Abstract
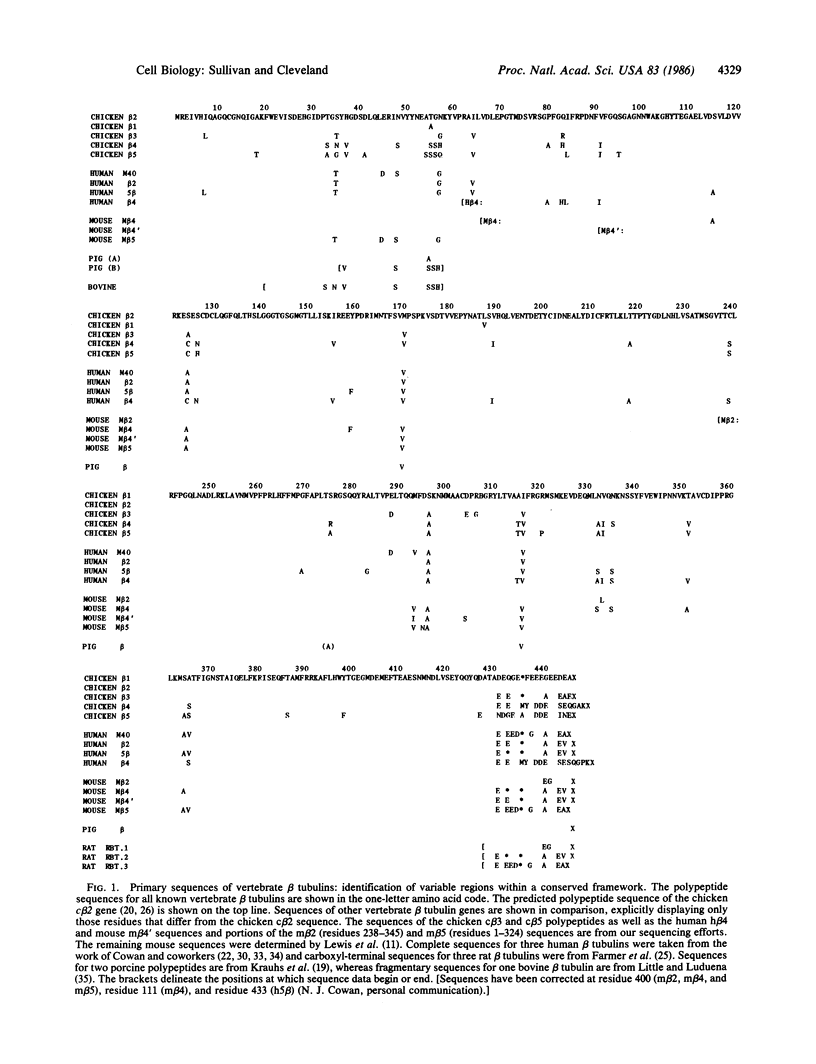
We report the determination of the complete sequences for two chicken beta tubulin genes, beta 3 and beta 5. Taken with the previously published efforts, we have determined the primary structures of five of the seven beta tubulin genes in this vertebrate species. A comparison of these sequences unambiguously reveals that amino acid sequence variations among different beta tubulin gene products are distinctly clustered within an otherwise highly conserved framework of the beta tubulin molecule. To determine the extent to which this pattern of structural heterogeneity is conserved among vertebrates, we have isolated novel beta tubulin sequences from human and mouse cDNA libraries and compared these and all other known vertebrate beta tubulin sequences with the family of chicken polypeptide sequences. What emerges from such comparison is the recognition of distinct, evolutionarily conserved isotypes of beta tubulin that are distinguished primarily by their characteristic carboxyl-terminal variable region sequence and, to a lesser extent, by sequence in an amino-terminal variable domain as well. These correlations represent a convincing demonstration that multiple beta tubulin genes in vertebrates encode a family of closely related but structurally distinct beta tubulin isotypes and further serve to define the sequences of four classes of polypeptide isotypes that constitute that family.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Luca F. C., Vallee R. B. Widespread cellular distribution of MAP-1A (microtubule-associated protein 1A) in the mitotic spindle and on interphase microtubules. J Cell Biol. 1984 Jan;98(1):331–340. doi: 10.1083/jcb.98.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. F., Fridovich-Keil J. L., Pillus L., Mulligan R. C., Solomon F. A chicken-yeast chimeric beta-tubulin protein is incorporated into mouse microtubules in vivo. Cell. 1986 Feb 14;44(3):461–468. doi: 10.1016/0092-8674(86)90467-8. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Sullivan K. F. Molecular biology and genetics of tubulin. Annu Rev Biochem. 1985;54:331–365. doi: 10.1146/annurev.bi.54.070185.001555. [DOI] [PubMed] [Google Scholar]
- Cumming R., Burgoyne R. D., Lytton N. A. Immunocytochemical demonstration of alpha-tubulin modification during axonal maturation in the cerebellar cortex. J Cell Biol. 1984 Jan;98(1):347–351. doi: 10.1083/jcb.98.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddé B., Jeantet C., Gros F. One beta-tubulin subunit accumulates during neurite outgrowth in mouse neuroblastoma cells. Biochem Biophys Res Commun. 1981 Dec 15;103(3):1035–1043. doi: 10.1016/0006-291x(81)90913-x. [DOI] [PubMed] [Google Scholar]
- Gard D. L., Kirschner M. W. A polymer-dependent increase in phosphorylation of beta-tubulin accompanies differentiation of a mouse neuroblastoma cell line. J Cell Biol. 1985 Mar;100(3):764–774. doi: 10.1083/jcb.100.3.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G. G., Kalnoski M. H., Bulinski J. C. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984 Oct;38(3):779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Hall J. L., Dudley L., Dobner P. R., Lewis S. A., Cowan N. J. Identification of two human beta-tubulin isotypes. Mol Cell Biol. 1983 May;3(5):854–862. doi: 10.1128/mcb.3.5.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havercroft J. C., Cleveland D. W. Programmed expression of beta-tubulin genes during development and differentiation of the chicken. J Cell Biol. 1984 Dec;99(6):1927–1935. doi: 10.1083/jcb.99.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden J. H., Allen R. D. Detection of single microtubules in living cells: particle transport can occur in both directions along the same microtubule. J Cell Biol. 1984 Nov;99(5):1785–1793. doi: 10.1083/jcb.99.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber G., Matus A. Differences in the cellular distributions of two microtubule-associated proteins, MAP1 and MAP2, in rat brain. J Neurosci. 