Abstract
Background & objectives:
Serum prostate specific antigen (PSA) though most commonly used for diagnosis of prostate cancer lacks specificity. This study was aimed at exploring the use of serum glyoxalase as a supplemental biomarker to differentiate between malignant vs non-malignant diseases of the prostate in patients with PSA in the range of 4-20 ng/ml.
Methods:
Serum glyoxalase and PSA were measured in 92 men (30 control, 31 cases of benign prostate hyperplasia (BPH) and 31 cases of adenocarcinoma of prostate). Of the latter group, 11 cases of prostate cancer in the PSA range of 4-20 ng/ml were included for studying the diagnostic utility of combination of both serum PSA and glyoxalase.
Results:
In prostate cancer cases with PSA in the range of 4-20 ng/ml, the glyoxalase was found to be 233.3 ± 98.6 μmol/min while for the non-malignant group it was 103.1 ± 19.7 μmol/min. A cut-off of 19.2 ng/ml PSA showed sensitivity of 9 per cent, specificity of 96.7 per cent, positive predictive value (PPV) of 50 per cent and negative predictive value (NPV) of 75 per cent. A serum glyoxalase cut-off of 141 μmol/min showed sensitivity of 81.8 per cent, specificity of 100 per cent, PPV of 100 per cent and NPV of 93.9 per cent. Further, ROC analysis showed a significant difference in the area under curve (AUC) for glyoxalase as compared to serum PSA (0.92 vs 0.57; P<0.001).
Interpretation & conclusions:
Serum glyoxalase appears to be predictive of prostate cancer in the PSA range of 4-20 ng/ml. Studies with larger number of participants would be required to confirm this finding.
Keywords: Glyoxalase, prostate cancer, prostate specific antigen
Serum prostate specific antigen (PSA) is considered to be the first line laboratory investigation in suspected cases of prostate cancer1. However, there are limitations associated with the diagnostic efficacy of PSA testing. In non-malignant diseases of the prostate such as benign prostate hyperplasia (BPH) and prostatitis, there is considerable elevation in the serum PSA levels, which frequently leads to unnecessary prostate biopsies1,2. The universally accepted diagnostic grey zone wherein non malignant and malignant prostate diseases coexist is in the PSA range of 4-10 ng/ml. However, as per our earlier observations3, the detection rate of prostate cancer is almost similar in the PSA range of 4-10 ng/ml as well as in 11-20 ng/ml range. Thus, the extension of diagnostic grey zone of PSA level till 20 ng/ml was recommended in the Indian setting, taking into consideration that non-specific elevation of PSA levels are more often observed than the occurrence of prostate cancer3.
Till date, several attempts have been made to improve the diagnostic efficacy of PSA testing. Supplementary markers to PSA such as free to total PSA ratio, percentage free PSA and complexed PSA were examined in recent studies. However, the predictive values of these tests are not adequate enough and thus search for new biomarkers for prostate cancer detection still continues4–6.
Abnormalities in the glyoxalase system have been linked with a number of human disease states including cancer, diabetes and muscle dystrophy7–10. Recent studies have identified the use of glyoxalase system in discriminating the non malignant and malignant diseases of the prostate. Galzigna et al11 performed a preliminary analysis of the serum glyoxalase activity in cases of prostate cancer as compared to a control group and reported a statistically significant difference in the activity of the glyoxalase system between normal individuals and prostate cancer patients.
One of the important features of actively proliferating cells such as tumours is high glycolytic activity, which results in production of high methyl glyoxal. The major function of the glyoxalase system is to detoxify α-ketoaldehydes, especially the potent and cytotoxic methylglyoxal (MG)7. MG, a byproduct of glycolysis, is produced through nonenzymatic phosphate elimination from the glycolytic intermediates, dihydroxyacetone phosphate and glyceraldehyde 3-phosphate. The glyoxalase system comprises of two enzymes glyoxalase I (lactoyl - glutathione lyase) and glyoxalase II (hydroxyacyl glutathione hydrolase) and a catalytic amount of reduced glutathione12. MG is converted to S-D-lactoylglutathione (SLG) by glyoxalase I with reduced glutathione as a cofactor, and SLG in turn is hydrolyzed to D-lactate along with regeneration of reduced glutathione by glyoxalase II.
