Abstract
Introduction
Diabetes is a disorder that is well known to delay wound repair resulting in the formation of colonized, chronic wounds. The resultant ulcers contribute to increased risk of morbidity, including osteomyelitis and amputations, and increased burden to the health care system.
Area Covered
The only active product approved for the treatment of diabetic ulcers, Regranex, has been shown to reduce amputation risk, but is not widely used due to minimal proven efficacy and recent warnings added to the Instructions for Use. Here, we overview the development of NorLeu3-angiotensin (1-7) (NorLeu3-A(1-7)) as an active agent for the treatment of diabetic wounds. NorLeu3-A(1-7) is an analogue of the naturally occurring peptide, angiotensin 1-7. The mechanisms of action include induction of progenitor proliferation and accelerated vascularization, collagen deposition and re-epithelialization.
Expert Opinion
Research to date has shown that NorLeu3-A(1-7) is highly effective in the closure of diabetic wounds and is superior to Regranex in animal studies. Further clinical development of this product as a topical agent for the healing of chronic wounds and investigation into the mechanisms by which this product accelerates healing are warranted.
Keywords: angiotensin, dermal, diabetes, NorLeu3-Angiotensin 1-7, wound healing
3. INTRODUCTION
Wounds result in tissue destruction and loss of vascularization at the site of injury. Normal healing processes involve cell proliferation and angiogenesis at the site of the wound. All wound healing of soft tissues follows a similar, orderly process.
Chronic wounds have a devastating effect on the health and lifestyle of over 8 million Americans a year. Approximately 900,000 people a year in the US suffer from diabetic ulcers, 915,000 suffer from venous stasis ulcers and 6.5 million people suffer from pressure ulcers. Chronic wounds can take years to heal and there is generally a high rate of wound recurrence. Ten to 15% of the population are refractory to conventional wound healing procedures and suffer continuously from chronic wounds. There is a frequent need for hospitalization of chronic wound patients and each year approximately 75,000 people in the US must undergo amputations as a result of their wounds.
4. OVERVIEW OF THE MARKET
Conventional procedures to treat chronic wounds involve protecting the wounds with dressings with occlusive dressing providing additional benefit to gauze. Debridement is also used to speed up the natural healing process. The only pharmacological agent approved by the FDA (1997) to treat diabetic wound healing is the human recombinant platelet derived growth factor in carboxymethyl cellulose (Regranex™). This drug is indicated for treatment of lower extremity diabetic neuropathetic ulcers. The drug was approved based on results of four large clinical studies in which Regranex™ was applied topically in a gel formulation. The primary endpoint of the studies was complete ulcer closure within 20 weeks. Twenty-five to 37% of patients treated with a placebo control achieved this endpoint while Regranex™ facilitated wound closure in 36-50% of the patients ([1]; Figure 1). In one of the four studies, conducted on 250 patients, Regranex™ did not achieve statistically significant improvement (36% improvement versus 32% improvement for placebo control). The relatively modest efficacy of Regranex, its high expense (average >$2000 per treatment cycle), and its limited use underscore the urgent need for more effective therapeutics for dermal tissue repair.
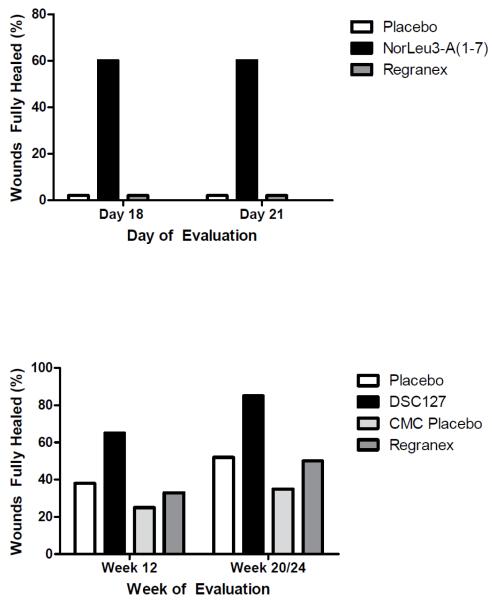
Figure 1.
