Abstract
The development of a new, less invasive, and more rapidly implemented method of quantifying endothelial cell density in tumors could facilitate experimental and clinical studies of angiogenesis. Therefore, we evaluated the utility of tumor fine needle aspiration (FNA) coupled with flow cytometry for assessment of tumor angiogenesis. Samples were obtained from cutaneous tumors of mice using FNA, then immunostained and assessed by flow cytometry to determine the number of CD31+ endothelial cells. Results of the FNA/flow cytometry technique were compared with quantification of tumor microvessel density using immunohistochemistry. The ability of the FNA/cytometry technique to quantify the effects of anti-angiogenic therapy and to monitor changes in tumor angiogenesis over time in individual tumors was also determined. We found that endothelial cell percentages determined in tumor tissue aspirates by flow cytometry correlated well with the percentages of endothelial cells determined in whole tumor digests by flow cytometry and with tumor microvessel density measurements by immunohistochemistry. Moreover, we found that repeated FNA sampling of tumors did not induce endothelial cell changes. Interestingly, by employing repeated FNA sampling of the same tumors we were able to observe a sudden and marked decline in tumor angiogenesis triggered when tumors reached a certain size. Thus, we conclude that the FNA/flow cytometry technique is an efficient, reproducible, and relatively non-invasive method of rapidly assessing tumor angiogenesis, which could be readily applied to evaluation of tumor angiogenesis in clinical settings in humans.
Keywords: endothelial, cancer, blood vessel, microvessel density, immunohistochemistry
Introduction
Angiogenesis is a critical process for tumor development, thus making measurement of tumor angiogenesis a valuable biomarker for evaluating tumor biology and assessing responses to anti-angiogenic therapy [1-3]. Currently, tumor angiogenesis is most often measured by CD31 immunostaining of tumor biopsies to quantify microvessel density (MVD) [4, 5]. However, determination of tumor MVD in mice requires that the animals be euthanized, while in humans, tissue biopsy is required. Thus, other non-invasive approaches to measurement of tumor angiogenesis have been explored, including imaging techniques such as X-ray angiography, positron emission tomography, and doppler ultrasound [6-8]. However, these modalities are often expensive and labor intensive techniques that are not feasible for animal studies and are often not realistic for repeated measurements in humans or mice [4].
Therefore, we sought to determine whether an alternative, relatively non-invasive, but easily applied technique could be used for routine, direct assessment of tumor angiogenesis. Previous studies have shown that flow cytometry could be used to assess angiogenesis when frozen and embedded tumor sections were used as the sample source [9]. However, this technique also requires relatively large amounts of tumor tissue for analysis. Fine needle aspirates (FNA) of tumors are routinely used to identify tumors by cytologic criteria, based on staining of cell samples prepared on glass slides. However, the FNA technique typically also collects infiltrating leukocytes and mesenchymal cells in addition to tumor cells in the sample. Therefore, we hypothesized that sufficient numbers of endothelial cells could be obtained in the FNA specimen to allow evaluation and quantification by flow cytometry.
To address this question, we developed an FNA approach to collect small samples of tumor tissue from cutaneous tumor tissues of mice to assess angiogenesis. The technique was optimized for sample collection, preparation, and analysis by flow cytometry for changes in angiogenesis. We sought to ensure that FNA analysis was comparable to conventional MVD analysis and that serial sampling of tumors would allow for evaluation of anti-angiogeneic drugs and assessment of changes in angiogenesis over time. Based on the results of these studies, we concluded that the FNA/flow cytometry technique could be used to accurately measure tumor angiogenesis in mice with cutaneous tumors.
Materials and Methods
Cell lines
The mouse tumor cell lines 4T1 (mammary carcinoma) and MCA-205 (fibrosarcoma) were maintained in C/5/5 MEM medium [MEM (Lonza, Walkersville, MD) supplemented with 1X MEM vitamin solution (Cellgro, Henderson, VA), 2 mM L-glutamine (Cellgro), 1 mM sodium pyruvate (Cellgro), 1X non-essential amino acid solution (Cellgro), 1X antibiotic/antimycotic (Cellgro), 5% heat inactivated fetal bovine serum (FBS, Atlas, Fort Collins, CO), and 5% heat inactivated newborn calf serum (Hyclone, Logan, UT)]. Once confluent, cells were washed with 1x phosphate-buffered saline and detached with 0.25% trypsin (Cellgro) supplemented with 0.5 mM EDTA.
Animals
All animal studies were performed in an AALAC-approved facility and were approved by the Colorado State University Institutional Animal Care and Use Committee. Female mice 6-8 weeks of age were used in all experiments and were purchased from Harlan Sprague Dawley (Indianapolis, IN) or Jackson Laboratories (Bar Harbor, ME). The 4T1 tumor cell line was injected in the mammary fat pad of BALB/c mice using 5×105 cells in 100 μL of PBS. MCA-205 tumor cells (2.5×105 cells per mouse) were injected subcutaneously into the rear flank of C57BL/6 mice.
