vitamin B12 status in a healthy elderly population. J Am Geriatr Soc. 1984;32:719–26. doi: 10.1111/j.1532-5415.1984.tb04170.x. [DOI] [PubMed] [Google Scholar]
- 7.Stabler SP, Lindenbaum J, Allen RH. Vitamin B-12 deficiency in the elderly: current dilemmas. Am J Clin Nutr. 1997;66:741–9. doi: 10.1093/ajcn/66.4.741. [DOI] [PubMed] [Google Scholar]
- 8.Bates CJ, Schneede J, Mishra G, Prentice A, Mansoor MA. Relationship between methylmalonic acid, homocysteine, vitamin B12 intake and status and socio-economic indices, in a subset of participants in the British National Diet and Nutrition Survey of people aged 65y and over. Eur J Clin Nutr. 2003;57:349–57. doi: 10.1038/sj.ejcn.1601540. [DOI] [PubMed] [Google Scholar]
- 9.Kaptan K, Beyan C, Ural AU, Cetin T, Avcu F, Gülşen M, et al. Helicobacter pylori- is it a novel causative agent in vitamin B12 deficiency? Arch Intern Med. 2000;160:1349–53. doi: 10.1001/archinte.160.9.1349. [DOI] [PubMed] [Google Scholar]
- 10.Refsum H, Yajnik CS, Gadkari M, Schneede J, Vollset SE, Orning L, et al. Hyperhomocysteinemia and elevated methylmalonic acid indicate a high prevalence of cobalamin deficiency in Asian Indians. Am J Clin Nutr. 2001;74:233–41. doi: 10.1093/ajcn/74.2.233. [DOI] [PubMed] [Google Scholar]
- 11.Lindenbaum J, Healton EB, Savage DG, Brust JC, Garrett TJ, Podell ER, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318:1720–8. doi: 10.1056/NEJM198806303182604. [DOI] [PubMed] [Google Scholar]
- 12.Gopalan C, Rama Sastri BV, Balasubramanian SC, Narasinga Rao BS, Deosthale YG, Pant KC. Nutritive value of Indian foods. Hyderabad: National Institute of Nutrition (ICMR); 1989. pp. 47–95. [Google Scholar]
- 13.USDA National Nutrient Database for Standard Reference, Release 22 - Nutrient Lists. [accessed on July 16, 2010]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=18877 .
- 14.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's disease (CERAD). Part 1. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 15.Ganguli M, Chandra V, Gilby JE, Ratcliff G, Sharma SD, Pandev R, et al. Cognitive test performance in a community-based nondemented elderly sample in rural India: the Indo-U.S Cross-National Dementia Epidemiology Study. Int Psychogeriatr. 1996;8:507–24. doi: 10.1017/s1041610296002852. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Ganguli M, Ratcliff G, Chandra V, Sharma S, Gilby J, Pandav R, et al. A Hindi version of the MMSE: The development of a cognitive screening instrument for a largely illiterate rural elderly population in India. Int J Geriatr Psychiatr. 1995;10:367–77. [Google Scholar]
- 18.Harkness J, Pennell BE, Schoua-Glusberg A. Survey Questionnaire translation and assessment. In: Presser S, Rothgeb J, Couper M, Lessler J, Martin E, Martin J, editors. Methods for testing and evaluating survey questionnaires. Hoboken, NJ: John Wiley; 2004. pp. 453–73. [Google Scholar]
- 19.Kumar N, Shekhar C, Kumar P, Kundu AS. Kuppuswamy's socioeconomic status scale-updating for 2007. Indian J Pediatr. 2007;74:1131–2. [PubMed] [Google Scholar]
- 20.Hoffbrand AV. Megaloblastic anemias. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al., editors. Harrison's principles of internal medicine. 17th ed. I. USA: McGraw-Hill Companies. Inc; 2008. p. 643. [Google Scholar]
- 21.Windelberg A, Arseth O, Kvalheim G, Ueland PM. Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylchloroformate derivatization and gas chromatography-mass spectrometry. Clin Chem. 2005;51:2103–9. doi: 10.1373/clinchem.2005.053835. [DOI] [PubMed] [Google Scholar]
- 22.Dwyer J. Nutritional requirements and dietary assessment. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al., editors. Harrison's principles of internal medicine. 17th ed. I. USA: McGraw-Hill Companies. Inc; 2008. p. 438. [Google Scholar]
- 23.Vogiatzoglou A, Oulhaj A, Smith AD, Nurk E, Drevon CA, Ueland PM, et al. Determinants of plasma methylmalonic acid in a large population: implications for assessment of vitamin B12 status. Clin Chem. 2009;55:2198–206. doi: 10.1373/clinchem.2009.128678. [DOI] [PubMed] [Google Scholar]
- 24.Yajnik CS, Deshpande SS, Lubree HG, Naik SS, Bhat DS, Uradey BS, et al. Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J Assoc Physicians India. 2006;54:775–82. [PubMed] [Google Scholar]
- 25.Bailey LB, Wagner PA, Christakis GJ, Araujo PE, Appledor FH, Davis CG, et al. Vitamin B12 status of elderly persons from urban low-income households. J Am Geriatr Soc. 1980;28:276–8. doi: 10.1111/j.1532-5415.1980.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 26.Elsborg L, Lund V, Bastrup-Madsen P. Serum vitamin B12 levels in the aged. Acta Med Scand. 1976;200:309–14. doi: 10.1111/j.0954-6820.1976.tb08237.x. [DOI] [PubMed] [Google Scholar]
- 27.Magnus EM, Bache-Wiig JE, Aanderson TR, Melbostad E. Folate and vitamin B12 (cobalamin) blood levels in elderly persons in geriatric homes. Scand J Haematol. 1982;28:360–6. doi: 10.1111/j.1600-0609.1982.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 28.Allen RH, Stabler SP, Savage D, Lindenbaum J. Diagnosis of cobalamin deficiency I: usefulness of serum methylmalonic acid and total homocysteine concentrations. Am J Hematol. 1990;34:90–8. doi: 10.1002/ajh.2830340204. [DOI] [PubMed] [Google Scholar]
- 29.Government of India, Ministry of Home Affairs, Office of the Registrar General & Census Commissioner, India. Census data. [accessed on July .. 2010]. Available from http://www.censusindia.gov.in/Census_Data_2001/India_at_glance/rural.aspx .
- 30.Census of India: Number of Villages. Office of the Registrar General and Census Commissioner, India. Available from: http://www.censusindia.gov.in/Census_Data_2001/Census_data_finder/A_Series/Number_of_Village.htm .


