Abstract
An estimated 300,000 babies are born each year with a severe inherited disease of haemoglobin and that over 80 per cent of these births occur in low- or middle-income countries. As these countries go through the epidemiological transition, characterized by a reduction in childhood and infant mortality due to improved public health measures, infants who had previously died of these conditions before they were recognised are now surviving to present for diagnosis and treatment. For a variety of reasons, even in the rich countries there are limited data about the true frequency, natural history, and survival of patients with these disorders, information that is absolutely critical towards providing governments and international health agencies with accurate information about the true global health burden of these conditions. The situation can only be improved by major action on the part of the rich countries together with the formation of partnerships between rich and poor countries and input from the major international health agencies and funding organisations.
Keywords: Haemoglobin, inherited disorders, phenotypic diversity, sickle cell anaemia, thalassaemia
An estimated 7,000,000 babies are born each year with either a congenital abnormality or a genetic disease and that over 90 per cent of these births occur in low- or middle-income countries1. Remarkably, of these births, approximately 25 per cent consist of only five disorders, two of which are monogenic diseases, the inherited disorders of haemoglobin and glucose-6-phosphate dehydrogenase deficiency.
Although the World Health Organization (WHO) has recognised the importance of the inherited disorders of haemoglobin, very little international action has been taken towards the development of services for the control and management of these conditions. Here, I will briefly outline the current state of knowledge about the potential global burden that they will pose and some of the steps that require to be taken urgently to try to improve the situation. These important issues have been discussed at greater length2–4.
Current global estimations of the global burden of the important haemoglobin disorders
A very approximate breakdown of the numbers of annual births of the important haemoglobin disorders is shown in the Table. Sickle cell anaemia is by far the commonest. The most important forms of β-thalassaemia are equally divided between β-thalassaemia major and haemoglobin E (HbE) β-thalassaemia, which occurs at a high frequency in parts of the Indian subcontinent, Bangladesh, Myanmar and throughout South-east Asia. The mild forms of α-thalassaemia, the α+-thalassaemias, occur at a variably high frequency throughout the tropical belt, while the more severe forms of α-thalassaemia, the α°-thalassaemias, are restricted to parts of the Mediterranean region and, in particular, to South-east Asia2–5.
Table.
Annual births of severe disorders of haemoglobin
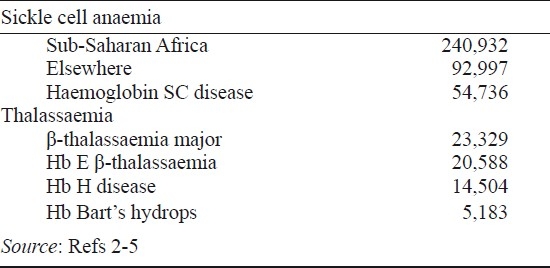
Unfortunately, the data summarised in the Table have to be interpreted with great caution. In particular, these are based on surveys that, in many cases, were carried out many years ago and based only on limited centres from many different countries. More recent studies utilizing micromapping, in which adequate sample sizes have been studied from many different centres within individual countries, indicate that all the haemoglobin disorders occur at very heterogeneous levels even within small geographical distances4. For example, even in the relatively small island population of Sri Lanka, there is considerable variation in the frequency of β-thalassaemia and HbE in different parts of the island6. Recent data obtained from two States in North-west India also show a remarkable variation in the frequency of β-thalassaemia over short distances and suggest that earlier data for the frequency of β-thalassaemia in the Indian subcontinent may have been underestimated7. Similar data showing a remarkable geographic heterogeneity of the thalassaemias have been obtained from Indonesia and Viet Nam. And from such data as are available it seems likely that the published figures for the numbers of births of babies with sickle-cell anaemia in India, approximately 25,000, may also be an underestimate5.
There are also very limited mortality data for the important haemoglobin disorders. In the case of sickle cell anaemia and related disorders some data are available on survival and on the improvements in survival following neonatal screening and the use of prophylactic antibiotics or vaccination in a few of the richer countries but, globally, there is a severe paucity of information of this type3. Similarly, data obtained from countries in which a full range of treatment for β-thalassaemia is available have shown a considerable improvement in survival over the last twenty years8 but, again, very scare data are available for countries where there are limited or no current forms of treatment available beyond blood transfusion. And with the exception of a few of the richer countries, very limited data are available regarding the course and natural history of these diseases.
