Abstract
Objective
To compare the nutritional intake of patients with acute and chronic spinal cord injury (SCI).
Design
Cross-sectional, observational study.
Setting
Spinal cord unit.
Methods
Twelve in-house patients of a spinal cord unit with acute SCI and paralysis duration of 5.3 ± 2.5 months (acute group) were compared with 12 subjects with chronic SCI (chronic group) with lesion duration of 55.5 ± 21.0 months. All subjects recorded their nutritional intake for 7 days, which was analyzed for intake of energy, proteins, fat, carbohydrates, vitamins, mineral nutrients, fluid, and dietary fiber. Resting energy expenditure (REE) and total body fat were also determined.
Results
The chronic group showed a significantly higher total body fat content compared to the acute group (19.4 ± 3.8 vs. 15.7 ± 4.3%). All other parameters were not significantly different between groups. Both groups ingested excessive fat and insufficient amounts of carbohydrates compared with common nutritional recommendations. Low intakes of vitamins C, D, E, biotin, folic acid, as well as potassium and iron were found.
Conclusions
No differences were found in the nutritional intakes of two comparable groups of subjects with acute and chronic SCI. Independent of lesion duration, subjects with SCI showed considerable deviations from the general accepted nutritional recommendations concerning macro- and micronutrients intake. Professional nutritional education for persons with SCI should start as soon as possible after injury to prevent nutrition-related secondary complications like cardiovascular diseases. Periodic determinations of body fat content and REE combined with a physical activity program might be helpful as well.
Keywords: Spinal cord injuries, Acute, Chronic, Paraplegia, Tetraplegia, Resting energy expenditure, Dietary intake, Body fat content, Nutrition, Education
Introduction
Spinal cord injury (SCI) causes a number of metabolic changes. The missing muscle innervation of paralyzed limbs leads to muscle atrophy with a parallel increase in relative body fat mass. Such changes in body composition1 in combination with inactivity are responsible for disturbances in carbohydrate and fat metabolism of patients with SCI. In these patients, cardiovascular risk factors such as glucose intolerance, insulin resistance, obesity, hyperinsulinemia, dyslipidemia, and hypertension are common.2 This is of importance as cardiovascular diseases are the main reason for the reduced life expectancy in patients with SCI.3 From a preventive point of view, balanced nutrition seems to play a key role in this context. Moreover, nutrition of patients with SCI has a major impact on secondary complications like pressure sores and prolonged wound healing,4 negative nitrogen balance,5 digestion problems,6,7 reduced immunofunction,8 as well as osteoporosis.2
Based on these findings, it is not surprising that nutritional information and support in this population is a big challenge. This is also of particular interest for nutritionists and health care professionals as malnutrition and obesity are well-known problems in this population.9 In fact, the nutritional behavior of patients with SCI has been investigated in several studies so far.4,9–14 However, all these studies were focussed on particular patient groups with either acute or chronic SCI. To our knowledge no study so far has concurrently investigated two comparable groups with acute and chronic SCI. The aim of the present study was to compare the nutritional intake of patients with acute (during first rehabilitation) and chronic (at least 2 years post-injury) SCI. This comparison is of interest as leaving the hospital after first rehabilitation is often associated with a change of environmental and life style factors. In many cases there is no predetermined daily routine or follow-up on the quality and quantity of nutritional intake, which might lead to a change of nutritional behavior based on individual preferences. Therefore, we expected a higher energy intake and a less balanced nutrition of the group with chronic SCI. The findings of the present study may provide helpful information for optimizing future nutritional education during and after the rehabilitation process of patients with SCI.
Methods
Subjects
In total 24 subjects with motor complete SCI (American Spinal Injury Association Impairment Scale (AIS) A or B) participated in the study. A frequency matching creating two groups was applied, whereas the confounders of sex (male or female) and lesion level (tetraplegia or paraplegia) were used as main matching criteria. Further, we aimed to align age, height, weight, and body mass index (BMI) between subjects of the two groups.
