Abstract
OBJECTIVE
To estimate whether prenatal exposure to acetaminophen is associated with risk of diagnosed asthma and asthma symptoms in children.
METHODS
The authors prospectively followed 1,505 pregnant women and their children until 6 years (±3 months) of life. Acetaminophen use in the first and third trimesters of pregnancy was assessed before 24 weeks of gestation and within 1 month of delivery, and asthma in children was assessed when the child was 6 years old. Adjusted odds ratios (aORs) were derived from logistic regression models controlling for potential confounders.
RESULTS
Acetaminophen was used by 69% of women during pregnancy. Use of acetaminophen did not significantly increase the risk of asthma (aOR 0.76, 95% confidence interval [CI] 0.53–1). Acetaminophen use during both the first and the third trimester was associated with a significantly reduced risk of asthma (aOR 0.59, 95% CI 0.36–0.98). There was no evidence of a dose response, and consumption greater than 10,400 mg (32 tablets) a month did not increase risk (aOR 0.99, 95% CI 0.19–5.30).
CONCLUSION
Our results suggest that acetaminophen use during pregnancy does not increase risk of asthma in children.
Acetaminophen is a widely used analgesic drug found in more than 100 products and having no known teratogenic effects.1,2 Thus, it is the most commonly used analgesic by pregnant women,3,4 in preference to other nonsteroidal antiinflammatory drugs, such as acetylsalicylic acid, ibuprofen, and naproxen, which have been associated with complications during pregnancy and infancy.5,6
Recently, several investigations reported that acetaminophen use in pregnancy may increase the risk of wheezing and asthma in the first year of life and in later childhood.7–12 Other studies reported associations between asthma and acetaminophen exposure during infancy,11,13,14 childhood,13 and adult life.15,16 A recent review by Allmers et al17 suggests that acetaminophen increases the risk of asthma and thus should be avoided. However, the relationship between acetaminophen use in pregnancy and asthma in children remains unclear and warrants further investigation.
To identify risk factors for childhood asthma, we analyzed data from a prospective cohort study of women followed through their pregnancies and subsequent follow-up of their children at 6 years of age to investigate the etiology of childhood asthma. With detailed classification and comprehensive reporting of acetaminophen, we aimed to estimate the role of prenatal exposure to acetaminophen in the development of asthma in children.
MATERIALS AND METHODS
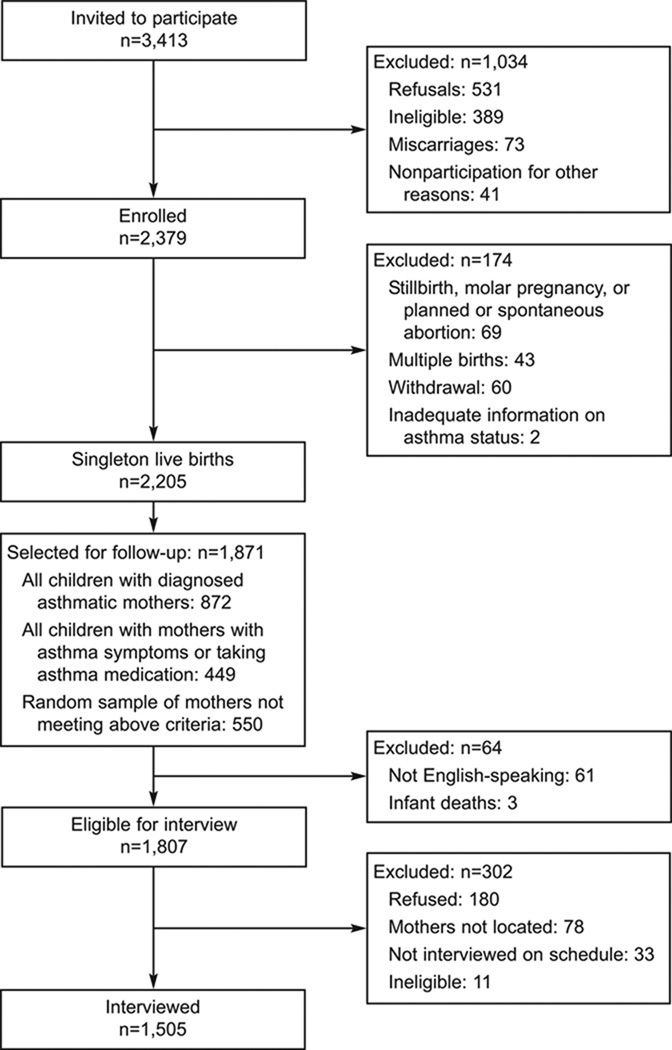
Between April 1997 and June 2000, a total of 3,413 women were invited to participate in a study of asthma in pregnancy (Fig. 1). Pregnant women with a history of physician-diagnosed asthma (n=1,343) and a simple random sample of pregnant women without asthma (n=2,070) were recruited while receiving prenatal care from 56 private obstetric practices and 15 clinics at six hospitals in southern New England (Bridgeport, Danbury, Hartford, and New Haven, Connecticut, and Springfield, Massachusetts). After accounting for refusals (n=531), ineligibility at the home interview, usually because they were more than 24 weeks pregnant (n=389), miscarriages (n=73), and nonparticipation for other reasons (n=41), 2,379 women (69.7%) were enrolled in the study. After enrollment, 174 women were excluded from analysis because of a stillborn fetus, molar pregnancy, a planned or spontaneous abortion (n=69), multiple birth (n=43), loss to follow-up or withdrawal (n=60), or inadequate information on asthma status (n=2). Of the singleton live births eligible for study, 1,871 children, including those with mothers with diagnosed asthma (n=872), mothers with asthma symptoms or taking asthma medication (n=449), and a random sample of mothers without asthma (n=550), were selected for subsequent follow-up. After excluding non-English speakers (n=61) and neonatal deaths (n=3), 1,807 women were eligible for the interview at 6-year follow-up. Of the eligible women, 302 women were excluded because of refusal, inability to locate, and missed interviews. Finally, 1,505 women were interviewed and included in the analysis.
Fig. 1.
Flowchart of patients showing inclusion and exclusions applied to the total cohort population for the acetaminophen and asthma analysis.
Kang. Prenatal Exposure to Acetaminophen and Asthma. Obstet Gynecol 2009
The Human Investigation Committee of Yale University (New Haven, Connecticut) approved this study, and all respondents provided informed consent before participation.
At enrollment, women were interviewed at home before 24 weeks of gestation. The standardized questionnaire administered included indepth questions about demographic factors, pregnancy history, health care use, smoking, asthma history, activity limitations due to asthma, household exposures, and other chronic conditions during the year before pregnancy and the period since conception. A postpartum interview was conducted in the hospital (n=1,344) or by telephone within 1 month of delivery (n=544). Data on pregnancy outcomes were abstracted from hospital delivery records. Information related to infancy and early childhood was collected during the structured interview (n= 1,505) when the child was 6 years (±3 months) of age.