FNA technique
Once tumors reached a minimal size of 5 mm in diameter, they were subjected to sampling using a 23# needle. Mice were anesthetized using isoflurane (Minrad, Bethlehem, PA) and a 23# needle (Beckton Dickinson, Franklin Lakes, NJ) (without syringe attached) was inserted into the tumor using a rotating motion. After insertion into the tumor, the needle was removed and the tissue sample was flushed from the needle by attaching a syringe filled with tissue culture medium and expelling the contents into a sterile eppendorf tube. This process was repeated twice more, each time inserting the needle at roughly perpendicular angles to the previous insertion, for a total of 3 samples collected from each tumor. The 3 collected tumor samples were then pooled and processed using collagenase digestion [collagenase (5mg/ml final concentration; Sigma-Aldrich, St. Louis, MO) dissolved in MEM (Lonza) with 0.0125 mg/ml DNase (bovine deoxyribonuclease I; Sigma-Aldrich), and 0.25 mg/ml trypsin inhibitor (Sigma-Aldrich)] at 37°C for 20 min in a water bath. The samples were then triturated using a 23# needle and 1 ml syringe to assure that any tissue clumps were disaggregated. Single cell suspensions obtained in this manner were washed once using FACS buffer (1x PBS with 2% FBS and 0.1% sodium azide) and then counted and resuspended in FACS buffer and kept on ice prior to immunostaining.
Immunostaining and flow cytometry
Single cell suspensions from tumor aspirates were placed in individual wells of 96-well round bottom plates at a concentration of 5×105 to 1×106 cells per well. Prior to immunostaining, non-specific staining was blocked using 10 μL unlabelled anti-mouse Fcr III antibody (CD16/32; clone 93; eBioscience, San Diego, CA) diluted in normal mouse serum (Jackson Immunoresearch). Immunostaining was performed at room temperature for 30 minutes using an anti-mouse CD31 antibody (FITC-conjugated; clone 390; eBioscience), anti-mouse CD11b antibody (Pacific blue-conjugated; clone M1/70; eBioscience, anti-mouse VEGFR2 antibody (PE-conjugated; clone Avas 12α1; BD Pharmingen, Franklin Lakes, NJ), and anti-mouse CD133 (APC-conjugated; clone 13A4; eBioscience). Samples were then washed twice and analyzed after addition of propidium iodide (5 μg/sample) for live-dead cell discrimination. Samples were analyzed using a CyAn ADP flow cytometer (Beckman-Coulter, Fullerton, CA) and analysis was performed using FlowJo software (Tree Star, Inc., Ashland, OR). Endothelial cells were identified as CD31+CD11b- cells that were also CD45-PI-. Late apoptotic and necrotic cells were excluded by removing PI+ cells from analysis.
Immunohistochemistry
Tumor tissues were embedded in OCT embedding medium (Sakura, Torrance, CA) and cryosectioned at a thickness of 4 μm onto Superfrost slides (VWR; West Chester, PA). Slides were rehydrated, non-specific binding blocked with appropriate serum, then incubated with anti-mouse purified CD31 antibody (clone 390; eBioscience) appropriately diluted primary antibody. After washing, sections were incubated with biotinylated donkey anti-rat (Jackson ImmunoResearch). A Vectastain ABC kit (Vector) and subsequent AEC peroxidase substrate kit (Vector) were used according to manufacturer’s instructions. Slides were then counterstained with hematoxalin and crystal mount (Biomeda, Foster City, CA) applied.
Microvessel density (MVD) was determined by imaging five random 20x-high power CD31-stained fields using a Zeiss AxioVision 2 microscope and Carl Zeiss AxioVision Software v4.6 (Zeiss, Thornwood, NY). Computerized determination of positively staining cells was performed by blanking against sections stained with an irrelevant isotype control antibody. The average of positively stained areas for the 5 fields was determined for each tumor.
Tumor Growth Inhibition with ZD6474
The VEGF receptor-2 inhibitor ZD6474 was kindly provided by Dr. Dan Gustafson (Colorado State University) and AstraZeneca (Macclesfield, UK). Three days after tumor cell injection in mice (n = 5 per group), treatment was initiated. One group of mice was treated with 25 mg/kg of ZD6474 dissolved in PBS with 1% Tween 80 vehicle, while a second group of mice was treated with PBS and Tween diluent alone [10, 11]. Treatment was administered daily for the duration of the study. Tumor aspirates were obtained from all mice 14 days post-tumor challenge as described above immediately before animals were euthanized.
