Abstract
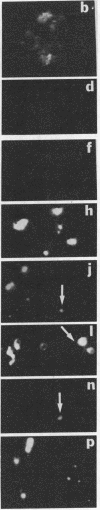
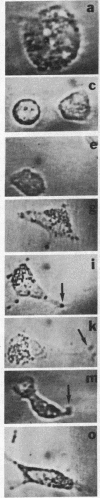
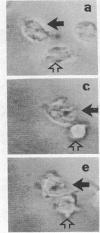
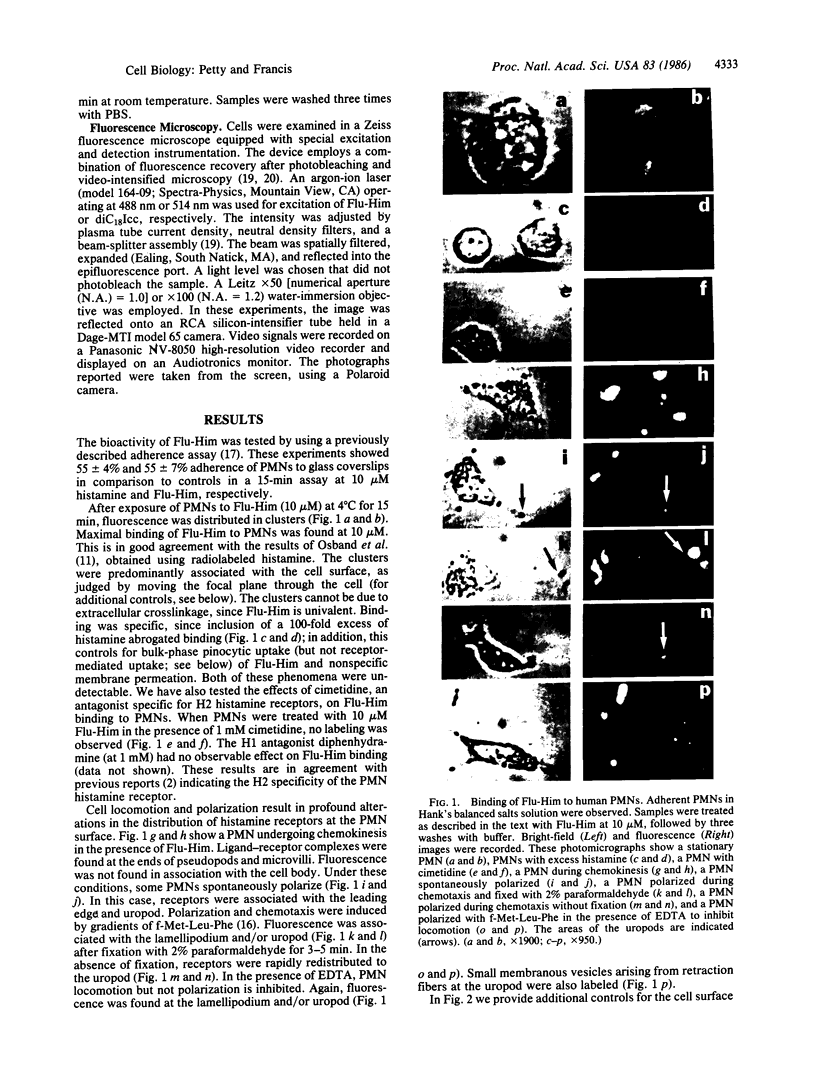
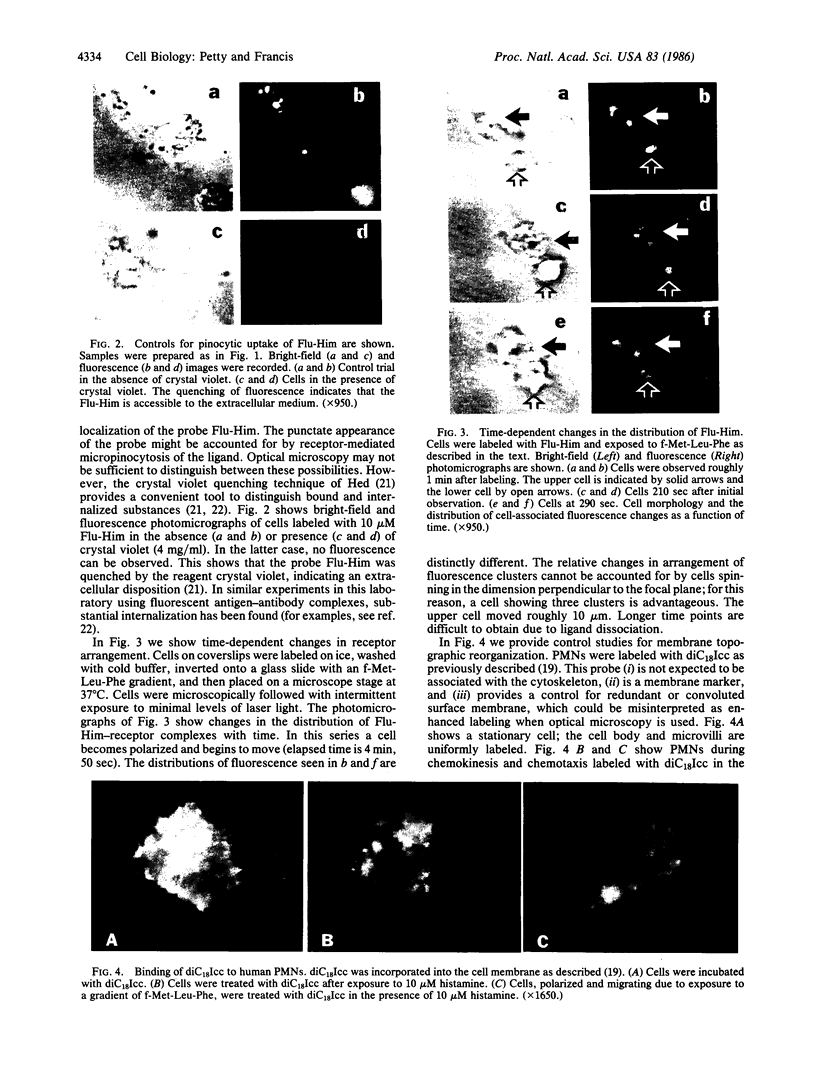
A univalent and bioactive fluorescent derivative of histamine bound to the surface of human polymorphonuclear leukocytes; free histamine was found to compete with this derivative for binding sites. Histamine H2-receptor specificity was indicated by binding inhibition experiments using cimetidine (H2-specific) but not diphenhydramine (H1-specific). Video-intensification fluorescence microscopy was used to determine the distribution of histamine receptors in living polymorphonuclear leukocytes. Receptors appeared as randomly distributed clusters upon stationary cells. During random locomotion, receptors were restricted to the ends of pseudopods, whereas chemotaxis led to receptor localization at lamellipodia and uropods. Ligand-receptor complexes were restricted to the cell surface, as shown by quenching exterior fluorescence with crystal violet. Therefore, pinocytic uptake cannot account for the observed receptor localization or clustering. As a further control, the lipid analog 1,1-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine remained uniformly distributed during all conditions. Histamine-mediated inhibition of adherence may be related to formation of ligand-receptor membrane domains at adherence sites.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie M., Heaysman J. E., Pegrum S. M. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971 Aug;67(2):359–367. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- Anderson R., Glover A., Rabson A. R. The in vitro effects of histamine and metiamide on neutrophil motility and their relationship to intracellular cyclic nucleotide levels. J Immunol. 1977 May;118(5):1690–1696. [PubMed] [Google Scholar]
- Beer D. J., Matloff S. M., Rocklin R. E. The influence of histamine on immune and inflammatory responses. Adv Immunol. 1984;35:209–268. doi: 10.1016/s0065-2776(08)60577-5. [DOI] [PubMed] [Google Scholar]