Information on acetaminophen use was obtained before 24 weeks of gestation from the following questions in the prenatal questionnaire: “Have you taken any medications for asthma, allergies, sinus, respiratory, or other breathing problems? Please include over-the-counter medication as well as prescription medications” and “Have you taken any medications or drugs (including over-the-counter and prescription drugs) other than those for respiratory conditions during the first 3 months of the pregnancy?” If a respondent answered affirmatively, a follow-up question resulted: “What is the name of the drug or medication that you used? Please include strength, form, and whether it was prescription or over-the-counter.” To measure frequency and dose of the medication, respondents were asked, “How often did you use this medication in the first, second, and third months of the pregnancy?” with responses in the following categories: none, 1–7 days per month, 8–14 days per month, more than 14 days (but not every day) per month, and every day. They were also asked, “How many tablets or doses of the medication did you usually take per day during this month?” The same set of questions was repeated during the post-partum interview to ascertain acetaminophen use during the last 3 months of the pregnancy.
All reported medications taken by the women were investigated to determine the active ingredients and, particularly, the total milligrams of acetaminophen contained, if any. This information was obtained from drug labels, the manufacturers, or other online drug indexes. An imputation method was used to estimate the total milligrams of acetaminophen in the drug where this information was not easily accessible or clearly evident (n=61). Uncertain amounts of acetaminophen in such drugs were substituted using total milligrams of acetaminophen of similar medications in the data set. Ever use of any medication that contained acetaminophen was classified as use of acetaminophen during the first and third trimester of pregnancy. Information on second-trimester exposure was not ascertained.
To explore the cumulative dose response, average monthly acetaminophen consumption during pregnancy was calculated. The dose was divided into six levels: 0, 1,300 or less, 1,301–2,600, 2,601–5,200, 5,201–10,400, and more than 10,400 mg per month, where 1,300 mg is equivalent to four tablets of regular-strength acetaminophen at 325 mg each. Additional exposure evaluations, based on the average consumption by the participant per day of use during the first and third trimesters, were performed. The dose was divided into four levels: 0, 650 or less, 651–1,300, and more than 1,300 mg per day of use. The categorical response for days per month (0, 1–7 days per month, 8–14 days per month, more than 14 days [but not every day] per month, and every day) were reassigned to their median values (0, 4, 11, 21, and 30 days per month) for the calculation of the dose and level for each participant. For women who used more than one medication containing acetaminophen in 1 month, it was assumed that the medications were taken on different days of the same month. Additional exposure evaluations, based on the assumption that multiple acetaminophen medications were taken on the same days of the month, were performed as a sensitivity analysis. The calculations and formulas are available on request.
When the child was 6 years (±3 months) old, the mothers were asked, “Has the child ever been diagnosed by a doctor or health professional as having asthma?” Mothers were also asked, “Has your child ever had wheezing or whistling in the chest at any time in the past?” and, if yes, “Has your child had wheezing or whistling in the chest in the last 12 months?” The primary outcome in this analysis was physician-diagnosed asthma ever with history of wheezing at the sixth year of age. Positive responses to both questions were considered a positive asthma outcome. In addition, phenotypes for persistent wheezing, ever wheezing, diagnosed bronchitis, sneezing/runny nose ever, and allergy were examined to allow more direct comparison with other reports in the literature.
Information on a large number of potential con-founding variables was collected from the interviews conducted during early pregnancy, postpartum, and at 6-year follow-up. Confounders measured during the first and second interviews were mother’s demo-graphics (age, ethnicity, marital status, and education), mother’s smoking and exposure to passive smoking during pregnancy, mother’s diagnosed asthma and other health conditions (asthma symptoms and high blood pressure during pregnancy, and diagnosed/treated allergies), offspring’s gestational age at birth and low birth weight, and preterm labor and delivery. Confounders measured during the interview at 6-year follow up were father’s ethnicity and education, history of father’s asthma and other health conditions (wheezing, allergies, and eczema), mother’s diagnosed or treated eczema, yearly household income, household exposures (mold/mildew growth at home and water leaks/damage at home during child’s first year, pets inside home and cockroaches observed in home during child’s first and sixth years), use of various home appliances (use of wood-burning stove, wood-burning fireplace, unvented gas fire-place/space heater, portable kerosene heater, gas stove, continuously burning pilot light, and air conditioner during sixth year), child’s ethnicity, number of biologic siblings, attendance at a program before elementary school, child’s asthma symptoms (wheezing and persistent cough in the first year, cough, shortness of breath, and chest tightness in the sixth year), use of emergency room and overnight stay at the hospital for asthma, allergy, or respiratory illnesses, use of neonatal intensive care unit and pediatric intensive care unit, use of intubation/ventilation in neonatal intensive care unit and pediatric intensive care unit, breastfeeding, child’s exposure to tobacco smoke for 2 hours or more ever, mother’s use of antibiotics while breastfeeding, child’s use of antibiotics and allergy medications, and child’s infections and respiratory illnesses (allergies, sneezing/ runny nose, hay fever, itchy rash, eczema, bronchitis, bronchiolitis, pneumonia, croup, ear infection, strep throat, sinus infection, respiratory syncytial virus, and tonsillitis).
Analysis of the effect of prenatal exposure to acetaminophen was conducted with logistic regression models using SAS 9.1 Proc Logistic (SAS Institute, Inc., Cary, NC). Odds ratios (ORs) were estimated for asthma, associated with different categories of acetaminophen use, compared with never use. For each combination of exposure and outcome, the unadjusted OR and adjusted OR were obtained, adjusting for potential confounders from pregnancy, the perinatal period, and early childhood. Covariates were initially selected for inclusion in the model by identifying those significantly associated with both the exposure and the outcome at P≤.10. Final adjusted models were constructed using a backward elimination statistical procedure that includes only those covariates resulting in a change of 10% or greater in the parameter estimate of acetaminophen exposure. The study was able to detect at least a 52% increased risk of asthma with 90% power.
RESULTS
Table 1 describes the study population at baseline and during the pregnancy. Twenty-one percent of the women were younger than 24 years of age, whereas 13% were 36 years of age or older. The majority of women in this study were white (74%), whereas 10% were African American and 13% were Hispanic. Approximately 26% were not married, and 89% of participants were at least high school graduates. Smokers during pregnancy constituted 17% of the study sample, and 27% and 47% of women were exposed to secondhand smoke in the first and third trimesters of pregnancy, respectively. By design of the study sample, nearly half of the study women (45%) reported physician-diagnosed asthma, and a greater proportion (64%) of the women reported any maternal asthma symptoms during the pregnancy. Other illnesses were also reported: 13% of women had high blood pressure during pregnancy, and 62% reported allergies diagnosed or treated by a physician.