The first group consisted of hospitalized patients with SCI with a lesion duration of less than 8 months who took part in an initial rehabilitation program (acute group), whereas the second group were outpatients with a lesion duration of at least 2 years (chronic group). Detailed information about subjects' characteristics can be found in Table 1.
Table 1.
Subjects' characteristics
| Subjects | Sex | Lesion level | Time post injury (months) | Age (year) | Weight (kg) | Height (cm) | BMI (kg/m2) |
|---|---|---|---|---|---|---|---|
| Acute group | |||||||
| 1 | f | C4 | 8 | 22 | 52 | 173 | 17.4 |
| 2 | f | Th4/5 | 6 | 24 | 56 | 178 | 17.7 |
| 3 | f | Th6 | 2.5 | 30 | 57.6 | 162 | 21.9 |
| 4 | m | C6 | 7.5 | 22 | 72.7 | 187 | 20.8 |
| 5 | m | C5 | 8 | 22 | 77.7 | 178 | 24.5 |
| 6 | m | C5/6 | 7 | 26 | 75.5 | 190 | 20.9 |
| 7 | m | C7 | 4 | 29 | 55 | 170 | 19 |
| 8 | m | Th5 | 1.5 | 24 | 83.3 | 176 | 26.9 |
| 9 | m | Th10 | 3.5 | 26 | 88 | 178 | 27.8 |
| 10 | m | Th6 | 1.5 | 28 | 65 | 188 | 18.4 |
| 11 | m | Th10 | 7 | 31 | 75 | 180 | 23.1 |
| 12 | m | Th5 | 7 | 48 | 80 | 180 | 24.7 |
| Mean | 5.3 | 27.7 | 69.8 | 178.3 | 21.9 | ||
| SD | 2.5 | 7.1 | 12.2 | 7.9 | 3.5 | ||
| Chronic group | |||||||
| 1 | f | C7 | 115 | 26 | 54.6 | 168 | 19.3 |
| 2 | f | Th12 | 60 | 27 | 56.5 | 168 | 20 |
| 3 | f | Th9 | 59 | 30 | 51.3 | 165 | 18.8 |
| 4 | m | C7 | 38 | 26 | 64.1 | 171 | 21.3 |
| 5 | m | C6/7 | 49 | 26 | 80.4 | 185 | 23.5 |
| 6 | m | C6 | 57 | 25 | 59 | 180 | 18.2 |
| 7 | m | C5 | 59 | 36 | 57 | 177 | 18.2 |
| 8 | m | Th5 | 50 | 22 | 55 | 176 | 20.7 |
| 9 | m | Th6 | 50 | 28 | 57.3 | 173 | 19.1 |
| 10 | m | Th5 | 32 | 24 | 90.8 | 176 | 29.3 |
| 11 | m | Th4/5 | 59 | 29 | 89.5 | 180 | 27.6 |
| 12 | m | Th5 | 38 | 46 | 72 | 176 | 23.2 |
| Mean | 55.5* | 28.8 | 66.4 | 174.7 | 21.6 | ||
| SD | 21 | 6.5 | 14.5 | 6.1 | 3.7 | ||
BMI: body mass index; f: female; m: male; C: cervical lesion; Th: thoracic lesion; SD: standard deviation.
*Significant difference between groups (P < 0.001).
The study was approved by the local ethics committee and subjects gave their written informed consent before the start of the study.
Nutritional protocol and analysis
Subjects were instructed to record their nutritional intake precisely during seven consecutive days. Subjects were provided with a booklet containing 7 prepared standard forms, and detailed instructions, as well as 26 photos showing different serving sizes of common foods. To ensure correct use of the booklet, the content was discussed with each subject in detail and subjects were advised to complete the nutritional protocol with diligence. The competed protocol was analyzed using the program EBISpro (Universität Hohenheim, Stuttgart, Deutschland), which allowed evaluation of energy intake, macronutrients and micronutrients (including dietary supplements) as well as fluid and dietary fiber intake.