Statistical analysis
Statistical analyses were performed using commercial software (Prism 5; GraphPad Software, La Jolla, CA). Statistical comparisons between two groups were performed using the Mann-Whitney two-tailed t-test. Tumor growth analysis was performed using a paired two-tailed t-test. Correlations were analyzed using a one-tailed Spearman correlation test. For all statistical analyses, p-values of less than 0.05 were considered statistically significant.
Results
Quantification of endothelial cells in tumor digests using flow cytometry
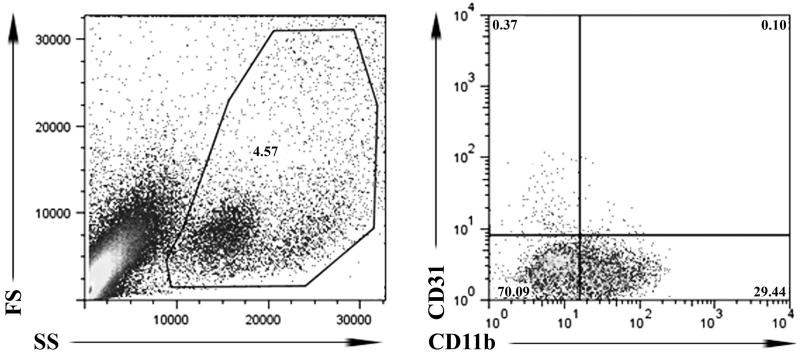
In this study we identified tumor endothelial cells as CD31+ cells that did not express the integrin CD11b, as described previously [12-15]. Cells that expressed CD11b were excluded from the endothelial cell population because some monocytes and macrophages are known to also express CD31 [15-17]. Propidium iodide staining was used to exclude dead cells from the analysis. Cells were first gated by FSC and SSC to exclude debris. Using this approach, we were able to routinely identify a distinct population of CD31+CD11b- cells in tumor aspirates and tumor digests (Figure 1).
Figure 1. Flow cytometric analysis of tumor endothelial cells.
Fine needle aspirates (FNA) of tumors were processed for immunostaining and analyzed by flow cytometry as described in Methods. Cells that were PI+ were first excluded to eliminate dead cells from analysis. Then, live cells were gated using forward and side scatter characteristics to eliminate cell debris, and subsequent analysis of CD31 and CD11b stained cells. Endothelial cells were classified as CD31+CD11b-.
We also assessed endothelial cell expression of other markers associated with tumor endothelium, such as CD133 and VEGFR2/Flk-1. However, these determinants were expressed on only a very small subset of CD31+ cells and did not contribute substantially to endothelial cell identification and therefore not used further (data not shown).
It should be noted that only tumors of a diameter of 5 mm or greater could be accurately aspirated using 23# needles. We experimented with smaller and larger gauge needles, but found that use of 23# needles gave superior results in terms of cell yield from small tumors (data not shown). Moreover, it was possible to determine when only normal skin was aspirated, because FNA/flow cytometry of normal skin typically yielded fewer than 1,000 total cell events for analysis (data not shown).
Comparison of endothelial cell percentages determined on FNA specimens versus whole tumor digests
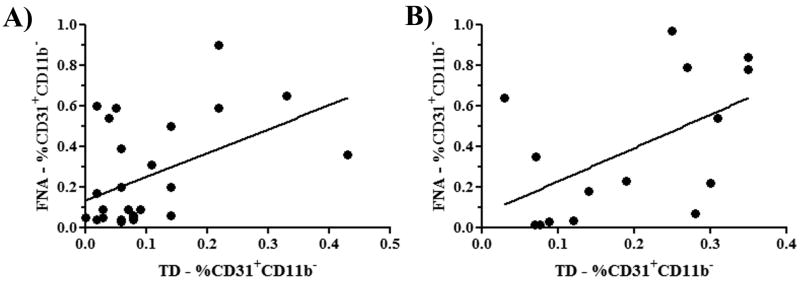
Using the flow cytometry analysis scheme noted above, we first asked whether FNA/flow cytometry samples of tumors yielded similar results in terms of percentages of endothelial cells as samples obtained by whole tumor digestion and flow cytometry. To address this question, FNA samples were collected from tumors of anesthetized mice (n = 5 per group), then the mice were euthanized and whole tumor tissues were collected and digested enzymatically. Single cell suspensions obtained by both techniques were then immunostained and analyzed by flow cytometry, using the protocol noted in the Methods. Endothelial cell populations from two different tumor types were compared. We found that the percentages of endothelial cells obtained by FNA and whole tumor digests of 4T1 breast carcinomas were significantly correlated (p<0.05), as determined by Spearman correlation analysis (Figure 2). In the case of the sarcoma cell line MCA-205, the two techniques also yielded results that were significantly correlated (p<0.05) (Figure 2). Thus, we concluded that flow cytometric analysis of endothelial cell populations in tumors using samples collected by FNA yielded results similar to those obtained using whole tumor digests.