Table 1.
| Characteristic | n‡ | Percent of Total N |
With Acetaminophen Exposure (%) |
OR | 95% CI |
|---|---|---|---|---|---|
| Total | 1,505 | 68.8 | |||
| Mother’s age at initial interview (y) | |||||
| 24 or younger | 296 | 20.9 | 65.5 | Ref | |
| 25–29 | 374 | 26.5 | 74.1 | 1.50 | 1.08–2.10 |
| 30–35 | 561 | 39.7 | 66.7 | 1.05 | 0.78–1.42 |
| 36 or older | 183 | 12.9 | 70.0 | 1.22 | 0.82–1.82 |
| Mother’s ethnicity | |||||
| White, non-Hispanic | 1,041 | 73.7 | 71.0 | Ref | |
| African American | 147 | 10.4 | 60.5 | 0.63 | 0.44–0.90 |
| Hispanic | 187 | 13.2 | 64.7 | 0.75 | 0.54–1.04 |
| Asian, other | 38 | 2.7 | 60.5 | 0.63 | 0.32–1.22 |
| Marital status | |||||
| Not married | 359 | 25.7 | 63.2 | Ref | |
| Married | 1,055 | 74.6 | 70.7 | 1.40 | 1.09–1.81 |
| Mother’s education | |||||
| Less than 12 y | 155 | 11.0 | 63.2 | Ref | |
| High school graduate | 218 | 15.4 | 72.0 | 1.50 | 0.96–2.33 |
| At least some college | 1,039 | 73.6 | 68.9 | 1.29 | 0.91–1.83 |
| Smoking during pregnancy | |||||
| Never | 1,171 | 83.0 | 67.8 | Ref | |
| First trimester only | 147 | 10.4 | 75.5 | 1.46 | 0.99–2.17 |
| First and third trimesters | 93 | 6.6 | 69.9 | 1.10 | 0.70–1.75 |
| Mother exposed to secondhand smoke during first trimester | |||||
| No | 1,031 | 72.9 | 67.3 | Ref | |
| Yes | 383 | 27.1 | 72.9 | 1.30 | 1.00–1.69 |
| Mother exposed to secondhand smoke during third trimester | |||||
| No | 699 | 53.2 | 64.8 | Ref | |
| Yes | 614 | 46.8 | 69.1 | 1.21 | 0.96–1.53 |
| Mother diagnosed with asthma | |||||
| No | 783 | 55.4 | 64.6 | Ref | |
| Yes | 631 | 44.6 | 74.0 | 1.56 | 1.24–1.96 |
| Any maternal asthma symptoms during pregnancy | |||||
| No | 506 | 35.8 | 58.5 | Ref | |
| Yes | 907 | 64.2 | 74.5 | 2.08 | 1.65–2.62 |
| Mother had persistent cough during first trimester | |||||
| No | 1,175 | 83.1 | 66.4 | Ref | |
| Yes | 239 | 16.9 | 80.8 | 2.13 | 1.51–3.00 |
| Mother had shortness of breath during first trimester | |||||
| No | 799 | 56.6 | 63.3 | Ref | |
| Yes | 614 | 43.5 | 75.9 | 1.82 | 1.44–2.30 |
| Mother had chest tightness during first trimester | |||||
| No | 1,090 | 77.2 | 66.3 | Ref | |
| Yes | 322 | 22.8 | 77.0 | 1.70 | 1.28–2.27 |
| Mother wheezing during first trimester | |||||
| No | 1,070 | 75.7 | 66.1 | Ref | |
| Yes | 344 | 24.3 | 77.3 | 1.75 | 1.32–2.32 |
| Mother had persistent cough during third trimester | |||||
| No | 1,105 | 83.7 | 64.3 | Ref | |
| Yes | 215 | 16.3 | 78.1 | 1.98 | 1.40–2.80 |
| Mother had shortness of breath during third trimester | |||||
| No | 777 | 58.8 | 63.6 | Ref | |
| Yes | 544 | 41.2 | 71.0 | 1.40 | 1.11–1.77 |
| Mother had tightness of chest during third trimester | |||||
| No | 1,092 | 82.7 | 64.8 | Ref | |
| Yes | 228 | 17.3 | 75.0 | 1.63 | 1.18–2.25 |
| Mother had wheezing during third trimester | |||||
| No | 1,010 | 76.5 | 64.2 | Ref | |
| Yes | 311 | 23.5 | 74.6 | 1.64 | 1.23–2.18 |
| Mother has high blood pressure | |||||
| No | 1,153 | 87.3 | 65.9 | Ref | |
| Yes | 168 | 12.7 | 72.0 | 1.33 | 0.93–1.91 |
| Mother had diagnosed/treated allergies | |||||
| No | 546 | 38.7 | 62.8 | Ref | |
| Yes | 866 | 61.6 | 72.6 | 1.57 | 1.25–1.98 |
| Gestational age at birth (wk)§ | |||||
| 37 or greater | 1,330 | 94.1 | 68.4 | Ref | |
| Less than 37 | 83 | 5.9 | 75.9 | 1.46 | 0.87–2.44 |
| Low birth weight (less than 2,500 g) | |||||
| No | 1,351 | 95.6 | 68.9 | Ref | |
| Yes | 62 | 4.4 | 66.1 | 0.88 | 0.51–1.51 |
| Preterm (less than 37 wk) labor and delivery‖ | |||||
| Neither | 1,141 | 86.4 | 65.8 | Ref | |
| Preterm labor only | 102 | 7.7 | 70.6 | 1.25 | 0.80–1.94 |
| Preterm delivery only | 46 | 3.5 | 80.4 | 2.14 | 1.02–4.47 |
| Both | 32 | 2.4 | 65.6 | 0.99 | 0.47–2.08 |
OR, odds ratio; CI, confidence interval; Ref, referent.
Ever use of acetaminophen during first and third trimesters of pregnancy.
Additional variables were evaluated for confounding, including yearly household income, household exposures (mold/mildew growth at home and water leaks/damage at home during child’s first year, pets inside home and cockroaches observed in home during child’s first and sixth years), use of various home appliances (use of wood-burning stove, wood-burning fireplace, unvented gas fireplace/space heater, portable kerosene heater, gas stove, continuously burning pilot light, and air conditioner during sixth year), mother’s diagnosed or treated eczema, father’s ethnicity and education, history of father’s asthma and other health conditions (wheezing, allergies, and eczema), child’s ethnicity, number of biologic siblings, attendance at a program before elementary school, child’s asthma symptoms (wheezing and persistent cough in the first year, cough, shortness of breath, and chest tightness in the sixth year), use of emergency department and overnight stay at the hospital for asthma, allergy, or respiratory illnesses, use of neonatal intensive care unit and pediatric intensive care unit, use of intubation/ventilation in neonatal intensive care unit and pediatric intensive care unit, breastfeeding, mother’s use of antibiotics while breastfeeding, child’s use of antibiotics and allergy medications, child’s exposure to tobacco smoke for 2 hours or more ever, and child’s infections and respiratory illnesses (allergies, sneezing/runny nose, hay fever, itchy rash, eczema, bronchitis, bronchiolitis, pneumonia, croup, ear infection, strep throat, sinus infection, respiratory syncytial virus, tonsillitis).