The higher activity of glyoxalase seen in malignant condition due to heightened cellular and metabolic activity warrants an understanding of this enzyme in diagnostic perspective.
This study aims to evaluate the efficacy of serum glyoxalase in differentiating non-malignant and malignant conditions of prostate specifically in patients with the PSA range of 4-20 ng/ml.
Material & Methods
This was a cross-sectional study on 92 male patients attending the urology out patient department of Lokmanya Tilak Municipal Medical College & General Hospital, a tertiary care teaching hospital at Mumbai, India, over a period of two years (June 2006 - May 2008). Written informed consent was obtained from all patients. The study protocol was approved by the Institutional Review Board and ethical clearance was obtained from Institutional Ethics Committee. On the basis of clinical and histological diagnosis, men included in the study were classified into one of the following three groups.
Malignant group: Thirty one clinically and histologically confirmed patients with prostate cancer were consecutively enrolled in the study as and when diagnosed. The baseline serum PSA level for each case was estimated. All cases with PSA level >4ng/ml were subjected to 12 core primary prostate biopsy for detection and confirmation of malignancy. Of the 31 men diagnosed with prostate cancer, 11 presented pre-treatment PSA levels in the range of 4-20 ng/ml (PSA levels <10 ng/ml in 1 case; 11-20 ng/ml in 10 cases) whereas the remaining 20 cases demonstrated PSA levels >20 ng/ml. These 11 cases were included in a separate group in whom efficacy of PSA + glyoxalase was tested as diagnostic tool. Histological grading was performed using Gleason score13 in each of the cancer case.
Non malignant group: A total of 31 aged matched cases presenting with BPH and/or prostatitis were included in this group who were diagnosed clinically by digital rectal examination (DRE) and/or transrectal ultrasonography (TRUS). The clinical symptoms were assessed by American Urological Association (AUA) scoring14 which was observed to be above 15 points. The patients specifically showing PSA levels in the range of 4-20 ng/ml were included in this group. In all these patients, malignancy was ruled out on the basis of histological diagnosis with tissue samples obtained by 12 core primary prostate TRUS guided biopsy. The patients with obstructive symptoms were subjected to transurethral resection of prostate (TURP) procedure also and the remnant tissue was again histologically examined. Patients already diagnosed as BPH and currently under treatment with antiandrogens were excluded from this study.
Control group: Thirty men age matched to patients’ groups not suffering from any prostatic disease were included as controls and enrolled during same period from Urology OPD. These men were suffering from erectile dysfunction, urolithiasis without bladder outlet obstruction and urinary tract infection. The inclusion criteria were age above 40 yr, negative DRE, AUA score less than 8 and PSA levels <4 ng/ml.
Pre-treatment blood samples were obtained for analysis of serum PSA levels and glyoxalase activity. Blood samples were collected prior to rectal examination or catheterization to exclude the possibility of influencing the results due to prostatic manipulation.
Serum PSA level was determined by commercially available biotin- avidin sandwich ELISA technique (CanAg PSA EIA kit, Cat No.340-10). Glyoxalase estimation was performed with continuous spectrophotometric technique and the rate of absorbance was recorded at 412 nm11. The results were expressed as μmol of GSH liberated per minute. Each of the estimations was performed by a separate group of laboratory personnel as part of blinding procedures introduced in the study.
Statistical analysis: Results were expressed as mean ± standard deviation (SD). Statistical tests such as 1 sample-KS test was performed to determine the normal distribution of the data. The data were log-transformed to normalize the distribution. One way Analysis of variance (ANOVA) was performed to test for the difference in serum PSA and glyoxalase levels between the study groups. Levene's test was performed to examine the homogeneity of variance for the PSA and glyoxalase levels between the study groups. Tamhane's post hoc test was adopted to compare the serum PSA and glyoxalase levels between the study groups, as equality of variance was not assumed by the data. These analyses were performed by SPSS.16 software (SPSS Inc., Chicago IL, USA).
With the help of MedCalc software (version 9.6.2.0; MedCalc Software, MariakerKe, Belgium) receiver operating characteristic (ROC) curve was constructed using sensitivity and specificity for both the serum PSA and Glyoxalase. The area under curve (AUA), positive predictive value (PPV), negative predictive value (NPV) were determined from the ROC curve for assessing the diagnostic efficacy of serum glyoxalase. P<0.05 was considered significant.