Figure 2. Flow cytometric analysis of tumor FNA specimens and whole tumor digests yield similar estimates of tumor angiogenesis.
Tumor specimens were collected by FNA or tumor biopsy from mice with established 4T1 tumors (A) or MCA-205 tumors (B), and analyzed by flow cytometry to determine percentages of CD31+ endothelial cells, as described in Methods. There was a significant (p = 0.0402) correlation between the percentage of CD31+ cells in mice (n = 27) with 4T1 tumors (A) and the percentage of CD31+ cells in mice (n = 15) with MCA-205 tumors (B, p = 0.0267). Multiple experiments were pooled for each tumor type in this analysis.
Correlation between FNA/flow cytometry and tumor MVD analysis
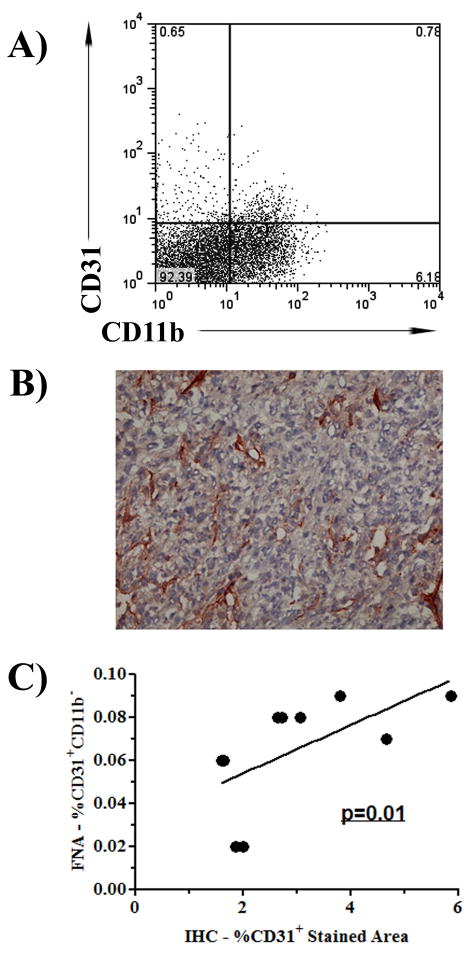
Next, we investigated the correlation between tumor angiogenesis assessed using FNA/flow cytometry and angiogenesis assessed using immunohistochemistry (IHC) and MVD analysis. As noted above, FNA specimens were collected from tumors before the tissues were collected for analysis by IHC. Results of endothelial cell analysis from FNA/flow cytometry samples were compared with MVD results from the same tumor sample. We observed a significant correlation (p<0.05) between endothelial cell percentages as determined by FNA/flow cytometry and tumor MVD for individual tumors in mice (Figure 3). In addition, we observed a significant correlation (p<0.05) between the results of whole tumor digest by flow cytometry and tumor MVD results (data not shown). This latter result is in agreement with a previous report correlating angiogenesis determination by flow cytometry on archived frozen tissues and tumor MVD analysis [9]. Thus, the estimates of tumor angiogenesis provided by the FNA/flow cytometry technique correlated well with tumor angiogenesis measurements determined by the conventional MVD assay.
Figure 3. Tumor angiogenesis assessed by FNA/flow cytometry correlates with angiogenesis measured by immunohistochemistry (IHC).
Fine needle aspirate samples were collected from mice (n= 10 per group) with cutaneous 4T1 tumors and numbers of CD31+ endothelial cells were analyzed by flow cytometry (A), as described in Methods. Mice were euthanized, and tumors isolated and analyzed for MVD by IHC (B) as described in Methods. Correlation between MVD and percentage of CD31+ cells was determined using Spearman correlation, which demonstrated a significant (p = 0.1) correlation between the values. Results are representative of two independent experiments.