Numbers may not sum to total due to missing data.
n=1,413.
n=1,321.
Overall, 68.8% of women used acetaminophen at least once during the first (53.4%) or third (45.5%) trimester of their pregnancy. Of the total sample, 32.3% of women reported use of acetaminophen during both the first and the third trimester. Among users, the mean reported monthly acetaminophen intake was 2,677 (standard deviation 4,650) mg during the first and third trimesters, with a median intake of 1,371 mg. Similarly, the mean reported acetaminophen intake on the day of use was 793 (standard deviation 415) mg during the first and third trimesters, with a median intake of 650 mg.
Several characteristics were associated with use of acetaminophen during pregnancy in unadjusted analyses (Table 1). Reported acetaminophen use was less common in African-American women compared with white women, and use was more common in women 25–29 years of age, married women, and women exposed to secondhand smoke during the first trimester. Women who were diagnosed with asthma, who had asthma symptoms during their pregnancy, or who had diagnosed or treated allergies were also more likely to use acetaminophen. Women who had preterm delivery, but not preterm labor, were twice as likely to use acetaminophen as women with neither preterm labor nor preterm delivery. Low birth weight did not seem to be associated with acetaminophen use in pregnancy.
Overall, 15% of the children in this study cohort were asthmatic, defined as physician-diagnosed asthma ever and wheezing at the sixth year of age. The risk of childhood asthma decreased with increasing maternal age (Table 2). African-American and Hispanic mothers had an increased risk of asthma in their children. Married and educated mothers had a decreased risk, whereas mothers who smoked during pregnancy or were exposed to secondhand smoke during the first trimester had an increased risk of asthma in children. Mother’s diagnosed asthma, asthma symptoms during pregnancy, and diagnosed or treated allergies increased the risk of asthma in children. Children who were born at less than 37 weeks of gestation had twice the risk of asthma, and those who were born with low birth weight had a threefold risk of asthma. Preterm delivery only did not have an effect, but preterm labor alone and both preterm labor and delivery were associated with an increased risk of asthma compared with neither preterm labor nor preterm delivery.
Table 2.
| Characteristic | n‡ | Percent of Total N |
With Asthma (%) |
OR | 95% CI |
|---|---|---|---|---|---|
| Total | 1,505 | ||||
| Mother’s age at initial interview (y) | |||||
| 24 or younger | 341 | 22.7 | 23.5 | Ref | |
| 25–29 | 394 | 26.3 | 14.2 | 0.54 | 0.37–0.79 |
| 30–35 | 578 | 38.5 | 12.5 | 0.46 | 0.33–0.66 |
| 36 or older | 188 | 12.5 | 8.0 | 0.28 | 0.16–0.51 |
| Mother’s ethnicity | |||||
| White, non-Hispanic | 1,081 | 72.1 | 11.6 | Ref | |
| African American | 166 | 11.1 | 22.9 | 2.27 | 1.51–3.41 |
| Hispanic | 212 | 14.1 | 25.0 | 2.55 | 1.77–3.66 |
| Asian, other | 41 | 2.7 | 17.1 | 1.58 | 0.68–3.63 |
| Marital status | |||||
| Not married | 406 | 27.1 | 24.9 | Ref | |
| Married | 1,094 | 72.9 | 11.1 | 0.38 | 0.28–0.50 |
| Mother’s education | |||||
| Less than 12 y | 174 | 11.6 | 26.4 | Ref | |
| High school graduate | 244 | 16.3 | 17.6 | 0.60 | 0.37–0.95 |
| At least some college | 1,081 | 72.1 | 12.4 | 0.39 | 0.27–0.58 |
| Smoking during pregnancy | |||||
| Never | 1,234 | 82.4 | 13.1 | Ref | |
| First trimester only | 170 | 11.4 | 21.8 | 1.84 | 1.23–2.75 |
| First and third trimesters | 94 | 6.3 | 25.5 | 2.27 | 1.39–3.71 |
| Mother exposed to secondhand smoke during first trimester | |||||
| No | 1,084 | 72.2 | 12.9 | Ref | |
| Yes | 417 | 27.8 | 19.9 | 1.68 | 1.24–2.26 |
| Mother exposed to secondhand smoke during third trimester | |||||
| No | 707 | 53.1 | 13.3 | Ref | |
| Yes | 624 | 46.9 | 14.6 | 1.11 | 0.82–1.52 |
| Mother diagnosed with asthma | |||||
| No | 830 | 55.3 | 9.3 | Ref | |
| Yes | 671 | 44.7 | 21.8 | 2.72 | 2.02–3.66 |
| Any maternal asthma symptoms during pregnancy | |||||
| No | 543 | 36.2 | 10.3 | Ref | |
| Yes | 957 | 63.8 | 17.5 | 1.84 | 1.33–2.54 |
| Mother had persistent cough during first trimester | |||||
| No | 1,242 | 82.7 | 13.9 | Ref | |
| Yes | 259 | 17.3 | 19.7 | 1.53 | 1.08–2.16 |
| Mother had shortness of breath during first trimester | |||||
| No | 844 | 56.3 | 12.0 | Ref | |
| Yes | 655 | 43.7 | 18.6 | 1.68 | 1.27–2.24 |
| Mother had chest tightness during first trimester | |||||
| No | 1,150 | 76.8 | 13.0 | Ref | |
| Yes | 348 | 23.2 | 21.0 | 1.77 | 1.30–2.41 |
| Mother wheezing during first trimester | |||||
| No | 1,129 | 75.2 | 12.8 | Ref | |
| Yes | 372 | 24.8 | 21.2 | 1.84 | 1.36–2.50 |
| Mother had persistent cough during 3rd trimester | |||||
| No | 1,118 | 83.6 | 12.6 | Ref | |
| Yes | 220 | 16.4 | 21.4 | 1.88 | 1.30–2.72 |
| Mother had shortness of breath during third trimester | |||||
| No | 789 | 58.9 | 13.2 | Ref | |
| Yes | 550 | 41.1 | 15.3 | 1.19 | 0.87–1.62 |
| Mother had tightness of chest during third trimester | |||||
| No | 1,108 | 82.8 | 13.0 | Ref | |
| Yes | 230 | 17.2 | 19.1 | 1.58 | 1.09–2.30 |
| Mother had wheezing during third trimester | |||||
| No | 1,026 | 76.6 | 12.7 | Ref | |
| Yes | 313 | 23.4 | 18.5 | 1.57 | 1.12–2.20 |
| Mother has high blood pressure | |||||
| No | 1,167 | 87.2 | 13.4 | Ref | |
| Yes | 172 | 12.9 | 18.6 | 1.48 | 0.97–2.25 |
| Mother had diagnosed/treated allergies | |||||
| No | 963 | 64.2 | 13.1 | Ref | |
| Yes | 536 | 35.8 | 18.1 | 1.47 | 1.10–1.96 |
| Gestational age at birth (wk)§ | |||||
| 37 or greater | 1,409 | 93.9 | 14.3 | Ref | |
| Less than 37 | 91 | 6.1 | 24.2 | 1.92 | 1.16–3.17 |
| Low birth weight (less than 2,500 g) | |||||
| No | 1,435 | 95.7 | 14.1 | Ref | |
| Yes | 64 | 4.3 | 32.8 | 2.98 | 1.73–5.13 |
| Preterm (less than 37 wk) labor and delivery‖ | |||||
| Neither | 1,155 | 86.3 | 12.6 | Ref | |
| Preterm labor only | 104 | 7.8 | 22.1 | 1.96 | 1.20–3.22 |
| Preterm delivery only | 46 | 3.4 | 19.6 | 1.68 | 0.80–3.56 |
| Both | 34 | 2.5 | 29.4 | 2.88 | 1.35–6.14 |
OR, odds ratio; CI, confidence interval; Ref, referent.