- Busse W. W., Sosman J. Histamine inhibition of neutrophil lysosomal enzyme release: an H2 histamine receptor response. Science. 1976 Nov 12;194(4266):737–738. doi: 10.1126/science.185696. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Gallin J. I., Kaplan A. P. The selective eosinophil chemotactic activity of histamine. J Exp Med. 1975 Dec 1;142(6):1462–1476. doi: 10.1084/jem.142.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. G., Munro A. J., Dracott B. N., Ife R. J., Vickers M. R. Histamine receptors on leukocytes: the binding of histamine serum albumin conjugates is non-specific. Int Arch Allergy Appl Immunol. 1985;76(1):9–15. doi: 10.1159/000233653. [DOI] [PubMed] [Google Scholar]
- Dereski W., Petty H. R. Role of macrophage cell surface sulphydryl groups in endocytosis, but not recognition of immune complexes. Immunology. 1985 Feb;54(2):397–399. [PMC free article] [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Gingell D., Todd I., Bailey J. Topography of cell-glass apposition revealed by total internal reflection fluorescence of volume markers. J Cell Biol. 1985 Apr;100(4):1334–1338. doi: 10.1083/jcb.100.4.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller H. U., Barandun S., Kistler P., Ploem J. S. Locomotion and adhesion of neutrophil granulocytes. Effects of albumin, fibrinogen and gamma globulins studied by reflection contrast microscopy. Exp Cell Res. 1979 Sep;122(2):351–362. doi: 10.1016/0014-4827(79)90311-2. [DOI] [PubMed] [Google Scholar]
- Lanni F., Waggoner A. S., Taylor D. L. Structural organization of interphase 3T3 fibroblasts studied by total internal reflection fluorescence microscopy. J Cell Biol. 1985 Apr;100(4):1091–1102. doi: 10.1083/jcb.100.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Berlin R. D. Surface and cytoskeletal events regulating leukocyte membrane topography. Semin Hematol. 1983 Oct;20(4):282–304. [PubMed] [Google Scholar]
- Osband M. E., Cohen E. B., McCaffrey R. P., Shapiro H. M. A technique for the flow cytometric analysis of lymphocytes bearing histamine receptors. Blood. 1980 Nov;56(5):923–925. [PubMed] [Google Scholar]
- Osband M. E., Cohen E. B., Miller B. R., Shen Y. J., Cohen L., Flescher L., Brown A. E., McCaffrey R. P. Biochemical analysis of specific histamine HI and H2 receptors on lymphocytes. Blood. 1981 Jul;58(1):87–90. [PubMed] [Google Scholar]
- Osband M., McCaffrey R. Solubilization, separation, and partial characterization of histamine H1 and H2 receptors from calf thymocyte membranes. J Biol Chem. 1979 Oct 25;254(20):9970–9972. [PubMed] [Google Scholar]