Repeated FNA sampling of tumors does not alter tumor angiogenesis measurements
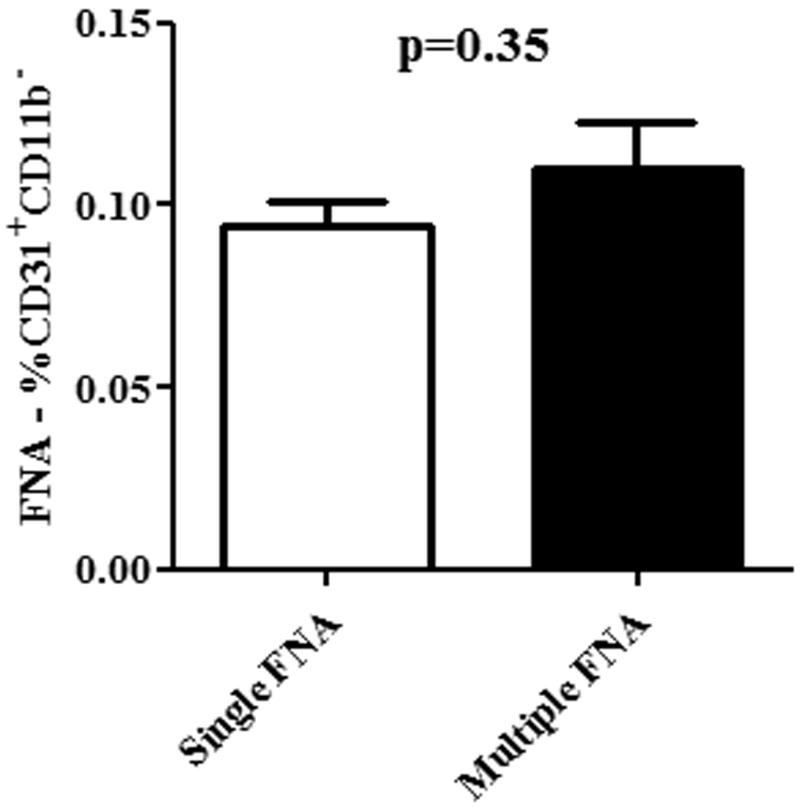
To determine whether repeated sampling of tumors by FNA introduced artifacts into the endothelial cell analysis, we compared endothelial cell percentages determined after a single tumor aspirate to values obtained after 3 repeated tumor aspirates in mice with tumors established for equal periods of time. Mice (n = 5-6 per group) with established 4T1 tumors were divided into two groups. In group 1, the tumor was only aspirated once, 7 days after tumor challenge. In group 2, tumors were aspirated on days 7, 10, and 13. Sixteen days after tumor implantation, FNA samples were collected from all mice in both groups and endothelial cell numbers compared by flow cytometry (Figure 4). The mean percentage of CD31+ endothelial cells in tumors only aspirated once was 0.09%, while the percentage of endothelial cell in tumors aspirated 3 times was 0.11%, and these percentages were not significantly different (p=0.35). Therefore, we concluded that repeated sampling of tumors by FNA did not introduce significant artifacts in endothelial cell numbers.
Figure 4. Repeated FNA sampling of tumors does not lead to endothelial cell artifacts.
BALB/c mice with established 4T1 tumors were divided into two groups (n = 5 per group). In Group 1 mice, a single FNA was performed, while in Group 2 mice, 3 separate FNAs were performed, each 3 days apart. The percentage of CD31+ cells (mean ± SEM) was compared between the two groups by Mann-Whitney test. Significant differences in endothelial cell percentages were not observed (p < 0.05). Results are representative of two independent experiments with 5 mice per group.
FNA/flow cytometry for assessment of tumor angiogenic responses to angiogenesis inhibitors
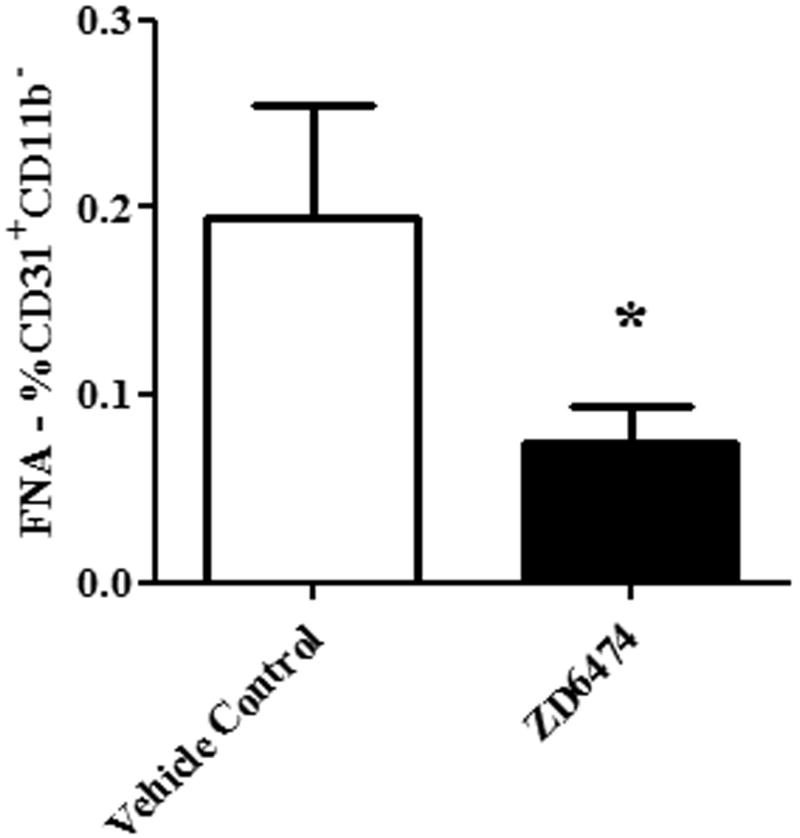
The FNA/flow cytometry technique would be particularly useful clinically if it could be used to assess tumor responses to anti-angiogenic agents. Therefore, we assessed the ability of FNA/flow cytometry to detect changes in tumor angiogenesis in mice treated with the angiogenesis inhibitor ZD6474, a dual inhibitor of VEGFR2 and EGFR [18-20]. Mice with established 4T1 tumors were treated by daily oral gavage with ZD6474, which was initiated 3 days after tumor cells were injected. Control mice were treated by daily oral gavage of dilution buffer. Treatment with ZD6474 induced a significant delay in tumor growth compared to mice treated with dilution buffer (data not shown). In addition, the percentage of endothelial cells was significantly reduced in tumors of ZD6474-treated mice compared to control mice, as assessed by FNA/flow cytometry (Figure 5). These results indicated that FNA/flow cytometry could realistically be used to monitor the efficacy of anti-angiogenic agents in vivo.