Definition of asthma: diagnosis of asthma and wheezing during sixth year (±3 months) of life.
See † footnote in Table 1.
Numbers may not sum to total due to missing data.
n=1,500.
n= 1,339.
The association of acetaminophen use ever during pregnancy with childhood asthma and the effect of potential confounders included in the final model were examined (Table 3). In the unadjusted model, the effect of acetaminophen use was not significant, with an OR of 0.89 (95% confidence interval [CI] 0.66–1.22). After adjustment by logistic regression, use of acetaminophen ever during pregnancy did not significantly decrease the risk of asthma, with an OR of 0.76 (95% CI 0.53–1.10). Nonwhite and non-Asian children were at increased risk of asthma, with His-panic children having the greatest risk. Mother’s history of physician-diagnosed asthma was associated with a twofold increase in the risk of asthma in children. Child’s sneezing/runny nose increased the risk threefold, and itchy rash increased the risk two-fold. Keeping pets inside home at the child’s sixth year of life was associated with a reduced risk of asthma.
Table 3.
Multivariate-Adjusted Model: Association of Ever Use of Acetaminophen During First and Third Trimesters of Pregnancy and Asthma*†
| Adjusted OR |
95% CI | |
|---|---|---|
| Ever use of acetaminophen during first and third trimesters | ||
| No | Ref | |
| Yes | 0.76 | 0.53–1.10 |
| Child’s ethnicity | ||
| White or Asian | Ref | |
| African American | 1.92 | 1.07–3.44 |
| Hispanic | 2.84 | 1.80–4.48 |
| Biracial | 2.32 | 1.31–4.10 |
| Mother had physician-diagnosed asthma | ||
| No | Ref | |
| Yes | 2.05 | 1.34–2.72 |
| Child ever had sneezing or runny nose | ||
| No | Ref | |
| Yes | 3.23 | 2.28–4.60 |
| Child had itchy rash | ||
| No | Ref | |
| Yes | 2.11 | 1.49–3.00 |
| Kept pets inside home at sixth year | ||
| No | Ref | |
| Yes | 0.69 | 0.49–0.98 |
OR, odds ratio; CI, confidence interval; Ref, referent.
Definition of asthma: diagnosis of asthma and wheezing during sixth year (±3 months) of life.
See † footnote in Table 1.
Table 4 presents the results of the logistic regres-sion models to assess the effect of acetaminophen use during different trimesters of pregnancy and the dose response of acetaminophen. In the unadjusted model, acetaminophen use in different trimesters of pregnancy was not significantly associated with the risk of asthma. Those who consumed an average of 1,300 mg/mo or less significantly decreased the risk of asthma compared with never users before adjustment, and those who used 650 mg per day of use or less significantly decreased the risk of asthma.
Table 4.
Association Between Use of Acetaminophen During Pregnancy and Asthma Diagnosis With Wheezing at Sixth Year of Life*
| Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|
| n | OR | 95% CI | OR | 95% CI | |
| Ever use of acetaminophen during first and third trimesters† | |||||
| Neither | 441 | Ref | Ref | ||
| First trimester only | 273 | 0.73 | 0.46–1.14 | 0.68 | 0.39–1.20 |
| Third trimester only | 174 | 1.11 | 0.69–1.78 | 0.91 | 0.48–1.69 |
| Both | 424 | 0.74 | 0.50–1.09 | 0.59 | 0.36–0.98 |
| Average monthly consumption of acetaminophen during first and third trimesters(mg/mo)‡‖ | |||||
| 0 | 452 | Ref | |||
| 1,300 or less | 327 | 0.63 | 0.41–0.99 | 0.60 | 0.31–1.15 |
| 1,301–2,600 | 165 | 0.69 | 0.40–1.20 | 0.72 | 0.32–1.64 |
| 2,601–5,200 | 133 | 0.67 | 0.36–1.23 | 0.76 | 0.32–1.81 |
| 5,201–10,400 | 54 | 1.16 | 0.54–2.48 | 0.58 | 0.19–1.80 |
| More than 10,400 | 25 | 1.08 | 0.36–3.23 | 0.99 | 0.19–5.30 |
| Average consumption of acetaminophen during first and third trimesters on the days of use (mg per day of use)§ | |||||
| 0 | 441 | Ref | Ref | ||
| 650 or less | 338 | 0.61 | 0.40–0.94 | 0.62 | 0.39–1.00 |
| 651–1,300 | 264 | 0.81 | 0.53–1.25 | 0.67 | 0.41–1.09 |
| More than 1,300 | 53 | 1.15 | 0.54–2.45 | 0.83 | 0.35–1.98 |
OR, odds ratio; CI, confidence interval; Ref, referent.
See † footnote in Table 1.
Adjusted for covariates:
(n=1,208) mother’s ethnicity, mother’s asthma, mother’s asthma symptoms during pregnancy, mother’s allergy, father’s wheezing, child’s ethnicity, child’s visit to emergency department for respiratory illnesses, child’s antibiotic use during first years of life, child’s exposure to tobacco smoke 2 hours or greater, child’s use of allergy medication, child’s allergy, child’s sneezy or runny nose, child’s bronchitis, respiratory syncytial virus, and tonsillitis;
(n=990) mother’s age and ethnicity, marital status, mother’s education, mother’s asthma and asthma symptoms during pregnancy, mother’s allergy, low birth weight, father’s ethnicity and education, father’s wheezing, mother’s eczema, household income, pets inside home at sixth year, gas stove use at sixth year, child’s ethnicity, sibling, cough at sixth year, wheezing in the first year, visit to emergency department for respiratory illnesses, breastfeeding child’s antibiotic use during first 2 years of life, child’s use of allergy medication, child’s exposure to tobacco 2 hours or greater, child’s sneezy or runny nose, child’s eczema and child’s tonsillitis;
(n=1,116) mother’s asthma, father’s education, father’s wheezing, mother’s eczema, program before elementary, child’s antibiotic use during first 2 years of life, child’s exposure to tobacco smoke 2 hours or greater, and child’s sneezy or runny nose.