Results
The mean age of men in control and the study groups was 62 ± 14.1 yr with a range of 40 to 90 yr. The AUA scores of patients in both non malignant and malignant cases was moderate to severe. Histological grading of patients in the malignant group revealed Gleason score of >7 in 75 per cent of the cases.
The serum PSA and glyoxalase levels (Table I) were log-transformed in the four groups as the cases in the prostate cancer groups were not showing normal distribution of the data as per 1 sample-KS test. Accordingly, the log transformed serum concentrations of total PSA analysed for control, non-malignant, prostate cancer, and prostate cancer in the PSA range of 4-20 ng/ml groups were -0.12 ± 0.56, 1.09 ± 0.16, 1.92 ± 0.76 and 1.15 ± 0.13 respectively. Similarly the log transformed serum glyoxalase levels were 1.9 ± 0.17, 2 ± 0.8, 2.36 ± 0.19 and 2.32 ± 0.20 μmol/min respectively.
Table I.
Serum PSA and glyoxalase in control and patients with non-malignant and malignant disease of the prostate
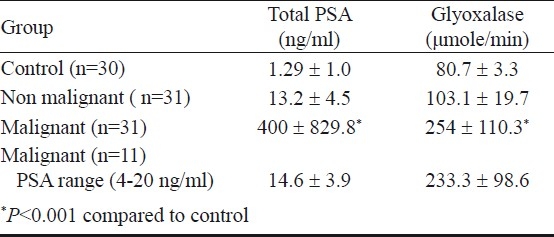
Comparison of log-transformed serum PSA and glyoxalase levels using one way ANOVA showed a statistically significant difference (P<0.001) between the four groups. To assess the pair-wise difference in the serum PSA and glyoxalase levels between the groups, non-parametric Tamhane's post hoc test was applied. This analysis revealed significantly higher serum PSA and glyoxalase levels in prostate cancer group as compared to that of controls and non malignant group (P<0.001).
In the prostate cancer group with PSA in the range of 4-20 ng/ml, the estimated glyoxalase activity was found to be significantly higher as compared to that of non malignant group (P<0.001) and almost similar to that of prostate cancer group. Conversely, serum PSA levels were found to be almost similar to that of non malignant group.
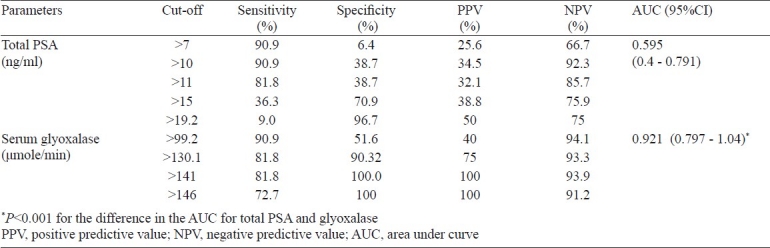
Diagnostic efficacy of serum glyoxalase as a biomarker for prostate cancer over the established serum PSA test was assessed by ROC curve in the PSA range of 4-20 ng/ml. The sensitivity, specificity, PPV and NPV for serum glyoxalase and PSA are depicted in the Fig. and Table II. For PSA cut-off of 19.2 ng/ml, the sensitivity was 9 per cent, specificity was 96.7 per cent with a PPV of 50 per cent and NPV of 75 per cent. Using the cut-off of 141 μmol/min for serum glyoxalase, the sensitivity was 81.8 per cent, specificity was 100 per cent and PPV of 100 per cent and NPV of 93.9 per cent. The area under curve (AUC) observed for glyoxalase was (0.92 for glyoxalase and 0.595 for serum PSA; P<0.001) significantly higher than that of total PSA. Our results showed that using PSA (in the range of 4-20 ng/ml) in combination with serum glyoxalase with a cut-off of 141 μmole/min identified 9 of 11 cases of prostate cancer accurately.
Fig.