In short, the information required to build up a true picture of the current global health burden of the haemoglobin disorders is very limited, a problem that requires urgent attention.
Population genetics and dynamics
Why are the inherited haemoglobin disorders so common and what is likely to happen to their frequency in the future? The extremely high frequency of the haemoglobin disorders compared with other monogenic diseases reflects several different processes1. First, and foremost, is natural selection whereby carriers for these diseases have been protected during evolution from severe malaria. Hence they have lived longer in malarious areas and produced more children until the numbers are balanced by the loss from the community of homozygotes. A second critical factor is the high frequency of consanguineous marriages which tend to increase the frequency of any Mendelian recessive disorder. The third factor, already mentioned, is the epidemiological transition whereby, as neonatal and childhood mortality figures decline due to improved social conditions, babies who would formerly have died with the serious haemoglobin disorders before they came to diagnosis are now surviving to present for management. Additional factors that maintain the high gene frequency include founder effects and gene drift.
There is now extensive evidence that the high frequency of many of the haemoglobin disorders reflects heterozygote, and in the case of α+-thalassaemia and HbC, homozygote protection against Plasmodium falciparum malaria. There is also at least some evidence that the high frequency of β-thalassaemia and HbE follows the same process9,10. Recent studies have underlined the complexity of the interactions between malaria and the haemoglobin disorders. For example, while the sickle cell trait and the heterozygous or homozygous state for α+ thalassaemia both provide considerable protection against malaria, in those who inherit both traits, malaria protection is cancelled out and individuals become equally susceptible to malaria as those who carry neither trait11. Such epistatic interactions have been modelled and go some way to explaining the extraordinary diversity of the frequency of different forms of haemoglobin disorder among communities12. But even if malaria were to be totally eradicated it would take generations for the gene frequencies of haemoglobin disorders to decline. And there is growing evidence that some forms of thalassaemia, certainly α-thalassaemia and HbE β-thalassaemia, may render individuals more susceptible to malaria due to P. vivax, which is becoming an increasingly serious problem in many tropical countries10.
While natural selection and consanguinity have played a major role in increasing the frequency of the haemoglobin disorders, the epidemiological and demographic transitions that are occurring in the poorer countries of the world will undoubtedly have a major influence in setting the frequency of these conditions in the future. In many cases increased survival of babies with severe haemoglobin disorders due to improvements in social conditions and public health are occurring in countries with the highest rate of increase in their populations. For example, it is currently estimated that the population of Africa has risen to approximately one billion and could reach close to two billion by 2050. It has been estimated recently that if early deaths due to infection were controlled there could be a population of patients with sickle cell anaemia in excess of six million in sub-Saharan Africa by the middle of the present century2. Although population growth has slowed down somewhat in India and China it is likely that their populations will also increase. Given these different issues it seems certain that there will be a major increase in the population of patients with severe thalassaemia over the next fifty years.
Phenotypic diversity of the haemoglobin disorders
One of the major problems in developing programmes for the control and management of the haemoglobin disorders is their remarkable phenotypic variability. This applies to both the sickle cell disorders and to all the different forms of thalassaemia.
There is clear genetic evidence that the sickle cell mutation arose at least twice, once in India or the Middle East and at least once and perhaps several times in sub-Saharan Africa13. Overall, the former form of the condition is clinically milder than the African form but both varieties show remarkable clinical heterogeneity. And although certain genetic modifiers such as the co-inheritance of α-thalassaemia or polymorphisms of genes involved in the regulation of foetal haemoglobin production may play an important role in phenotypic modification, much remains to be explained. For example, the role of the environment in modifying the phenotype has remained largely unexplored.