1,300 mg is equivalent to four tablets of regular-strength (325 mg) acetaminophen.
After adjustment, acetaminophen use during both trimesters significantly reduced the risk of asthma (OR 0.59, 95% CI 0.36–0.98). Analysis of acetaminophen dose demonstrated that none of the cumulative acetaminophen doses were significantly associated with risk of asthma. Average acetaminophen con sumption per day of use nonsignificantly reduced the risk of asthma at all doses.
Sensitivity analysis was performed using the dose calculated on the assumption that women who used more than one acetaminophen medication in the same month would take multiple medications on the same days of the month. The results of this sensitivity analysis are not shown, but final estimates did not differ significantly from the results based on the assumption that multiple medications were taken on different days of the month.
Asthma symptoms and other respiratory health outcomes in children were modeled to further explore the effect of acetaminophen use during pregnancy (Table 5). Before adjusting for potential confounders, acetaminophen use during pregnancy did not have a significant association with the outcomes of ever wheezing, persistent wheezing, or diagnosed bronchitis. After adjustment, acetaminophen use seems to significantly decrease the risk of persistent wheezing (OR 0.67, 95% CI 0.46–0.98). Acetaminophen use during pregnancy significantly increased the risk of sneezing/runny nose and allergy in children in the unadjusted models. After adjusting for potential confounders, acetaminophen use during pregnancy was not significantly associated with these symptoms and conditions.
Table 5.
Risk of Various Health Outcomes in Children According to Acetaminophen Use During Pregnancy*
| Ever Use of Acetaminophen During First and Third Trimesters |
||||||
|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
|||||
| n Dis | n Exp | OR | 95% CI | OR | 95% CI | |
| Total (N= 1,505) | ||||||
| Persistent wheezing†‡ | 172 | 113 | 0.86 | 0.61–1.20 | 0.67 | 0.46–0.98 |
| Ever wheezing§ | 650 | 450 | 1.04 | 0.83–1.31 | 0.80 | 0.61–1.04 |
| Diagnosed bronchitis‖ | 272 | 200 | 1.33 | 0.99–1.79 | 1.04 | 0.74–1.46 |
| Sneezing/runny nose ever¶ | 568 | 413 | 1.36 | 1.08–1.72 | 0.97 | 0.70–1.34 |
| Allergy# | ||||||
| Yes | 592 | 421 | 1.30 | 1.02–1.65 | 1.04 | 0.75–1.44 |
| Unsure | 152 | 112 | 1.48 | 1.00–2.20 | 1.28 | 0.79–2.07 |
n Dis, number of individuals having the disease; n Exp, number of exposed subjects among those who have the disease; OR, odds ratio; CI, confidence interval.
See † footnote in Table 1.
Wheezing at first and sixth years of life.
Adjusted for covariates:
(n=1,387) maternal asthma symptoms during pregnancy, child’s ethnicity, child’s sneezy or runny nose, child’s bronchitis and respiratory syncytial virus (RSV);
(n=1,342) mother’s asthma symptoms, income, child’s runny or sneezy nose, child’s bronchitis, croup, and RSV;
(n=1,305) mother’s ethnicity, exposure to secondhand smoke during pregnancy, mother’s symptoms during pregnancy, mother’s allergy, father’s wheezing, child’s antibiotic use during first 2 years of life, child’s allergy, child’s runny or sneezy nose, child’s itchy rash, child’s sinus infection;
(n=1,270) marital status, mother’s diagnosis, mother’s allergy, father’s allergy, mother’s eczema, use of air conditioner at sixth year, child’s antibiotic use during 4 years of life, child’s exposure to tobacco smoke 2 hours or greater, child’s allergy, child’s hay fever, child’s itchy rash, child’s bronchitis, sinus infection, RSV, and tonsillitis;
(n=1,233) mother’s allergy, asthma symptoms during pregnancy, mother’s allergy, father’s ethnicity, mother’s eczema, income, pets inside home at sixth year, use of wood-burning stove at sixth year, use of air conditioner at sixth year, child’s antibiotic use during 4 years of life, child’s use of allergy medication, child’s runny or sneezy nose, child’s itchy rash, child’s bronchitis and respiratory syncytial virus.
DISCUSSION
Our findings contradict results from several previous studies, and we discuss below the possibility that positive associations reported in these studies may be due to residual confounding, possible phenotype differences in asthma diagnosis, and incomplete acetaminophen ascertainment.
Shaheen et al8,9 reported that frequent antenatal acetaminophen use was positively associated with childhood asthma and wheezing. Koniman et al11 performed a matched patient–sibling case–control study and found acetaminophen use during pregnancy and early months of life may increase the risk of allergic and nonallergic asthma in children. Garcia-Marcos et al12 performed a retrospective cohort study and concluded that frequent use of acetaminophen during pregnancy was associated with wheezing in offspring during preschool years. Persky et al10 reported significant associations with wheezing, but not asthma, during the first year of life. These studies all used a single asthma symptom or simpler definitions of asthma than the definition used in this study. Asthma is a complex clinical manifestation that is difficult to diagnose before age 6 years, and wheezing in early infancy is not always a good predictor of subsequent asthma development.18
We modeled outcomes that more closely replicated earlier studies. This analysis, however, also did not find any evidence that acetaminophen increased the risk of persistent wheezing, ever wheezing, and diagnosed bronchitis. A significant protective effect was present for persistent wheezing after adjusting for potential confounders. Acetaminophen seemed to increase the risk of sneezing/runny nose ever and allergy in unadjusted models; however, these effects were no longer significant in adjusted models, suggesting the presence of residual confounding in previous studies.
Collection of retrospective information may have resulted in recall bias; this can lead to erroneous estimates of increased risk. Further, no previous studies measured acetaminophen exposure as inclusively and accurately as the current study. Respondents stated yes or no to whether they had taken acetaminophen or selected acetaminophen from a list of drugs shown to them. When exposure is prelimited, under-reporting of acetaminophen exposure is possible.
Previous studies measured few potential con-founders. This study shows very strong associations of maternal asthma and asthma symptoms with acetaminophen use. This and the strong associations of maternal asthma with asthma in the child could, without careful adjustment, produce strong confounding favoring an apparent association of maternal acetaminophen use and childhood asthma.