- Ozaki Y., Kume S., Ohashi T. Effects of histamine agonists and antagonists on luminol-dependent chemiluminescence of granulocytes. Agents Actions. 1984 Oct;15(3-4):182–188. doi: 10.1007/BF01972347. [DOI] [PubMed] [Google Scholar]
- Patrone F., Dallegri F., Lanzi G., Sacchetti C. Reversal by cimetidine of histamine-induced inhibition of true chemotaxis in neutrophil polymorphonuclears. Res Exp Med (Berl) 1980;176(3):201–205. doi: 10.1007/BF01855840. [DOI] [PubMed] [Google Scholar]
- Petty H. R., Francis J. W. Novel fluorescence method to visualize antibody-dependent hydrogen peroxide-associated "killing" of liposomes by phagocytes. Biophys J. 1985 Jun;47(6):837–840. doi: 10.1016/S0006-3495(85)83987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty H. R., Smith L. M., Fearon D. T., McConnell H. M. Lateral distribution and diffusion of the C3b receptor of complement, HLA antigens, and lipid probes in peripheral blood leukocytes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6587–6591. doi: 10.1073/pnas.77.11.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligmann B. E., Fletcher M. P., Gallin J. I. Histamine modulation of human neutrophil oxidative metabolism, locomotion, degranulation, and membrane potential changes. J Immunol. 1983 Apr;130(4):1902–1909. [PubMed] [Google Scholar]
- Smart L. M., Kay A. B. Histamine receptors on human peripheral blood leucocytes. Clin Exp Immunol. 1981 Jun;44(3):581–586. [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Walker J. R. The effects of some antirheumatic drugs on an in vitro model of human polymorphonuclear leucocyte chemokinesis. Br J Pharmacol. 1980 Jul;69(3):473–478. doi: 10.1111/j.1476-5381.1980.tb07037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. A., Mandell G. L. Motility of human polymorphonuclear neutrophils: microscopic analysis of substrate adhesion and distribution of F-actin. Cell Motil. 1983;3(1):31–46. doi: 10.1002/cm.970030104. [DOI] [PubMed] [Google Scholar]
- Thulin H., Hanifin J. M., Bryant R. Leukocyte adherence in atopic dermatitis: diminished responses to histamine and isoproterenol. Acta Derm Venereol. 1980;60(3):235–238. [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. The visualization of fluorescent proteins in living cells by video intensification microscopy (VIM). Cell. 1978 Mar;13(3):501–507. doi: 10.1016/0092-8674(78)90323-9. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977 Nov;75(2 Pt 1):606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]