Similar heterogeneity exists in the case of the different forms of thalassaemia. The most remarkable example is HbE β-thalassaemia, in which patients with similar β-thalassaemia mutations may have phenotypes varying from transfusion-dependent anaemia for life to conditions characterized by reasonable growth and development, albeit at lower haemoglobin levels, without the need for transfusion14. While α-thalassaemia and the level of HbF have also been found to be important genetic modifiers of this condition it is clear that many of the complications are also modified by genetic factors and that both developmental changes and differences in adaptation to anaemia are important factors. There is also increasing evidence that the environment, particularly exposure to malaria and other infections, are important modifiers. There is also growing evidence that the different severe forms of α-thalassaemia, notably HbH disease, show remarkable heterogeneity, particularly related to the underlying molecular forms.
Until we have a better understanding of the genetic and environmental basis for this phenotypic heterogeneity it will be difficult to develop programmes for the control and management of many of these conditions.
Current approaches to control and management
The only approach to the radical cure of the important haemoglobin disorders is bone marrow transplantation in cases where suitable donors are available. In many countries premarital screening followed by prenatal diagnosis has reduced the numbers of births of affected babies. And there have been genuine improvements in the symptomatic management of all these conditions over the last twenty years.
Because of the uncertainties of the prognosis both marrow transplantation and prenatal diagnosis have been applied less to the sickle cell anaemias than to the thalassaemias. A major advance in the control of this condition has followed neonatal screening programmes after which affected babies are given either vaccines or prophylactic antibiotics, with a marked reduction in death from infection during the first years of life. Symptomatic improvement has undoubtedly followed the use of hydroxyurea and by the use of prophylactic transfusion to reduce the frequency of vascular complications, particularly those involving the brain. In centres with adequately trained staff there have undoubtedly been improvements in the management of many of the other complications of sickle cell disease15.
So far, population screening and marital advice alone have not had a major effect on the numbers of births of babies with the different forms of thalassaemia. However, well designed national prenatal screening programmes followed by prenatal diagnosis have undoubtedly reduced the frequency of births in many of the rich countries. Regular transfusion with the use of chelating agents has significantly improved the survival of patients with severe forms of α- and β- thalassaemia16.
The current position in poorer countries
The current position regarding the control and management of thalassaemia in Asian countries, as compiled by the Asian Thalassaemia Network, has been reported recently4.
In short, relatively few countries have national thalassaemia control programmes, and although in a few cases there are centres of genuine expertise for the control and management of the disease, in many countries these facilities are only available to those who can afford them and the overall position for many countries is a complete lack of provision for any aspects of the disease.
Less is known about the global position regarding sickle cell disease although it is apparent that facilities for the control and management of this condition in sub-Saharan Africa are extremely limited and the bulk of babies are still dying early in life. While there appear to be some centres with expertise in the Middle East and India, the overall position is not clear though it seems likely that in many countries the facilities for treatment are extremely limited.
The future
Possible ways forward for improvements in the global control of the haemoglobin disorders have been published recently3,4. It is clear that the WHO, NGOs and international funding agencies, though they may be becoming more aware of the problem of the haemoglobin disorders, are not likely to take any action in the immediate future. It is vital, therefore, that the international haematology community takes the initiative.
In the case of the thalassaemias, though to a lesser degree in the case of the sickle cell disorders, there have been many successful examples of North/South partnerships over the last twenty years, in which teams from the richer countries have paired up with those in the developing countries to initiate thalassaemia programmes which, at least in some cases, have been successful and resulted in the evolution of national programmes for the control of the disease3,4. The natural follow up of this approach, that is the South/South partnership, in which countries which have developed expertise can form links with those where no such expertise exists has been proposed as an effective way forward4. In very large countries like India this might include the development of similar partnerships within the country. While this approach has been approved by the WHO, it has not made any efforts in this direction and hence it will be up to the haematology community to evolve its own partnerships of this type.
The objective of the South/South partnerships is to develop training programmes for countries where there is limited or no knowledge of the control and management of the haemoglobin disorders. Such programmes would have the objective of evolving micromapping studies in different countries to provide evidence about the actual burden of disease in their populations, followed by instruction in the control and management of these conditions and advice to their governments. While a start has been made over the last five years in developing an Asian Thalassaemia Network4 with these objectives, the main problem has been in evolving sufficient funding through international health agencies although some slow progress is being made.