Some studies report positive associations between asthma and exposure to acetaminophen after birth. These studies deserve additional caution because reverse causality may be present. Children who show early symptoms of asthma may be more likely to be treated with acetaminophen, a drug commonly used for pain relief and fever in children. In the multivariate logistic regression model (Table 3), keeping pets inside the home at the sixth year of the child’s life was associated with a decreased risk of asthma in children (OR 0.69, 95% CI 0.49–0.98). This is an example of reverse causality in that homes with asthmatic children may avoid keeping pets inside.
Evidence for biologic plausibility supporting the causal relationship between acetaminophen and asthma is conflicting. Acetaminophen crosses the placenta in an unconjugated form and is primarily metabolized by sulfation in the fetus (Levy G, Garrettson LK, Soda DM. Evidence of placental transfer of acetaminophen [letter]. Pediatrics 1975;55:895). One proposed mechanism through which prenatal exposure to acetaminophen causes asthma in children is by reduction of pulmonary glutathione levels and inhibition of the cyclooxygenase (COX) pathway19–24 (Levy et al, Pediatrics and Varner A. Paracetamol and asthma [letter]. Thorax 2000;55:882–3; author reply 883–4). However, this hypothesis is based on in vitro findings, and it is unclear whether this effect is found in vivo. One study showed that extracellular glutathione peroxidase is actually increased in the asthmatic airway.25 Moreover, the period of glutathione resynthesis in the lungs is very short, making it unlikely that oxidative stress would cause significant damage.26
A second mechanistic hypothesis concerns lack of suppression of the COX pathway in cases of inflammation. Induction of the COX pathway leads to production of prostaglandin E2. The argument that acetaminophen is not antiinflammatory is questionable, because other studies demonstrate antiinflammatory activities of acetaminophen.27–30
There were several strengths to our study. Differential exposure misclassification was unlikely in this investigation because reported drug use was recorded before birth. Some nondifferential misclassification may have occurred, but acetaminophen consumption was high and the risk estimates were below 1, suggesting that any effect of misclassification would be small. Ascertainment, interviewer, and indication bias were unlikely because neither interviewer nor respondents knew about the study hypothesis.
This study examined and assessed the impact of many covariates to a greater degree than previous studies. Because many mothers had diagnosed asthma, they were knowledgeable of asthma and likely to accurately report respiratory symptoms. This study measured acetaminophen exposure more accurately than other studies by asking more openended questions for any respiratory and nonrespiratory drugs consumed during pregnancy and by precisely calculating the dose and daily frequency of acetaminophen use. A total of 64 different drugs containing acetaminophen were reported by respondents. Among those who responded, 69% ever consumed acetaminophen in first or third trimester of pregnancy, a higher rate of use than in previous studies except that of Persky et al, who reported 70%.
Our study had several limitations. First, acetaminophen exposure during the second trimester was not ascertained. It may be important to learn the effect of exposure throughout the entire pregnancy. Women who only used acetaminophen in the second trimester, likely a small group, would be classified as nonusers, moving our result closer to the null. Second, because exposure is self-reported, actual consumption of acetaminophen could not be verified. Also, asthma of the mother and child are reported by the mother and not confirmed by medical records. Nonetheless, the definition of asthma used here is common in epidemiologic research. Finally, our study did not control for cotherapy. However, antibiotics are the only class of drugs associated with asthma risk, but with increased risk. In this study, where a decreased risk of asthma was observed, it is highly unlikely that antibiotics are confounding. We are not aware of any prenatal drug exposures that reduce the risk of asthma. Thus, confounding by other drugs is unlikely.
Our study showed that acetaminophen use during pregnancy does not increase the risk of asthma in children, and there is no dose–response effect of acetaminophen. Although some of our analysis showed a protective effect of the drug, we are unaware of any mechanism supporting a real effect. Acetaminophen has antiinflammatory effects27–31 and in theory may be expected to reduce asthma, but we are unaware of any literature to support this. An observed protective effect may also be attributed to a random effect. Crude ORs for high doses (more than 1,300 mg/d of use and more than 5,200 mg/mo) showed an increased risk of asthma, albeit nonsignificantly. After adjusting for potential confounders, the risk decreased, suggesting that there were confounders that other studies were unable to take into account.
In 2005, Eneli et al20 advised that recommending changes in acetaminophen use among adults and children is premature. However, in 2009, Allmers et al17 suggested that the general public should be warned of possible risk associated with acetaminophen. Based on the results of this study, it may still be premature for public warnings of acetaminophen and increased risk of asthma. Acetaminophen is the drug of choice for pain relief during pregnancy and early childhood, and this study offers reassurance that antenatal acetaminophen has limited, if any, effects on asthma development in children.
Acknowledgments
Supported by grants AI41040 and DA05484 from the National Institutes of Health.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Rebordosa C, Kogevinas M, Horvath-Puho E, Norgard B, Morales M, Czeizel AE, et al. Acetaminophen use during pregnancy: effects on risk for congenital abnormalities. Am J Obstet Gynecol. 2008;198:178.e1–178.e7. doi: 10.1016/j.ajog.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 2.Rathmell JP, Viscomi CM, Ashburn MA. Management of nonobstetric pain during pregnancy and lactation. Anesth Analg. 1997;85:1074–1087. doi: 10.1097/00000539-199711000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Headley J, Northstone K, Simmons H, Golding J. ALSPAC Study Team Medication use during pregnancy: data from the Avon Longitudinal Study of Parents and Children. Eur J Clin Pharmacol. 2004;60:355–361. doi: 10.1007/s00228-004-0775-7. [DOI] [PubMed] [Google Scholar]
- 4.Werler MM, itchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol. 2005;193(pt 1):771–777. doi: 10.1016/j.ajog.2005.02.100. [DOI] [PubMed] [Google Scholar]