Body fat and resting energy expenditure
Body fat of the subjects was determined by means of the bioimpedance technique using the device Bodystat QuadScan 4000 (Bodystat Ltd, Douglas, Isle of Man, UK) and resting energy expenditure (REE) was measured via indirect calorimetry with the MedGem apparatus (Health Tech Inc., Golden, USA). All measurements were performed in the supine position after lying relaxed for 5–10 minutes. In order to obtain reliable data, test preparations and measurement conditions were standardized. Thus, subjects were instructed to avoid food or fluid intake 4–5 hours before the measurement, as well as to abstain from caffeine or alcohol intake the last 24 hours. Further, physical exercise was prohibited for 12 hours before the measurements. All subjects fulfilled this measurement conditions.
Statistics
Data are presented as means ± standard deviation (SD). For group comparisons an unpaired t-test was applied. All statistical analysis was performed using SYSTAT (Version 10, SPSS Inc., Richmond, CA, USA). Statistical significance was set at P < 0.05.
An undersupply of micronutrients was assumed if values were more than 20% lower compared to generally accepted guidelines.15
Results
Subjects
Concerning lesion duration, there was a significant difference between the acute and chronic group (P < 0.001). However, no significant differences were found for age (P = 0.701), weight (P = 0.544), height (P = 0.236), or BMI (P = 0.827) between groups (Table 1).
Nutritional analysis
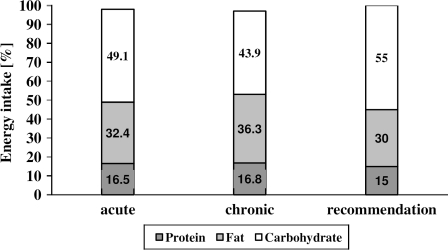
No significant difference (P = 0.467) was found for average daily energy intake between the acute (7.71 ± 0.91 MJ/d (1842 ± 217 kcal)) and the chronic group (7.43 ± 0.98 MJ/d (1775 ± 234 kcal)). Whereas the acute group ingested 74.6 ± 10.0 g/d of protein, 67.8 ± 15.3 g/d of fat, and 223.7 ± 56.3 g/d of carbohydrates corresponding average intakes in the chronic group were 71.4 ± 7.9, 71.5 ± 9.8, and 193.8 ± 55.9 g/d, respectively, and not significantly different (protein: P = 0.399; fat: P = 0.490; and carbohydrates: P = 0.205) between groups. Average percentage caloric daily intake of ingested macronutrients protein, fat, and carbohydrate can be found in Fig. 1 and was comparable and not significantly different (protein: P = 0.776; fat: P = 0.151; and carbohydrates: P = 0.180) between groups. Further subjects of the acute group consumed on average 15.6% of their daily carbohydrate intake as soft drinks, whereas it was only 7.4% in the chronic group.
Figure 1.
Mean percentage caloric daily intake of ingested macronutrients in the acute and chronic groups compared to generally accepted recommendations.
Mean daily micronutrients intake showed no significant differences between groups. Detailed information are presented in Table 2 and also shown in relation to the conventional recommendations for able-bodied subjects.15 For the acute as well as for the chronic group intake of six vitamins (C, D, E, folic acid, pantothenic acid, and biotin), potassium and iron were remarkable below the recommendations (more than 20%) for able-bodied subjects (Table 2).
Table 2.