These figure summarizes data comparing the efficacy of NorLeu3-A(1-7) (active pharmaceutical ingredient in DSC 127) with Regranex in preclinical model of delayed healing in diabetic mice (db/db) [from 26] and clinical studies [from 1 {20 weeks for Regranex} and 27 {24 weeks for DSC127}]. NorLeu3-A(1-7) is superior to Regranex in the closure of diabetic wounds.
5. INTRODUCTION TO NorLeu3-A(1-7)
Active peptides of the renin angiotensin system (RAS), angiotensin II (All) and angiotensin 1-7 (A(1-7)) have been shown to accelerate the healing of injuries [2-7]. A complete RAS has been shown to reside within human skin [8,9]. Studies by multiple laboratories have reported alterations in the level of RAS components by injury and the contribution of the RAS to wound healing through increased collagen deposition and transactivation of the epidermal growth factor receptor [8-17]. Angiotensin receptors are expressed both in rat neonates and adults, with the receptor phenotype changing with maturation. Upon injury the expression of receptors is up-regulated, and down regulated as the tissue heals [10-13]. In human skin, AT1 and AT2 receptors were found in the epidermis and in dermal vessel walls [8]. Recently, an upregulation of the Mas receptor, the functional receptor for A(1-7), in the skin after injury has been shown [18] (Figure 2). The same expression pattern was found for angiotensinogen, the parent protein, renin and angiotensin converting enzyme (ACE). All components were also demonstrated at the mRNA level in cultured primary keratinocytes, melanocytes (except AT2 receptors), dermal fibroblasts and dermal microvascular endothelial cells.
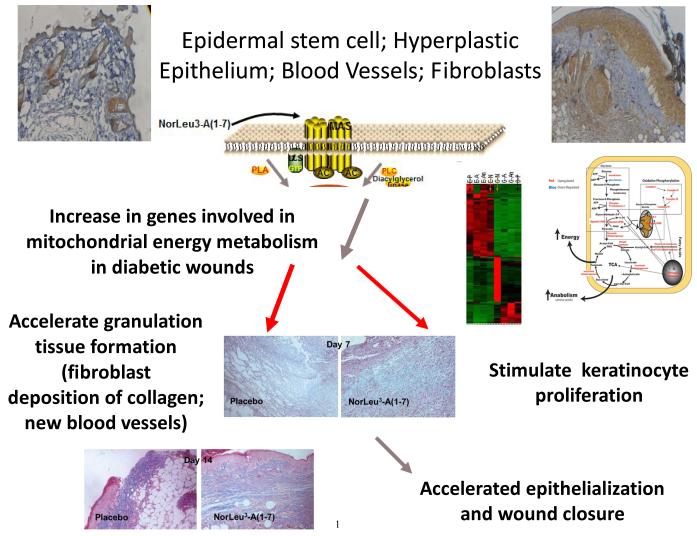
Figure 2.
This is a cartoon summarizing the current hypothesis regarding the mechanisms by which NorLeu3-A(1-7) accelerates healing of diabetic wounds. With injury, expression of the receptor for NorLeu3-A(1-7), Mas, increases in skin. The cellular components with upregulated expression include cells of the hair follicle, hyperplastic epidermis and dermis [18]. Unpublished studies using microarray technology show that a cassette of genes is upregulated in granulation tissue of wounds treated with NorLeu3-A(1-7) (compare first lane from the right [G-P; granulation-placebo] with the fourth lane from the right [G-N; granulation-NorLeu3-A(1-7)]. Analysis of the genes upregulated indicated a normalization of energy metabolism in the treated granulation tissue (schema next to microarray). These changes may contribute to the accelerated formation of new blood vessels and granulation tissue (day 7 photomicrograph) and re-epithelialization (day 14 photomicrograph).