The WHO have approved a similar approach for the better control of sickle cell disease, at least in Africa, but again it is being left to individual haematology groups to try to evolve partnerships of this type. While the evolution of these partnerships seems to be the obvious way forward for the control of the haemoglobin disorders in the future, these will not be successful until international health agencies and funding bodies are convinced of the global health burden that is likely to be presented by these conditions. Given the continued problem of communicable disease, and the epidemics of ‘Western’ diseases like coronary artery disease and diabetes in many developing countries, it will not be easy to emphasise the importance of genetic disease. But if this is not done the position is unlikely to improve and the only way forward is to continue to try to collect better data about the frequency of these conditions in the poorer countries of the world and hence to try to convince their governments and the appropriate international bodies about their importance.
Acknowledgments
The author thanks Jeanne Packer and Liz Rose for their help in preparing this paper.
References
- 1.Christianson A, Howson CP, Modell B. March of Dimes global report on birth defects. New York: March of Dimes Birth Defects Foundation; 2006. [Google Scholar]
- 2.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86:480–7. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weatherall DJ, Greenwood BM, Chee H-L, Wasi P. Science and technology for disease control: Past, present and future. In: Jamison DT, Breman JG, Measham AR, et al., editors. Disease control priorities in developing countries. 2nd ed. New York: Oxford University Press and the World Bank; 2006. pp. 119–38. [Google Scholar]
- 4.Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115:4331–6. doi: 10.1182/blood-2010-01-251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Williams TN, et al. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat Comms. 2010;1:104. doi: 10.1038/ncomms1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Silva S, Fisher CA, Premawardhena A, Lamabadusuriya SP, Peto TE, Perera G, et al. Thalassaemia in Sri Lanka: implications for the future health burden of Asian populations. Sri Lanka Thalassaemia Study Group. Lancet. 2000;355:786–91. doi: 10.1016/s0140-6736(99)08246-x. [DOI] [PubMed] [Google Scholar]
- 7.Colah R, Gorakshakar A, Phanasgaonkar S, D’Souza E, Nadkami A, Surve R, et al. Epidemiology of beta-thalassaemia in Western India: Mapping the frequncies and mutations in sub-regions of Maharashtra and Gujarat. Br J Haematol. 2010;149:739–47. doi: 10.1111/j.1365-2141.2010.08131.x. [DOI] [PubMed] [Google Scholar]
- 8.Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89:1187–93. [PubMed] [Google Scholar]
- 9.Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77:171–92. doi: 10.1086/432519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weatherall DJ. Genetic variation and susceptibility to infection: the red cell and malaria. Br J Haematol. 2008;141:276–86. doi: 10.1111/j.1365-2141.2008.07085.x. [DOI] [PubMed] [Google Scholar]
- 11.Williams TN, Mwangi TW, Wambua S, Peto TE, Weatherall DJ, Gupta S, et al. Negative epistasis between the malaria-protective effects of alpha+-thalassemia and the sickle cell trait. Nat Genet. 2005;37:1253–7. doi: 10.1038/ng1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penman BS, Pybus OG, Weatherall DJ, Gupta S. Epistatic interactions between genetic disorders of hemoglobin can explain why the sickle-cell gene is uncommon in the Mediterranean. Proc Natl Acad Sci USA. 2009;106:21242–6. doi: 10.1073/pnas.0910840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serjeant GR. Geographic heterogeneity of sickle cell disease. In: Steinberg MH, Forget BG, Higgs DR, Nagel RL, editors. Disorders of hemoglobin. Cambridge: Cambridge University Press; 2001. pp. 895–905. [Google Scholar]
- 14.Olivieri NF, Muraca GM, O’Donnell A, Premawardhena A, Fisher C, Weatherall DJ. Studies in haemoglobin E beta-thalassaemia. Br J Haematol. 2008;141:388–97. doi: 10.1111/j.1365-2141.2008.07126.x. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg MH, Forget BG, Higgs DR, Weatherall DJ, editors. Disorders of hemoglobin. 2nd ed. New York: Cambridge University Press; 2009. [Google Scholar]
- 16.Weatherall DJ, Clegg JB. The thalassaemia syndromes. 4th ed. Oxford: Blackwell Science; 2001. [Google Scholar]