Mean daily vitamin and mineral nutrient intake in acute (acute group; n = 12) and chronic (chronic group; n = 12) SCI in comparison to widely accepted recommendations expressed in percent
| Acute group (Mean ± SD) | Recommendation (%) | Chronic group (Mean ± SD) | Recommendation (%) | P value | |
|---|---|---|---|---|---|
| Vitamin | |||||
| A (μg/d) | 1056.8 ± 249.8 | 132.1 | 1101.2 ± 338.0 | 137.7 | 0.718 |
| D (μg/d) | 1.7 ± 1.6 | 34.3 | 1.9 ± 2.0 | 38.0 | 0.804 |
| E (mg/d) | 7.6 ± 2.4 | 63.3 | 8.9 ± 2.4 | 74.2 | 0.167 |
| K (μg/d) | 214.3 ± 82.0 | 357.2 | 195.2 ± 51.2 | 325.3 | 0.502 |
| B1 (mg/d) | 1.0 ± 0.3 | 100.8 | 1.3 ± 0.8 | 133.3 | 0.234 |
| B2 (mg/d) | 1.6 ± 0.4 | 129.9 | 1.8 ± 1.1 | 146.5 | 0.566 |
| B6 (mg/d) | 1.5 ± 0.4 | 123.6 | 2.1 ± 1.5 | 171.5 | 0.216 |
| B12 (μg/d) | 2.8 ± 0.9 | 92.8 | 3.0 ± 1.4 | 100.0 | 0.656 |
| Niacin (mg/d) | 12.3 ± 3.5 | 94.6 | 15.2 ± 5.6 | 116.9 | 0.143 |
| Folic acid (μg/d) | 88.2 ± 25.0 | 22.1 | 96.1 ± 32.4 | 24.0 | 0.508 |
| Pantothenic acid (mg/d) | 4.3 ± 1.1 | 70.8 | 4.5 ± 1.2 | 75.1 | 0.582 |
| Biotin (μg/d) | 31.8 ± 8.5 | 70.6 | 36.2 ± 18.0 | 80.5 | 0.451 |
| C (mg/d) | 79.1 ± 37.7 | 79.1 | 89.3 ± 61.7 | 89.3 | 0.631 |
| Mineral nutrient | |||||
| Sodium (mg/d) | 2402.4 ± 533.5 | 120.1 | 2647.5 ± 616.3 | 132.4 | 0.246 |
| Potassium (mg/d) | 2692.3 ± 925.3 | 76.9 | 2375.8 ± 570.9 | 67.9 | 0.315 |
| Calcium (mg/d) | 1075.1 ± 255.7 | 107.5 | 1042.5 ± 311.7 | 104.3 | 0.927 |
| Magnesium (mg/d) | 319.6 ± 78.2 | 106.5 | 329.6 ± 54.9 | 109.9 | 0.542 |
| Phosphor (mg/d) | 1226.3 ± 246.5 | 175.2 | 1119.6 ± 181.1 | 159.9 | 0.402 |
| Iron (mg/d) | 11.0 ± 2.9 | 73.1 | 12.7 ± 7.6 | 84.9 | 0.394 |
| Zinc (mg/d) | 9.7 ± 1.4 | 139.1 | 9.6 ± 2.0 | 137.7 | 0.781 |
Note that there were no significant differences between groups.
SD: standard deviation; P-values represent the comparison between the acute and the chronic group.
Fluid intake was 2.6 ± 0.8 l/d in the acute and 3.1 ± 1.2 l/d in the chronic group but did not reach statistical significance (P = 0.205) between groups. The acute group ingested an average amount of 14.4 ± 4.9 g and the chronic group of 15.6 ± 2.4 g of dietary fiber per day, which revealed no significant difference (P = 0.455) between groups.
Body fat and REE
The acute group showed a significant (P = 0.038) lower body fat content (15.7 ± 4.3%) compared to the chronic group (19.4 ± 3.8%). No significant difference (P = 0.356) was found for REE between the acute (5.92 ± 1.37 MJ (1414 ± 327 kcal) per day) and the chronic group (5.46 ± 0.97 MJ (1304 ± 232 kcal) per day).
Discussion
No significant differences were found between the nutritional behavior of comparable groups of subjects with acute and chronic SCI. The only difference between the two groups concerned the higher body fat content of the subjects with longer lesion duration. However, there are some interesting aspects, which might be helpful for future nutritional consulting during and after the rehabilitation process of patients with SCI.