As stated, active peptides of the RAS are potent stimulators of the proliferation of early progenitor cells which then can evolve, based upon the body’s need, into more specialized cells [2,4]. It has been shown in this and other laboratories that immature stem and progenitor cells, e.g. epidermal stem cells or hematopoietic progenitors, are most sensitive to induction of proliferation and the effect of the peptides in vivo after injury [2-5; 19]. In fact, one of the earliest markers of stem cells resulting in the blood and immune systems (hemangioblast) and epidermis is the expression of ACE, a key enzyme in the RAS [20-24]. Because of the increased sensitivity of more immature cells to the proliferative effects of angiotensin peptides, unique therapeutic opportunities with the potential to enhance tissue regeneration have been conceptualized. The description of multipotential adult progenitor cells with the capacity to contribute to the repair of multiple tissues after injury provides a basic framework for the mechanisms by which this occurs. That is, the broad spectrum of activities observed with these peptides may be due to actions on early progenitor cells.
As might be predicted by the hypothesized mechanism by which these peptides act, angiotensin peptides have a broad range of actions in facilitating tissue healing. Substantial work has also been conducted to assess the ability of angiotensin peptides to stimulate epithelial healing. Currently, our lead pharmaceutical ingredient, NorLeu3-A(1-7) is in clinical trials formulated as a topical gel in hydroxyethyl cellulose (DSC127) for accelerated healing of diabetic foot ulcers.
5.1 Efficacy of NorLeu3-A(1-7)
NorLeu3-A(1-7) has been evaluated in 3 models of dermal repair (rat full thickness excision, rat full thickness incision and full thickness excision in a diabetic mouse; [25]). The first animal model examined was the rat full-thickness excision model. NorLeu3-A(1-7) was applied to wounds for up to 14 days and effects on wound healing were compared to those induced by A(1-7) and by Regranex. NorLeu3-A(1-7) reduced the wound size by greater than 60% compared to placebo controls. A(1-7) was less effective, causing a 45% reduction in wound size. Regranex only reduced the wound size by 20%. Thus, NorLeu3-A(1-7) was three times more effective in wound healing than the FDA-approved drug .
In similar studies in the diabetic mouse full thickness excision model, NorLeu3-A(1-7) was again more effective in wound healing than either A(1-7) or Regranex. NorLeu3-A(1-7) greatly accelerated wound healing. By 18 days of treatment, NorLeu3-A(1-7) completely healed approximately 60% of the wounds and reduced total wound area by greater than 80% [25](Figure 1). In contrast, none of the diabetic animals that received Regranex were fully healed and approximately 20% were fully healed with A(1-7) by Day 18. We hypothesize that the increased efficacy of the novel peptide is due to either increased stability of the peptide in the wound or increased receptor binding. NorLeu3-A(1-7) is stable in the presence of common wound enzymes and fragments of NorLeu3-A(1-7) are active [7]. Thus, NorLeu3-A(1-7) formulated with HEC (DSC127) is superior to the FDA approved drug in wound healing (Regranex), and is undergoing clinical evaluation (Figure 1).
Studies in the rat full thickness incision model also showed that NorLeu3-A(1-7) was highly effective in wound healing. It reduced inflammation, wound dehiscence and scar formation if applied to the wound prior to closure with Dermabond [25,26]. Microscopic analysis of the wound area after systemic administration of NorLeu3-A(1-7) showed reduced scarring, reduced inflammation, accelerated healing and a normalized appearance in the full thickness incision model.
5.2 Cellular Mechanisms of Accelerated Healing
To begin to evaluate the mechanisms by which NorLeu3-A(1-7) accelerated healing, it was shown that the receptor antagonist for Mas, A779, blocked the effects of NorLeu3-A(1-7) on wound repair [26] (Figure 2). Unpublished studies show that administration of NorLeu3-A(1-7) to diabetic wounds normalizes expression of genes in the mitochondria associated with energy metabolism (Figure 2).