Figure 5. FNA analysis can be used to assess the effects of anti-angiogenic therapy (ZD6474).
BALB/c mice (n=5 per group) with established mammary fat pad 4T1 tumors were treated daily with ZD6474 (25 mg/kg) or with control buffer by oral gavage. Percentages of CD31+ endothelial cells in treated and sham-treated tumors were determined (mean ± SEM) using FNA/flow cytometry, as described in Methods. The percentage of CD31+ endothelial cells was significantly decreased (p < 0.05) in ZD6474-treated tumors, compared to control tumors, as assessed by Mann-Whitney test. Results are representative of two independent experiments.
Repeated FNA sampling to assess changes in tumor angiogenesis over time
Finally, we evaluated the potential for repeated FNA/flow cytometry analysis of individual tumors over time to provide insights into how tumor angiogenesis may change over time. Currently, to assess changes in tumor angiogenesis over time in mice, groups of mice with tumors must be serially sacrificed and tumor angiogenesis quantified by MVD analysis of each individual tumor tissue sample. The entire process is therefore costly in terms of mouse usage and analysis time. For clinical studies in humans, direct evaluation of changes in tumor angiogenesis over time is very difficult to accomplish because of the need for repeated tumor biopsies. Therefore, use of the FNA approach could offer substantial advantages over current technologies for repeated and relatively non-invasive assessment of tumor angiogenesis.
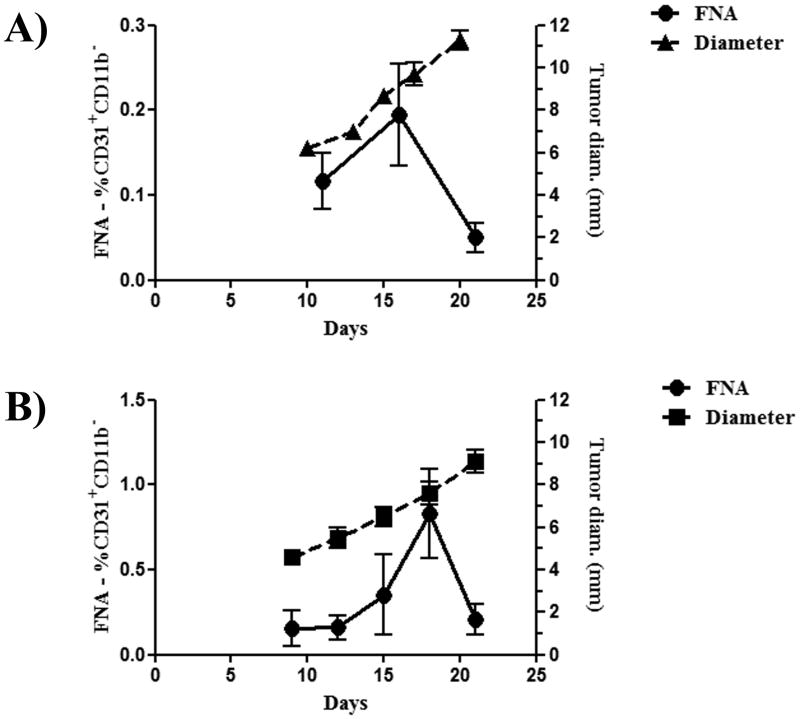
To address this question, we established cutaneous tumors (either 4T1 tumors in BALB/c mice or MCA-205 tumors in C57Bl/6 mice) in mice (n = 5 per group) and then serially evaluated tumor angiogenesis using tumor FNA samples collected from each individual tumor every 3 days using flow cytometry. We correlated tumor angiogenesis measurements with changes in tumor size. Results of the flow cytometric analysis showed that the percentage of CD31+ endothelial cells in 4T1 tumors increased steadily up until the point where the mean tumor diameter reached approximately 9 mm (Figure 6). At that point, the percentage of endothelial cells decreased dramatically, dropping from an average of 0.19% CD31+ cells to 0.05% CD31+ cells. A very similar phenomenon was observed in mice with MCA-205 tumors (Figure 6). For example, in the MCA-205 tumor model, the average number of CD31+ endothelial cells in tumors decreased from 0.83% to 0.21% once tumors reached a mean diameter of 7.6 mm. These results suggested that reaching a critical tumor size was associated with a rapid and pronounced reduction in endothelial cell density. Analysis of necrotic/apoptotic cells was performed by determination of PI+ events by flow cytometry, and determined that there was no significant change in apoptosis/necrosis at the time points assessed (data not shown). This suggests that the decrease in angiogenesis observed is not due to apoptosis and/or necrosis. Furthermore, serial sacrifice of mice at similar time points and determination of MVD by computer aided analysis was performed. Traditional analysis of MVD revealed similar changes as those observed by FNA/flow cytometry (Supplementary Figure 1). It should be noted that FNA results were obtained using a total of 20 mice (groups of 5 mice for each tumor type, with one repeat each), whereas a similar experiment using serial sacrifice of groups of mice, with a repeat, would have required the use of 80 mice.