- 5.Corby DG. Aspirin in pregnancy: maternal and fetal effects. Pediatrics. 1978;62(pt 2) suppl:930–937. [PubMed] [Google Scholar]
- 6.Hernandez-Diaz S, Garcia-Rodriguez LA. Epidemiologic assessment of the safety of conventional nonsteroidal anti-inflammatory drugs. Am J Med. 2001;110 suppl 3A:20S–27S. doi: 10.1016/s0002-9343(00)00682-3. [DOI] [PubMed] [Google Scholar]
- 7.Rebordosa C, Kogevinas M, Sorensen HT, Olsen J. Pre-natal exposure to paracetamol and risk of wheezing and asthma in children: a birth cohort study. Int J Epidemiol. 2008;37:583–590. doi: 10.1093/ije/dyn070. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen SO, Newson RB, Henderson AJ, Headley JE, Stratton FD, Jones RW, et al. ALSPACStudy Team Prenatal paracetamol exposure and risk of asthma and elevated immunoglobulin E in childhood. Clin Exp Allergy. 2005;35:18–25. doi: 10.1111/j.1365-2222.2005.02151.x. [DOI] [PubMed] [Google Scholar]
- 9.Shaheen SO, Newson RB, Sherriff A, Henderson AJ, Heron JE, Burney PG, et al. Paracetamol use in pregnancy and wheezing in early childhood. Thorax. 2002;57:958–963. doi: 10.1136/thorax.57.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persky V, Piorkowski J, Hernandez E, Chavez N, Wagner-Cassanova C, Vergara C, et al. Prenatal exposure to acetaminophen and respiratory symptoms in the first year of life. Ann Allergy Asthma Immunol. 2008;101:271–278. doi: 10.1016/S1081-1206(10)60492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riece K, Yiong Huak C, Teng Nging T, Van Bever HP. A matched patientsibling study on the usage of paracetamol and the subsequent development of allergy and asthma. Pediatr Allergy Immunol. 2007;18:128–134. doi: 10.1111/j.1399-3038.2006.00484.x. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Marcos L, Sanchez-Solis M, Perez-Fernandez V, Pas-tor-Vivero MD, Mondejar-Lopez P, Valverde-Molina J. Is the effect of prenatal paracetamol exposure on wheezing in pre- school children modified by asthma in the mother? Int Arch Allergy Immunol. 2008;149:33–37. doi: 10.1159/000176304. [DOI] [PubMed] [Google Scholar]
- 13.Beasley R, Clayton T, Crane J, von Mutius C, Lai CK, Montefort S, et al. ISAACPhase Three Study Group. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6–7 years: analysis from phase three of the ISAAC programme. Lancet. 2008;372:1039–1048. doi: 10.1016/S0140-6736(08)61445-2. [DOI] [PubMed] [Google Scholar]
- 14.Cohet C, Cheng S, MacDonald C, Baker M, Foliaki S, Huntington N, et al. Infections, medication use, and the prevalence of symptoms of asthma, rhinitis, and eczema in childhood. J Epidemiol Community Health. 2004;58:852–857. doi: 10.1136/jech.2003.019182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaheen SO, Sterne JA, Songhurst CE, Burney PG. Frequent paracetamol use and asthma in adults. Thorax. 2000;55:266–270. doi: 10.1136/thorax.55.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barr RG, Wentowski CC, Somers SC, Curhan GC, Stampfer MJ, Schwartz J, et al. Prospective study of acetaminophen use and newly diagnosed asthma among women. Am J Respir Crit Care Med. 2004;169:836–841. doi: 10.1164/rccm.200304-596OC. [DOI] [PubMed] [Google Scholar]
- 17.Allmers H, Skudlik C, John SM. Acetaminophen use: a risk for asthma? Curr Allergy Asthma Rep. 2009;9:164–167. doi: 10.1007/s11882-009-0024-3. [DOI] [PubMed] [Google Scholar]
- 18.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 19.Rollins DE, von Bahr C, Glaumann H, Moldeus P, Rane A. Acetaminophen: potentially toxic metabolite formed by human fetal and adult liver microsomes and isolated fetal liver cells. Science. 1979;205:1414–1416. doi: 10.1126/science.38505. [DOI] [PubMed] [Google Scholar]
- 20.Eneli I, Camargo C, Sadri K, Jr, Barr RG. Acetaminophen and the risk of asthma: the epidemiologic and pathophysiologic evidence. Chest. 2005;127:604–612. doi: 10.1378/chest.127.2.604. [DOI] [PubMed] [Google Scholar]
- 21.Varner AE, Busse WW, Lemanske RF., Jr Hypothesis: decreased use of pediatric aspirin has contributed to the increasing prevalence of childhood asthma. Ann Allergy Asthma Immunol. 1998;81:347–351. doi: 10.1016/S1081-1206(10)63127-4. [DOI] [PubMed] [Google Scholar]
- 22.Kozer E, Evans S, Barr J, Greenberg R, Soriano I, Bulkowstein M, et al. Glutathione, glutathione-dependent enzymes and antioxidant status in erythrocytes from children treated with high-dose paracetamol. Br J Clin Pharmacol. 2003;55:234–240. doi: 10.1046/j.1365-2125.2003.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larrey D, Letteron P, Foliot A, Descatoire V, Degott C, Geneve J, et al. Effects of pregnancy on the toxicity and metabolism of acetaminophen in mice. J Pharmacol Exp Ther. 1986;237:283–291. [PubMed] [Google Scholar]
- 24.Levy G, Khanna NN, Soda DM, Tsuzuki O, Stern L. Pharmacokinetics of acetaminophen in the human neonate: formation of acetaminophen glucuronide and sulfate in relation to plasma bilirubin concentration and D-glucaric acid excretion. Pediatrics. 1975;55:818–825. [PubMed] [Google Scholar]
- 25.Comhair SA, Bhathena PR, Farver C, Thunnissen FB, Erzurum SC. Extracellular glutathione peroxidase induction in asthmatic lungs: evidence for redox regulation of expression in human airway epithelial cells. FASEB J. 2001;15:70–78. doi: 10.1096/fj.00-0085com. [DOI] [PubMed] [Google Scholar]
- 26.Duan X, Plopper C, Brennan P, Buckpitt A. Rates of glutathione synthesis in lung subcompartments of mice and monkeys: possible role in species and site selective injury. J Pharmacol Exp Ther. 1996;277:1402–1409. [PubMed] [Google Scholar]
- 27.Skjelbred P, Album B, Lokken P. Acetylsalicylic acid vs paracetamol: effects on post-operative course. Eur J Clin Pharmacol. 1977;12:257–264. doi: 10.1007/BF00607424. [DOI] [PubMed] [Google Scholar]
- 28.Skjelbred P, Lokken P. Paracetamol versus placebo: effects on post-operative course. Eur J Clin Pharmacol. 1979;15:27–33. doi: 10.1007/BF00563555. [DOI] [PubMed] [Google Scholar]
- 29.Rezende RM, Franca DS, Menezes GB, dos Reis WG, Bakhle YS, Francischi JN. Different mechanisms underlie the analgesic actions of paracetamol and dipyrone in a rat model of inflammatory pain. Br J Pharmacol. 2008;153:760–768. doi: 10.1038/sj.bjp.0707630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seegers AJ, Jager LP, Zandberg P, van Noordwijk J. The anti-inflammatory, analgesic and antipyretic activities of non-narcotic analgesic drug mixtures in rats. Arch Int Pharmaco-dyn Ther. 1981;251:237–254. [PubMed] [Google Scholar]
- 31.Lee YS, Kim H, Brahim JS, Rowan J, Lee G, Dionne RA. Acetaminophen selectively suppresses peripheral prostaglandin E2 release and increases COX-2 gene expression in a clinical model of acute inflammation. Pain. 2007;129:279–286. doi: 10.1016/j.pain.2006.10.020. [DOI] [PubMed] [Google Scholar]