To further investigate the cellular mechanisms by which NorLeu3-A(1-7) accelerated healing we evaluated the quality of tissue repair in the same model in diabetic mice. The results of these studies are described in detail in [19, 25-26]. One factor essential in wound healing is the deposition of collagen in the wound area to allow for restructuring of the tissue. The natural wound repair process in diabetic mice involves the slow but gradual increase in collagen in the diabetic wounds. NorLeu3-A(1-7) accelerated the deposition of collagen to speed up the wound repair process through the formation of granulation tissue (Figure 2). Further histological examination revealed that the collagen present was more mature at later time points with a basket-weave appearance consistent with a more regenerative process. NorLeu3-A(1-7) was either equivalent or superior to AII and A(1-7) in accelerating collagen deposition.
The peptide also greatly accelerated dermal re-epithelialization at the wound site. Epidermal regrowth was seen as early as four days after the treatment started. NorLeu3-A(1-7) caused between 4–6-fold increases in epidermal growth and accelerated the maturation of wound healing such that by 18 days after NorLeu3-A(1-7) treatment, wounds were fully healed and fully re-epithelialized (Figures 1 and 2).
For wounds to heal they must start to receive nutrients and growth factors from the blood stream which requires new vascularization of the wound site. Revascularization is essential for wound repair and NorLeu3-A(1-7) has demonstrated accelerated neovascularization at the wound site [25,26]. Thus, NorLeu3-A(1-7) accelerated wound repair through enhanced angiogenesis as well.
The cumulative results of these extensive in vivo wound healing studies indicate that NorLeu3-A(1-7) is highly effective in dermal repair. We found it to be far superior to Regranex as well as to AII and to A(1-7) in animal studies. In every model system we tested NorLeu3-A(1-7) was effective in wound repair.
6. CHEMISTRY
NorLeu3-A(1-7) is a heptapeptide that is a non-hypertensive analogue of the naturally occurring heptapeptide Angiotensin 1-7 (molecular mass 913.1 Da), with the formula H2N-Asp-Arg-NorLeu-Tyr-Ile-His-Pro-OH. NorLeu3-A(1-7) is manufactured under GMP conditions by Bachem (Torrance, CA). It is soluble in water, alcohol, propylene glycol, and organic solvents, and is hydrolyzed by strong acids and by bases above pH 9.5. It is soluble in aqueous solutions at pH 5-8.
6.1 Formulation in Development
Formulation work was conducted to optimize the delivery of NorLeu3-A(1-7) in a biocompatible vehicle with preservatives. Release rates, as a measure of potential bioavailability, have undergone initial assessment in possible vehicles. The release rates from these vehicles were significantly different. The release characteristics for HEC were optimal for this peptide. Biocompatibility studies showed no inflammatory reaction with the placement of HEC in a wound, while inflammation was observed with other cellulose-based viscoelastic gel formulations. Efficacy studies confirmed the suitability of HEC as a vehicle for topical formulations in our full thickness excision model in diabetic mice.
In consideration of the clinical use of this product, it was determined that a multi-use, ready-to-use, sterile tube would be most acceptable to the patient with a diabetic ulcer. To meet the requirements for a multi-use tube we needed to add preservatives to the formulation. A number of preservative combinations were tested for both preservative effect and compatibility with the optimal healing in response to NorLeu3-A(1-7) in a diabetic full thickness excision model. The optimal formulation was chosen for clinical development.