Energy intake
We found similar energy intakes for subjects with SCI as described earlier in the literature,11 which seem to be clearly below the values for able-bodied subjects.16 In the study of Levine et al.11 the energy intake in subjects with SCI was 75% of the general recommendations for able-bodied persons. This is not surprising and reflects the reduced activity of subjects with SCI as well as the changed body composition.17 However, one has to keep in mind that daily activity plays a key role in energy consumption and therefore, could markedly influence dietary intake in subjects with SCI. Thus, the recording of physical activity would have been of interest for our study as well. Although such data are not available, we are confident that the study outcome was not significantly influenced by this limitation as we studied two comparable, frequency-matched groups.
In general, one has to take also into account that compared to able-bodied persons the REE of subjects with SCI is reduced17,18 and thus a lower daily energy intake is sufficient to meet the caloric needs of this particular population. Moreover, subjects with SCI often suffer from gastrointestinal problems, which compromise nutritional intake.19 Further reasons for a reduced energy intake might also be a reduced appetite or an earlier ‘satiety feeling’ in subjects with SCI.13
Macronutrients
In contrast to our expectations, no significant differences were found in percentage macronutrient intake between the acute and the chronic group (Fig. 1). A possible explanation might be that subjects with chronic SCI are aware of the consequences of possible changes in body composition, whereas patients with acute SCI need to be sensitized to their new situation. Subjects with a chronic SCI may pay more attention to nutritional aspects, which seems to be supported by the fact that – compared to the acute group – subjects of the chronic group consumed only half the amount of soft drinks. One could argue that nutritional consulting of subjects with acute SCI should be intensified and the food provided by the hospital might be reconsidered and adapted to even better meet the nutritional requirements of in-house patients.
However, both groups ingested a too high percentage of fat (acute group: 32%; chronic group: 36%) and an insufficient amount of carbohydrates (49 vs. 43%) compared with the general accepted recommendations (carbohydrates: 55–60%; fat 25–30%) for balanced nutrition.15 Our results are in line with data of a recently published study reporting macronutrient intake in 73 subjects with SCI at least 1 year post-injury16 and seem also not to differ in the nutritional behavior of the general able-bodied population of our country.20 Taking into account that obesity16 and cardiovascular diseases are common in patients with SCI,3 a reduction of fat intake is strongly recommended in order to minimize cardiovascular risk factors reported by Baumann and Spungen.2
Protein supplementation was about 17% in both groups (Fig. 1) corresponding to an average of 1.1 g of protein per kg body weight per day. To prevent complications like pressure sores and tissue atrophy in persons with SCI, a daily protein intake of 2 g per kg body weight was recommended.5 Thus, protein intake of our subjects should be slightly increased to supply the higher protein needs due to SCI.
Micronutrients
As a result of the reduced energy intake reported in subjects with SCI18 an adequate intake of micronutrients seems to be critical for this population. In fact, our study suggests very low intakes of vitamins C, D, E, folic acid, pantothenic acid, biotin, potassium and iron for the acute as well as the chronic group, with no significant differences between groups (Table 2). However, although subjects were instructed in detail about completion of the nutritional protocol, some sources of error cannot be entirely excluded (e.g. underreporting or unsatisfactory estimation of serving size by the subjects; limited data set of foods or non-consideration of storage mode and detailed preparation of vegetables for data analysis), which might have lead to a moderate underestimation of reported values. Nevertheless, the present results provide some helpful information concerning micronutrient intake in people with SCI and are in line with results of recently published studies,14,16 which also reported numerous nutrient inadequacies in adults with chronic SCI. However, going into more detail, the amount of ingested micronutrients between different studies seems to vary widely. Whereas in the present study the amount of ingested folic acid reached on average 88–96 µg/d (corresponding to 22–24% of daily recommendation), subjects consumed 75–79% of the daily recommended amount in the study of Walters et al.14 and 211–424 µg/d (corresponding to 53–106%) in the study of Groah et al..16 Similar observations can be found for example for vitamin D. For this vitamin the present study found ingested amounts of 34–38% of the daily recommendation, whereas Walters et al.14 reported 25–69% and Groah et al.16 44–96%, respectively. As a consequence, optimal consulting of patients with SCI concerning micronutrient intake should ideally be based on previous analysis of individual nutritional habits.