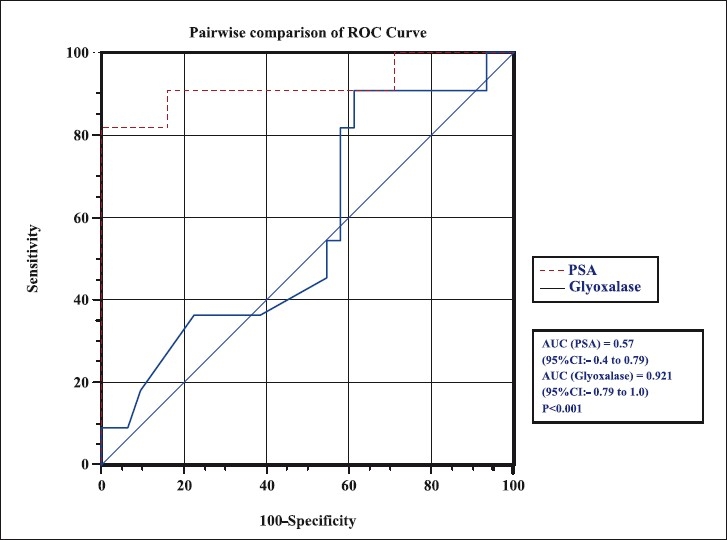
ROC analysis for serum PSA and glyoxalase of patients in the PSA range of 4-20 ng/ml.
Table II.
Sensitivity, specificity and AUC for total PSA and glyoxalase for patients in the PSA range of 4- 20 ng/ml
Discussion
In search of a biomarker that could potentially supplement serum PSA, for accurate diagnosis and sharper differentiation between non malignant and malignant states of the prostate specifically in the PSA range of 4-20 ng/ml, we assessed the diagnostic efficacy of serum glyoxalase activity along with serum PSA.
In our study, the serum glyoxalase activity in patient with PSA in the range of 4-20 ng/ml was found to be significantly higher in malignant condition of prostate than that in the non malignant prostatic diseases. Galzigna et al11 also demonstrated significant difference in the glyoxalase activity in patients with prostate cancer and healthy adult men. Davidson et al15 found higher glyoxalase 1 activity in cancerous than in non cancerous specimens, suggesting that it may play a role in prostate cancer homeostasis and survival.
In this study, the conventional cut-off of 10 ng/ml for serum PSA yielded a sensitivity of 90.9 per cent and specificity of a mere 38.7 per cent whereas a cut-off of 19.2 ng/ml yielded sensitivity of 9 per cent only which could be due to other contributing factors beside prostate cancer while the specificity increased to 96.7 per cent. Serum glyoxalase with a cut-off of 141 μmol/min yielded a sensitivity of 81.8 per cent and a specificity of 99.99 per cent and thus proved to be a better biomarker. ROC analysis showed that the AUC for serum glyoxalase was significantly higher than that for PSA.
Of the 31 patients in the non malignant group having serum PSA in the range of 4-20 ng/ml, 19 presented PSA levels above 10 ng/ml in whom the serum glyoxalase was less than 141 μmol/min. Similarly, of the 11 prostate cancer patients having PSA in the range of 4-20 ng/ml, 10 presented PSA levels above 10 ng/ml and 9 of them showed serum glyoxalase above 141 μmol/min. This suggests that in patients presenting with PSA levels >10 ng/ml, use of serum glyoxalase as a supplemental biomarker might be useful to differentiate malignant and non malignant diseases of the prostate.
Increase in the glyoxalase activity has been shown to be an indicator of increased cellular and metabolic activity. Rulli et al16 reported an increased glyoxalase activity and their corresponding gene expression in breast carcinoma tumour compared to that of normal tissue and suggested that measurement of glyoxalase activity in breast cancer tumours could become an important diagnostic tool14. A recent study has demonstrated that testosterone has an influence over the expression pattern of glyoxalase system genes and cell proliferation of both androgen dependent and androgen independent prostate cancer cell lines17.
The present study had limitation of small sample size of prostate cancer patients showing PSA in the range of 4-20 ng/ml. Further prospective studies are warranted with large number of cases to accentuate the reliability of serum glyoxalase as a supplemental marker over the existing complementary tests such as free/total PSA ratio, complexed PSA, and percentage free PSA in addition to traditional serum total PSA.