7. CLINICAL EFFICACY OF NorLeu3-A(1-7)
7.1 Phase I
A Phase l safety study was conducted in normal volunteers using 0.3% NorLeu3-A(1-7) in hydroxyethyl cellulose gel with paraben preservatives. No adverse effects associated with drug use were observed
7.2 Phase II
A Phase II clinical study evaluated the safety and preliminary efficacy of NorLeu3-A(1-7) (DSC127) in subjects with diabetic foot ulcers. In a double-blind, placebo-controlled, multi-center clinical trial, 77 patients were randomized to receive one of two dose strengths of DSC127 (0.03% and 0.01%) or vehicle placebo control [27]. After 14 days of best standard-of-care (including off-loading) to evaluate ulcer healing and ensure the wounds were non-healing, subjects randomized into the study received 4 weeks of once daily active treatment followed by 8 weeks of observation and assessment, and a further 12 weeks of follow up to assess durability of healed ulcers. In the Intent-to-Treat (“ITT”) population, the preliminary results show that 54% of the diabetic wounds treated with the 0.03% dose (high dose) of DSC127 achieved 100% closure in 12 weeks or less, compared with 33% of subjects receiving placebo control, and 30% of subjects receiving the 0.01% dose (low dose) of DSC127. By 24 weeks 73% of wounds treated with the 0.03% dose (high dose) of DSC127 achieved 100% closure compared with only 46% in the placebo (p=0.058) and 48% in the 0.01% dose respectively. Based on odds ratio analysis, subjects treated with DSC127 0.03% were 2.3 times more likely to have their wounds heal completely by 12 weeks and 3.1 times more likely by 24 weeks compared with subjects treated with placebo/standard of care. In the Per-Protocol (“PP”) population, the preliminary results show that 65% of the diabetic wounds treated with the 0.03% dose of DSC127 achieved 100% closure in 12 weeks or less, compared with 38% of subjects receiving placebo control, and 28% of subjects receiving the 0.01% dose of DSC127 (Figure 1). By 24 weeks 85% of wounds treated with the 0.03% dose (high dose) of DSC127 achieved 100% closure compared with only 52% in the placebo (p=0.032) and 50% in the 0.01% dose respectively. Based on odds ratio analysis, subjects treated with DSC127 0.03% were 3.0 times more likely to have their wounds heal completely by week 12 and 5 times more likely by week 24 compared with subjects treated with placebo/standard of care. Ulcers in the 0.03% dose group healed on average within 10 weeks in the ITT population as compared with ulcers treated with placebo which took on average 23 weeks to heal, and in the PP population the 0.03% group healed in 8.5 weeks as compared to the placebo group at 22 weeks. The drug was well tolerated with no differences in the safety parameters assessed across the three groups, and there were no significant adverse events associated with DSC127 treatment.
8. SAFETY AND TOLERABILITY
8.1 Preclinical Safety and Clinical Experience with NorLeu3-A(1-7)
A series of pharmacology and toxicology studies were performed with NorLeu3-A(1-7) and its formulated drug product DSC127. These studies were performed to demonstrate safety in support of initial human studies with DSC127 used as a topical gel. Cardiovascular safety pharmacology studies with NorLeu3-A(1-7) in conscious dogs indicated no risk of adverse hypertensive properties following subcutaneous administration. Four systemic toxicology, two dermatoxicology, four teratology, three in vitro mutagenicity and one in vivo genotoxicity studies were also conducted.
The daily parenteral administration of NorLeu3-A(1-7) to rats and dogs did not affect clinical parameters, body weights, or food intake. No changes in hematology parameters, serum chemistry values, or urinalysis were noted. In addition, there were no treatment-related histopathologic changes in any of the tissues or organs examined. The maximum no-observed-adverse-effect level in rats and dogs following repeat-dosing was 1000 μg/kg/d or higher. NorLeu3-A(1-7) was not genotoxic in in vitro tests evaluating point mutation in bacteria and chromosomal aberrations in Chinese hamster ovary cells. NorLeu3-A(1-7) was not genotoxic in a bone marrow micronucleus assay performed in mice. No cardiovascular changes were seen in a conscious dog cardiovascular safety pharmacology study (up to 10 mg/kg/day) of NorLeu3-A(1-7). Due to the absence of the 8th amino acid of AII, A(1-7) and analogues do not elevate blood pressure. Dermatotoxicity studies in rabbits and sensitization studies in guinea pigs showed no effects of NorLeu3-A(1-7). A Segment ll reproductive toxicology (teratology) study was conducted for NorLeu3-A(1-7) in rats and rabbits. No effects were seen in fetal development and no teratological effects were seen with doses up to 1 mg/kg/day. A favorable safety profile has been demonstrated with NorLeu3-A(1-7) in preclinical toxicology studies.