In general, an undersupply of micronutrients in combination with SCI has to be avoided as this may contribute to the development of complications already mentioned. In this context, the question arises whether an additional, well-directed micronutrient supplementation (e.g. tablets) should be recommended for persons with SCI. However, the first goal should be to sensitize and educate subjects with SCI in order to change their individual dietary behavior toward balanced nutrition. Furthermore, interactions between medication and nutritients have to be specifically considered as most subjects receive long-term medication (e.g. laxatives).
Fluid intake
Fluid intake in the acute as well as in the chronic group seemed to be adequate and met the general recommendations for able-bodied subjects.15 An interesting finding was, that the acute group consumed a much higher (more than double) amount of soft drinks compared to the chronic group. In the acute group 15.6% of the total daily carbohydrate intake was covered by soft drink ingestion compared to 7.4% in the chronic group. During personal interviews most subjects indicated a conscious additional consumption of calories as the main reason. Not all subjects liked the food provided by the hospital and therefore tried to compensate the lacking calories by means of soft drinks. Based on the fact that excessive soft drink consumption has received considerable notoriety with regard to obesity, nutritionists should keep these findings in mind during counselling interviews with subjects with SCI.
Moreover, soft drinks were obviously the main reason why subjects reached a far too high phosphate intake (Table 2), which might negatively influence bone metabolism.21 Indeed osteoporosis and the concomitant increase in fracture risk are known to be one of the main secondary complications in SCI.22 It seems to be worthwhile and necessary to inform subjects about this fact and to provide some alternatives.
Dietary fiber intake
Many patients with SCI suffer from gastrointestinal complications and thus a sufficient supply with dietary fiber has to be carefully considered.23,24 Whereas Badiali et al.6 reported positive effects of a daily dietary fiber intake of 18 g in subjects with SCI, a daily intake of more than 31 g/d is not recommended in this population.7 It seems to be difficult to provide generally accepted recommendations25 and further studies are needed to clarify this issue. In the present study, mean daily dietary fiber intake of the acute group was 14.4 and 15.6 g in the chronic group. These subject groups might benefit from a slightly increased intake of dietary fiber with respect to cholesterol metabolism.26
Body fat content
Although no differences were found in body weight, BMI, and nutritional behavior between groups, the chronic group showed a higher body fat content compared to the acute group. Possibly, the main reason for this finding is the significant difference in lesion duration between groups (Table 1). This assumption is supported by the study of Spungen et al.,27 where a direct relationship between time post-injury and total body fat content was demonstrated. The question arises if the continuously increasing body fat content of subjects with a long-term SCI can be avoided or at least retarded by means of dietary interventions, which might be helpful in reducing the risk for cardiovascular diseases in this population. In this respect, an additional well-directed physical activity program might also show beneficial effects. However, to prove our speculations further research is needed.
Although no difference in BMI between groups was found, body fat content was significantly higher in the chronic group. This finding argues against the use of BMI as an estimation of fat mass in the SCI population and is in line with results of former publications.28 Therefore, in order to monitor changes in body composition in persons with SCI, e.g. during medical checkups, the application of bioelectrical impedance analysis was recommended.29
Limitations of the study
The limited number of subjects is a limitation of this study. Further, study results have to be interpreted with caution as a selective sample (only motor complete; AIS impairment scale A or B) was studied. Before a generalization of the findings to the broader population with SCI, a larger number of subjects including further patient groups with SCI (e.g. subjects with incomplete SCI) is necessary. However, the present study might serve as pilot work for future projects, as to our knowledge; it was the first study so far directly comparing two similar groups of subjects with acute and chronic SCI at the same time. This approach seems necessary to warrant valid information.