Figure 6. Repeated measurement of tumors by FNA reveals angiogenic collapse of the tumor.
Syngeneic mice were challenged with 4T1 (A) or MCA-205 (B) tumors. Tumor measurements were initiated to coincide with the first FNA. FNA were taken every three days after the initial FNA until the first mouse had a maximal tumor diameter of 10 mm, at which time all mice were sacrificed. Tumors from mice challenged with 4T1 and MCA-205 tumors had a rapid decrease in the percentage of endothelial cells present suggestive of an angiogenic collapse. Results depict the mean (±SEM) of endothelial cells as determined by FNA and tumor growth as represented by maximal tumor diameter. Results are representative of two independent experiments.
Discussion
Most standard assays of tumor angiogenesis require sufficient amounts of tumor tissue to section in order to perform IHC analysis. This generally requires obtaining tumor biopsies (humans) or removal of the entire tumor from euthanized animals (mice). Thus, techniques for quantifying tumor angiogenesis using much smaller specimens would greatly facilitate clinical and experimental studies. In this report, we provide results to support the idea that FNA/flow cytometry, using very small tumor specimens, is an effective alternative to conventional tumor biopsy and IHC for assessing tumor angiogenesis. The major findings from the current study were that the FNA/flow cytometry technique could be adapted for use in mouse tumor studies, that the results of angiogenesis assays using FNA/flow cytometry correlated well with estimates of tumor angiogenesis provided by tumor MVD (analyzed by IHC) and whole tumor digest samples (analyzed by flow cytometry), and that the FNA/flow cytometry approach could be used to repeatedly assess tumor angiogenesis over time.
The ability to assess angiogenesis relatively non-invasively and repeatedly using a technique such as FNA/flow cytometry represents an important advance, since it greatly reduces the number of mice required for animal experiments. Equally important, it is reasonable to assume that the same approach could also be used to assess angiogenesis in solid tumors of humans, so long as the tumor was accessible by needle for aspiration. Thus, the approach could be used for tumor staging and as a readily obtained biomarker for evaluation of anti-angiogenic treatments and new drugs.
Key variables for performing the FNA/flow cytometry technique were identified in the current study. Among these were tumor size, needle size, and sample digestion after procurement. For example, we found that tumors < 5 mm in diameter could not be reliably sampled. Thus, the technique could only be applied to analysis of more advanced tumors. Several different needle sizes were evaluated for their ability to obtain reliable tumor FNA specimens and we found that a 23# needle gave the most reliable results. Larger needles were too large to use on mouse tumors, whereas smaller needles failed to yield sufficient cells for analysis. In addition, pooling aspirated specimens from a single tumor obtained by inserting the needle in 3 different orientations also improved the cell yield and served to decrease sample-to-sample variability. Finally, we found that a brief digestion step, using collagenase, increased the yield of cells overall and specifically endothelial cells from the FNA specimens.