9. CONCLUSION
NorLeu3-A(1-7) is an analogue of the active member of the RAS, A(1-7). The peptide accelerates and normalizes the healing of dermal injuries in multiple animal models. In initial clinical studies, NorLeu3-A(1-7) increased full closure of diabetic foot ulcers at 12 weeks. Additional clinical studies in other indications are now ongoing.
10. Expert Opinion
NorLeu3-A(1-7) is a novel therapy for the closure of chronic, non-healing diabetic foot ulcers. The efficacy of this product was seen as early as 4 weeks with a reduction in wound volume. This observation is consistent with that observed in preclinical studies where granulation tissue was rapidly deposited (Figure 2). By week 12, there was an absolute increase in full wound closure in the Phase II trial of 27% (Figure 1). The benefit continued over the durability phase of the trial where at 24 weeks after initiation of treatment (20 weeks since the last exposure), the absolute increase in complete wound closure was 33% even in the face of less rigorous clinical oversight and off-loading requirements. During this same period some Placebo and 0.01% wounds began to expand, but no 0.03% wounds expanded, suggesting a more enduring effect on tissue repair or the cells engaged in it than has historically been reported. This therapy has the potential to be an improvement over the current active therapy available to accelerate healing of diabetic foot ulcers, Regranex., in a number of aspects. Regranex is a topical treatment for the acceleration of healing diabetic foot ulcers. The active pharmaceutical ingredient in the currently approved therapy is made by recombinant DNA technology whereas NorLeu3-A(1-7) is produced by peptide synthesis. The vehicle, at least in our studies, for this new therapy has increased biocompatibility. Further, there is clear improvement in efficacy in animal studies comparing Regranex with NorLeu3-A(1-7). Finally, NorLeu3-A(1-7) accelerates healing through a number of mechanisms rather than simply through an increase in granulation tissue (Figure 2). For example, the increase in genes related to energy metabolism suggests a fundamental mechanism of the drug that may cross all aspects of tissue repair and regeneration, generating a variety of hypotheses worthy of future testing. In contrast, Regranex was shown to increase the formation of granulation tissue, but to have little effect on keratinocyte growth in preclinical studies. As NorLeu3-A(1-7) not only accelerates the formation of granulation tissue through accelerated blood vessel formation and collagen deposition, but increases the proliferation of both progenitor cells at the base of the hair follicle and in the basal keratinocyte layer in vivo as well as keratinocytes in vitro. These preclinical studies are consistent with the clinical observations of an initial reduction of wound volume followed by a reduction in wound surface area. The formulation in development was further shown to be safe in a Phase I study and to significantly increase the proportion of chronic, non-healing wounds that fully healed at 24 weeks in a Phase II study. If Phase III studies confirm the outcomes in the available data from Phase II studies, there is a 3 fold increase in the likelihood of ulcer closure in 12 weeks. In 5 years, it is anticipated that this product will have completed FDA review for marketing.
Based on the homology of RAS expression in dermal tissue of all mammalian systems studied to date including humans, topical administration of NorLeu3-A(1-7) should fulfill a wide variety of unmet clinical needs. Clinical studies have shown the pharmaceutical formulation of NorLeu3-A(1-7) delivered in an easily administered, multiple use tube to be safe. Additional clinical studies in other indications are now ongoing.
11. DRUG SUMMARY BOX
| Drug Name: NorLeu3-Angiotensin (1-7) |
| Phase: Phase II Clinical Trial |
| Indication: Acceleration of closure of diabetic foot ulcer |
| Pharmacological Description/Mechanism of Action: The mechanisms of action include induction of progenitor proliferation and accelerated vascularization, collagen deposition and re-epithelialization. |
| Route of administration: Topical |
| Chemical Structure: H2N-Asp-Arg-NorLeu-Tyr-Ile-His-Pro-OH |
| Pivotal trial(s): To be initiated |
Footnotes
Declaration of interest:
K Rodgers is an inventor on patents covering this peptide and may have royalties associated. She was the principal investigator on the SBIR grant that funded much of the work.