References
- 1.Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–90. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 2.Kehinde EO, Sheikh M, Mojimoniyi OA, Francis I, Anim JT, Nkansa-Dwamena D, et al. High serum prostate-specific antigen levels in the absence of prostate cancer in Middle-Eastern men: the clinician's dilemma. BJU Int. 2003;91:618–22. doi: 10.1046/j.1464-410x.2003.04199.x. [DOI] [PubMed] [Google Scholar]
- 3.Chavan PR, Chavan SV, Chavan NR, Trivedi VD. Detection rate of prostate cancer using prostate specific antigen in patients presenting with lower urinary tract symptoms: a retrospective study. J Postgrad Med. 2009;55:17–21. doi: 10.4103/0022-3859.43548. [DOI] [PubMed] [Google Scholar]
- 4.Leung HY, Lai LC, Day J, Thomson J, Neal DE, Hamdy FC. Serum free prostate-specific antigen in the diagnosis of prostate cancer. Br J Urol. 1997;80:256–9. doi: 10.1046/j.1464-410x.1997.00230.x. [DOI] [PubMed] [Google Scholar]
- 5.Okegawa T, Noda H, Nutahara K, Higashihara E. Comparisons of the various combinations of free, complexed, and total prostate-specific antigen for the detection of prostate cancer. Eur Urol. 2000;38:380–7. doi: 10.1159/000020312. [DOI] [PubMed] [Google Scholar]
- 6.Sakai I, Harada K, Hara I, Eto H, Miyake H. Limited usefulness of the free-to-total prostate-specific antigen ratio for the diagnosis and staging of prostate cancer in Japanese men. Int J Clin Oncol. 2004;9:64–7. doi: 10.1007/s10147-003-0365-1. [DOI] [PubMed] [Google Scholar]
- 7.Thornalley PJ, Hooper NI, Jennings PE, Florkowski CM, Jones AF, Lunec J, et al. The human red blood cell glyoxalase system in diabetes mellitus. Diabetes Res Clin Pract. 1989;7:115–20. doi: 10.1016/0168-8227(89)90101-0. [DOI] [PubMed] [Google Scholar]
- 8.Thornalley PJ. Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification - a role in pathogenesis and antiproliferative chemotherapy. Gen Pharmacol. 1996;27:565–73. doi: 10.1016/0306-3623(95)02054-3. [DOI] [PubMed] [Google Scholar]
- 9.Vander Jagt DL, Hunsaker LA, Campos NM, Baack BR. D-lactate production in erythrocytes infected with Plasmodium falciparum. Mol Biochem Parasitol. 1990;42:277–84. doi: 10.1016/0166-6851(90)90171-h. [DOI] [PubMed] [Google Scholar]
- 10.Kar NC, Pearson CM. Glyoxalase enzyme system in human muscular dystrophy. Clin Chim Acta. 1975;65:153–5. doi: 10.1016/0009-8981(75)90348-4. [DOI] [PubMed] [Google Scholar]
- 11.Galzigna L, Nyandiska HS, Burlina A. A new colorimetric assay of the serum glyoxalase system. Experientia. 1974;3:317–8. doi: 10.1007/BF01934853. [DOI] [PubMed] [Google Scholar]
- 12.Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J. 1990;269:1–11. doi: 10.1042/bj2690001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gleason DF. The Veteran's Administration Cooperative Urologic Research Group: histologic grading and clinical staging of prostatic carcinoma. In: Tannenbaum M, editor. Urologic pathology: The prostate. Philadelphia: Lea and Febiger; 1977. pp. 171–98. [Google Scholar]
- 14.Barry MJ, Fowler FJ, Jr, O’Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia.The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–57. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 15.Davidson SD, Cherry JP, Choudhury MS, Tazaki H, Mallouch C, Konno S. Glyoxalase I activity in human prostate cancer: A potential marker and importance in chemotheraphy. J Urol. 1999;161:690–1. [PubMed] [Google Scholar]
- 16.Rulli A, Carli L, Romani R, Baroni T, Giovannini E, Rosi G, et al. Expression of glyoxalase I and II in normal and breast cancer tissues. Breast Cancer Res Treat. 2001;166:67–72. doi: 10.1023/a:1010632919129. [DOI] [PubMed] [Google Scholar]
- 17.Antognelli C, Del Buono C, Baldracchini F, Talesa V, Cottini E, Brancadoro C, et al. Alteration of glyoxalase genes expression in response to testosterone in LNCaP and PC3 human prostate cancer cells. Cancer Biol Ther. 2007;6:1880–8. doi: 10.4161/cbt.6.12.4961. [DOI] [PubMed] [Google Scholar]