Conclusions
This study reveals no apparent differences in the nutritional behavior between subjects with acute and chronic SCI. Independent of lesion duration, subjects with SCI showed considerable deviations from the general accepted nutritional recommendations concerning macro- and micronutrients intake. As a consequence, professional nutritional education for persons with SCI should start as soon as possible after injury to prevent nutrition-related secondary complications like cardiovascular diseases. In addition, determination of body fat content and REE on regular time intervals combined with a physical activity program might be helpful as well.
References
- 1.Spungen AM, Wang J, Pierson RN, Jr, Baumann WA. Soft tissue body composition differences in monozygotic twins discordant for spinal cord injury. J Appl Physiol 2000;88(4):1310–15 [DOI] [PubMed] [Google Scholar]
- 2.Bauman WA, Spungen AM. Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am 2000;11(1):109–40 [PubMed] [Google Scholar]
- 3.Zlotolow SP, Levy E, Bauman WA. The serum lipoprotein profile in veterans with paraplegia: the relationship to nutritional factors and body mass index. J Am Paraplegia Soc 1992;15(3):158–62 [DOI] [PubMed] [Google Scholar]
- 4.Aquilani R, Boschi F, Contardi A, Pistarini C, Achilli MP, Fizzotti G, et al. Energy expenditure and nutritional adequacy of rehabilitation paraplegics with asymptomatic bacteriuria and pressure sores. Spinal Cord 2001;39(8):437–41 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez DJ, Benzel EC, Clevenger FW. The metabolic response to spinal cord injury. Spinal Cord 1997;35(9):599–604 [DOI] [PubMed] [Google Scholar]
- 6.Badiali D, Bracci F, Castellano V, Corazziari E, Fuoco U, Habib FI, et al. Sequential treatment of chronic constipation in paraplegic subjects. Spinal Cord 1997;35(2):116–20 [DOI] [PubMed] [Google Scholar]
- 7.Cameron KJ, Nyulasi IB, Collier GR, Brown DJ. Assessement of the effect of increased dietary fibre intake on bowl function in patients with spinal cord injury. Spinal Cord 1996;34(5):277–83 [DOI] [PubMed] [Google Scholar]
- 8.Cruse JM, Lewis RE, Dilioglou S, Roe DL, Wallace WF, Chen RS. Review of immune function, healing of pressure ulcers, and nutritional status in patients with spinal cord injury. J Spinal Cord Med 2000;23(2):129–35 [DOI] [PubMed] [Google Scholar]
- 9.Lynch AC, Palmer C, Anthony A, Roake JA, Frye J, Frizelle FA. Nutritional and immune status following spinal cord injury: case controlled study. Spinal Cord 2002;40(12):627–30 [DOI] [PubMed] [Google Scholar]
- 10.Barboriak JJ, Rooney CB, El Ghatit AZ, Spuda K, Anderson AJ. Nutrition in spinal cord injury patients. J Am Paraplegia Soc 1983;6(2):32–6 [DOI] [PubMed] [Google Scholar]
- 11.Levine AM, Nash MS, Green BA, Shea JD, Aronica MJ. An examination of dietary intakes and nutritional status of chronic healthy spinal cord injured individuals. Paraplegia 1992;30(12):880–9 [DOI] [PubMed] [Google Scholar]
- 12.Tomey KM, Chen DM, Wang X, Braunschweig CL. Dietary intake and nutritional status of urban community-dwelling men with paraplegia. Arch Phys Med Rehabil 2005;86(4):664–71 [DOI] [PubMed] [Google Scholar]
- 13.Laven GT, Huang CT, DeVivo MJ, Stover SL, Kuhlemeier KV, Fine PR. Nutritional status during the acute stage of spinal cord injury. Arch Phys Med Rehabil 1989;70(4):277–82 [PubMed] [Google Scholar]