S Verco was the project manager for a clinical trial for US Biotest
G diZerega is an inventor on patents covering this peptide and may have royalties associated. He is the owner of the company that received the SBIR grant that funded much of the work.
Bibliography
- 1.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet derived growth factor-BB (becaplermin) in patients with chronic neuropathic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21(5):822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 2.Mrug M, Stopka T, Julian BA, et al. Angiotensin II stimulates proliferation of normal early erythroid progenitors. J Clin Invest. 1997;100:2310–14. doi: 10.1172/JCI119769. PMCID: PMC508427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodgers KE, Abiko M, Girgis W, et al. Acceleration of dermal tissue repair by angiotensin II. Wound Repair & Regeneration. 1997;5:175–83. doi: 10.1046/j.1524-475X.1997.50210.x. [DOI] [PubMed] [Google Scholar]
- 4.Rodgers KE, Xiong S, Steer R, diZerega GS. Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem Cells. 2000;18:287–94. doi: 10.1634/stemcells.18-4-287. [DOI] [PubMed] [Google Scholar]
- 5.Rodgers KE, Xiong S, diZerega GS. Effect of angiotensin II and angiotensin (1-7) on hematopoietic recovery after intravenous chemotherapy. Cancer Chemother Pharmacol. 2003;51:97–106. doi: 10.1007/s00280-002-0509-4. [DOI] [PubMed] [Google Scholar]
- 6.Ellefson DE, diZerega GS, Espinoza T, et al. Synergistic effects of co-administration of angiotensin 1-7 and Neupogen on hematopoietic recovery in mice. Cancer Chemo Pharmacol. 2004;53:15–24. doi: 10.1007/s00280-003-0710-0. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers KE, Ellefson DE, Espinoza T, et al. Fragments of Nle3-Angiotensin(1-7) accelerate healing in dermal models. J Peptide Res. 2005;66(Suppl 1):41–47. [Google Scholar]
- 8.Steckelings UM, Wollschläger T, Peters J, et al. Human skin: source of and target organ for angiotensin II. Exp Dermatol. 2004;13(3):148–54. doi: 10.1111/j.0906-6705.2004.0139.x. [DOI] [PubMed] [Google Scholar]
- 9.Steckelings UM, Henz BM, Wiehstutz S, et al. Differential expression of angiotensin receptors in human cutaneous wound. Br J Dermatol. 2005;153(5):887–93. doi: 10.1111/j.1365-2133.2005.06806.x. *Describes the Renin Angiotensin System in the Human Skin and Its Response to Injury
- 10.Abiko M, Rodgers KE, Campeau JD, et al. Alterations In Angiotensin II Receptor Levels In Full Thickness Excisional Wounds In Rat Skin. Wound Repair and Regeneration. 1996;4(3):363–367. doi: 10.1046/j.1524-475X.1996.40313.x. [DOI] [PubMed] [Google Scholar]
- 11.Abiko M, Rodgers KE, Campeau JD, et al. Alterations In Angiotensin II Receptor Levels In Sutured Wounds In Rat Skin. J Invest Surg. 1996;9:447–453. doi: 10.3109/08941939609025862. [DOI] [PubMed] [Google Scholar]
- 12.Kimura B, Sumners C, Phillips MI. Changes in skin Angiotensin II receptors in rats during wound healing. BBRC. 1992;187:1083–90. doi: 10.1016/0006-291x(92)91308-d. [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan M, Saavedra JM. Expression of Angiotensin II AT2 receptors in rat skin during experimental wound healing. Peptides. 1992;13:783–36. doi: 10.1016/0196-9781(92)90187-8. PMID: 1437716. [DOI] [PubMed] [Google Scholar]