1984 Jan;4(1):151–160. doi: 10.1523/JNEUROSCI.04-01-00151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S. Cell division and the mitotic spindle. J Cell Biol. 1981 Dec;91(3 Pt 2):131s–147s. doi: 10.1083/jcb.91.3.131s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi H. C., Chu D., Buxbaum R. E., Heidemann S. R. Tension and compression in the cytoskeleton of PC 12 neurites. J Cell Biol. 1985 Sep;101(3):697–705. doi: 10.1083/jcb.101.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauhs E., Little M., Kempf T., Hofer-Warbinek R., Ade W., Ponstingl H. Complete amino acid sequence of beta-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4156–4160. doi: 10.1073/pnas.78.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Hernault S. W., Rosenbaum J. L. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985 Jan 15;24(2):473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Loomis C., Cowan N. J. Sequence of an expressed human beta-tubulin gene containing ten Alu family members. Nucleic Acids Res. 1984 Jul 25;12(14):5823–5836. doi: 10.1093/nar/12.14.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I. R., Farmer S., Racaniello V. R., Sharp P. A. Nucleotide sequence and evolution of a mammalian alpha-tubulin messenger RNA. J Mol Biol. 1981 Sep 5;151(1):101–120. doi: 10.1016/0022-2836(81)90223-0. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Gilmartin M. E., Hall J. L., Cowan N. J. Three expressed sequences within the human beta-tubulin multigene family each define a distinct isotype. J Mol Biol. 1985 Mar 5;182(1):11–20. doi: 10.1016/0022-2836(85)90023-3. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Lee M. G., Cowan N. J. Five mouse tubulin isotypes and their regulated expression during development. J Cell Biol. 1985 Sep;101(3):852–861. doi: 10.1083/jcb.101.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M., Ludueña R. F. Structural differences between brain beta 1- and beta 2-tubulins: implications for microtubule assembly and colchicine binding. EMBO J. 1985 Jan;4(1):51–56. doi: 10.1002/j.1460-2075.1985.tb02316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Havercroft J. C., Chow L. T., Cleveland D. W. Four unique genes required for beta tubulin expression in vertebrates. Cell. 1983 Mar;32(3):713–724. doi: 10.1016/0092-8674(83)90057-0. [DOI] [PubMed] [Google Scholar]
- Ludueña R. F., Roach M. C., Trcka P. P., Little M., Palanivelu P., Binkley P., Prasad V. beta 2-Tubulin, a form of chordate brain tubulin with lesser reactivity toward an assembly-inhibiting sulfhydryl-directed cross-linking reagent. Biochemistry. 1982 Sep 14;21(19):4787–4794. doi: 10.1021/bi00262a041. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Wallis K. T. Isolation of microtubule protein from chicken erythrocytes and determination of the critical concentration for tubulin polymerization in vitro and in vivo. J Biol Chem. 1983 Jul 10;258(13):8357–8364. [PubMed] [Google Scholar]
- Parysek L. M., Asnes C. F., Olmsted J. B. MAP 4: occurrence in mouse tissues. J Cell Biol. 1984 Oct;99(4 Pt 1):1309–1315. doi: 10.1083/jcb.99.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. F., Cleveland D. W. Sequence of a highly divergent beta tubulin gene reveals regional heterogeneity in the beta tubulin polypeptide. J Cell Biol. 1984 Nov;99(5):1754–1760. doi: 10.1083/jcb.99.5.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. F., Lau J. T., Cleveland D. W. Apparent gene conversion between beta-tubulin genes yields multiple regulatory pathways for a single beta-tubulin polypeptide isotype. Mol Cell Biol. 1985 Sep;5(9):2454–2465. doi: 10.1128/mcb.5.9.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan J. A., Solomon F. Reformation of the marginal band of avian erythrocytes in vitro using calf-brain tubulin: peripheral determinants of microtubule form. J Cell Biol. 1984 Dec;99(6):2108–2113. doi: 10.1083/jcb.99.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Reese T. S., Sheetz M. P. Movement of organelles along filaments dissociated from the axoplasm of the squid giant axon. Cell. 1985 Feb;40(2):449–454. doi: 10.1016/0092-8674(85)90159-x. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]