- 14.Walters JL, Buchholz AC, Martin Ginis MA. Evidence of dietary inadequacy in adults with chronic spinal cord injury. Spinal Cord 2009;47(4):318–22 [DOI] [PubMed] [Google Scholar]
- 15.DACH (Deutsche Gesellschaft für Ernährung, Oesterreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährungsforschung, Schweizerische Vereinigung für Ernährung) Referenzwerte für die Nährstoffzufuhr. Frankfurt am Main: Umschau Braus Verlag; 2000 [Google Scholar]
- 16.Groah MD, Nash MS, Ljungberg IH, Libin A, Hamm LF, Ward E, et al. Nutrient intake and body habitus after spinal cord injury: an analysis by sex and level of injury. J Spinal Cord Med 2009;32(1):25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr 2003;77(2):371–8 [DOI] [PubMed] [Google Scholar]
- 18.Monroe MB, Tataranni PA, Pratley R, Manore MM, Skinner JS, Ravussin E. Lower daily energy expenditure as measured by a respiratory chamber in subjects with spinal cord injury compared with control subjects. Am J Clin Nutr 1998;68(6):1223–7 [DOI] [PubMed] [Google Scholar]
- 19.Shizgal HM, Roza A, Leduc B, Droudin G, Villenmure JG, Yaffe C. Body composition in quadriplegic patients. JPEN 1986;10(4):364–8 [DOI] [PubMed] [Google Scholar]
- 20.Eichholzer M, Bovey F, Jordan P, Probst-Hensch N, Stoffel-Kurt N. Data on overweight and nutrition in the 2007 Swiss Health Survey. Praxis 2010;99(1):17–25 [DOI] [PubMed] [Google Scholar]
- 21.Sax L. The institute of medicine's ‘dietary reference intake’ for phosphorus: a critical perspective. J Am Coll Nutr 2001;20(4):271–8 [DOI] [PubMed] [Google Scholar]
- 22.Eser P, Frotzler A, Zehnder Y, Denoth J. Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil 2005;86(3):498–504 [DOI] [PubMed] [Google Scholar]
- 23.De Looze D, Van Laere M, De Muynck M, Beke R, Elewaut A. Constipation and other chronic gastrointestinal problems in spinal cord injury patients. Spinal Cord 1998;36(1):63–6 [DOI] [PubMed] [Google Scholar]
- 24.Han TR, Kim JH, Kwon BS. Chronic gastrointestinal problems and bowel dysfunction in patients with spinal cord injury. Spinal Cord 1998;36(7):485–90 [DOI] [PubMed] [Google Scholar]
- 25.Dapoigny M, Stockbrügger RW, Azpiroz F, Collins S, Coremans G, Müller-Lissner S, et al. Role of alimentation in irritable bowel syndrome. Digestion 2003;67(4):225–33 [DOI] [PubMed] [Google Scholar]
- 26.Kirby RW, Anderson JW, Sieling B, Rees ED, Chen WJ, Miller RE, et al. Oat-bran intake selectively lowers serum low-density lipoprotein cholesterol concentrations of hypercholesterolemic men. Am J Clin Nutr 1981;34(5):824–9 [DOI] [PubMed] [Google Scholar]
- 27.Spungen AM, Adkins RH, Stewart CA, Wang J, Pierson RN, Jr, Waters RL, et al. Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J Appl Physiol 2003;95(6):2398–407 [DOI] [PubMed] [Google Scholar]
- 28.Buchholz AC, Bugaresti JM. A review of body mass index and waist circumference as markers of obesity and coronary heart disease risk in persons with chronic spinal cord injury. Spinal Cord 2005;43(9):513–8 [DOI] [PubMed] [Google Scholar]
- 29.Desport JC, Preux PM, Guinvarc'h S, Rousset P, Salle JY, Daviet JC, et al. Total body water and percentage fat mass measurements using bioelectrical impedance analysis and anthropometry in spinal cord-injured patients. Clin Nutr 2000;19(3):185–90 [DOI] [PubMed] [Google Scholar]