- 14.Kurosaka M, Suzuki T, Hosono K, et al. Reduced angiogenesis and delay in wound healing in angiotensin II type 1a receptor-deficient mice. Biomed. Pharmacother. 2009;63(9):627–34. doi: 10.1016/j.biopha.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhang GY, Li X, Yi CG, et al. Angiotensin II activated connective tissue growth factor and induces extracellular matrix changes involving Smad/activation and p38 mitogen-activated protein kinase signaling pathways in human dermal fibroblasts. Exp. Dermatol. 2009;18(11):947–53. doi: 10.1111/j.1600-0625.2009.00880.x. [DOI] [PubMed] [Google Scholar]
- 16.Tang HT, Cheng DC, Jia YT, et al. Angiotensin II induces type I collagen gene expression in human dermal fibroblasts through an AP-1/TGF-beta 1-dependent pathway. Biochem. Biophys. Res. Commun. 2009;385(3):418–423. doi: 10.1016/j.bbrc.2009.05.081. [DOI] [PubMed] [Google Scholar]
- 17.Wu HF, Liu HW, Cheng B, et al. The change in angiotensin II production and its receptor expression during wound healing: possible role of angiotensin II in wound healing. Zhonghua Zheng Xing Wai Ke Za Shi. 2011;27(2):124–8. [PubMed] [Google Scholar]
- 18.Jadhav SS, Sharma N, Espinoza T, et al. Effects of combined thermal and radiation injury on the renin angiotensin system. Wound Repair and Regeneration. doi: 10.1111/j.1524-475X.2012.00867.x. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodgers KE, Xiong S, Felix JC, et al. Development of angiotensin (1-7) as an agent to accelerate dermal regeneration. Wound Repair and Regeneration. 2001;9:241–250. doi: 10.1046/j.1524-475x.2001.00238.x. [DOI] [PubMed] [Google Scholar]
- 20.Jokubaitis VJ, Sinka L, Driessen R, et al. Angiotensin-converting enzyme (CD143) marks hematopoietic stem cells in human embryonic, fetal and adult hematopoietic tissues. Blood. 2008;111(8):4055–63. doi: 10.1182/blood-2007-05-091710. [DOI] [PubMed] [Google Scholar]
- 21.Savary K, Michaud A, Favier J, et al. Role of the renin-angiotensin system in primitive erythropoiesis in the chick embryo. Blood. 2008;105(1):103–10. doi: 10.1182/blood-2004-04-1570. [DOI] [PubMed] [Google Scholar]
- 22.Parks T, Zambidis ET. A role for the renin-angiotensin system in hematopoiesis. Hamaetologica. 2009;94(6):745–7. doi: 10.3324/haematol.2009.006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zambidis ET, Park TS, Yu W, et al. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112:3601–14. doi: 10.1182/blood-2008-03-144766. PMCID: PMC2572789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lui HW, Cheng B, Li, et al. Characterization of angiotensin-converting enzyme expression during epidermis morphogenesis in humans: a potential marker for epidermal stem cells. Br. J. Dermatol. 2009;160(2):250–8. doi: 10.1111/j.1365-2133.2008.08970.x. [DOI] [PubMed] [Google Scholar]
- 25.Rodgers KE, Roda N, Felix JC, et al. Histological evaluation of the effects of angiotensin peptides on wound repair in diabetic mice. Exp Dermatol. 2003;12:784–90. doi: 10.1111/j.0906-6705.2003.00087.x. **Describes ability of NorLeu3-A(1-7) to heal through changes in granulation tissue, collagen deposition, and epithelialization.
- 26.Rodgers KE, Espinoza T, Felix J, et al. Acceleration of healing, reduction of fibrotic scar and normalization of tissue architecture by angiotensin analog, NorLeu3-A(1-7) Plast Recon Surg. 2003;111:1195–1206. doi: 10.1097/01.PRS.0000047403.23105.66. **Describes ability of NorLeu3-A(1-7) to heal normal and diabetic wounds and to reduce scarring.
- 27.Balingat P, Reyzelman A, Armstrong D, et al. Randomized Trial of a Novel Angiotensin Analogue on Diabetic Foot Ulcer Healing. Foot Global Conference; Los Angeles, California. 23-26 March 2011. [Google Scholar]