A potential limitation to the FNA/flow cytometry technique was identified in the small number of events used for analysis. For example, the overall number of endothelial cells detected in each sample was relatively small, thus limiting the assessment of complex subpopulations of endothelial cells. However, despite the small yield of endothelial cells, we still observed a relatively small and manageable variability in tumor-to-tumor endothelial cell numbers. In the case of human tumor samples, this limitation might be partially overcome by simply performing more needle aspirates of a tumor, which is reasonable given the much larger overall size of tumors in humans relative to mice. Regional variation in intra-tumoral angiogenesis did not appear to introduce significant problems with the FNA/flow cytometry technique, presumably because specimens were collected from several different regions of the tumor for each sample. Moreover, repeated sampling of the same tumor at 3-day intervals also did not appear to introduce significant angiogenic artifacts.
Use of the FNA/flow cytometry approach to repeatedly measure angiogenesis in individual tumors also provided us with a unique tool to assess changes in angiogenesis over time. Such information has not previously been reported in mouse or human tumor studies and generally can only be inferred by comparing levels of angiogenesis between tumors collected from carefully controlled groups of mice at different time points. Thus, previous studies assessing tumor angiogenesis may overlook rapidly occurring changes in angiogenesis [4]. In the present study, we noted a precipitous drop in the percentage of endothelial cells within tumors as tumor size reached a critical level of approximately 8-9 mm in diameter. This critical threshold of tumor size was detected in repeated experiments involving different types of tumors. This result was confirmed by serial sacrifice of mice and traditional analysis of MVD. Thus, the time and tumor size frame of such changes, such as this apparent vascular collapse phenomenon, can be documented using FNA/flow cytometry.
In summary, we describe here a new technique for simple, repeated and relatively non-invasive assessment of tumor angiogenesis in mice, which is also readily applicable to study of human tumors. Use of the FNA/flow cytometry technique should greatly reduce the number of mice required for experiments. Repeated, closely spaced sampling of angiogenesis in individual tumors using the technique may also provide unique insights into more rapidly occurring angiogenic processes.
Supplementary Material
Syngeneic mice challenged with MCA-205 tumors (n = 3-4 mice per time point) were serially sacrificed and tumors collected for immunohistochemical determination of microvessel density. Analysis of MVD revealed similar dynamics of CD31+ cells to those observed by FNA (A). Representative immunohistochemical sections immunostained for CD31 are shown (B).
Acknowledgments
We would like to thank Dr. Daniel Gustafson and AstraZeneca for providing the ZD6474 used for these experiments.
Funding: These studies were supported in part by the Harbers Foundation and by a grant from the Colorado State University Cancer Supercluster.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest
References
- 1.Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis: a possible control point in tumor growth. Ann Intern Med. 1975;82:96–100. doi: 10.7326/0003-4819-82-1-96. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Staton CA, Reed MW, Brown NJ. A critical analysis of current in vitro and in vivo angiogenesis assays. Int J Exp Pathol. 2009;90:195–221. doi: 10.1111/j.1365-2613.2008.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermeulen PB, Gasparini G, Fox SB, et al. Quantification of angiogenesis in solid human tumours: an international consensus on the methodology and criteria of evaluation. Eur J Cancer. 1996;32A:2474–2484. doi: 10.1016/s0959-8049(96)00379-6. [DOI] [PubMed] [Google Scholar]
- 6.Cai W, Niu G, Chen X. Imaging of integrins as biomarkers for tumor angiogenesis. Curr Pharm Des. 2008;14:2943–2973. doi: 10.2174/138161208786404308. [DOI] [PubMed] [Google Scholar]
- 7.Charnley N, Donaldson S, Price P. Imaging angiogenesis. Methods Mol Biol. 2009;467:25–51. doi: 10.1007/978-1-59745-241-0_2. [DOI] [PubMed] [Google Scholar]
- 8.Pearlman JD, Laham RJ, Post M, et al. Medical imaging techniques in the evaluation of strategies for therapeutic angiogenesis. Curr Pharm Des. 2002;8:1467–1496. doi: 10.2174/1381612023394395. [DOI] [PubMed] [Google Scholar]
- 9.Baeten CI, Wagstaff J, Verhoeven IC, et al. Flow cytometric quantification of tumour endothelial cells; an objective alternative for microvessel density assessment. Br J Cancer. 2002;87:344–347. doi: 10.1038/sj.bjc.6600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafson DL, Bradshaw-Pierce EL, Merz AL, et al. Tissue distribution and metabolism of the tyrosine kinase inhibitor ZD6474 (Zactima) in tumor-bearing nude mice following oral dosing. J Pharmacol Exp Ther. 2006;318:872–880. doi: 10.1124/jpet.106.102376. [DOI] [PubMed] [Google Scholar]
- 11.Troiani T, Serkova NJ, Gustafson DL, et al. Investigation of two dosing schedules of vandetanib (ZD6474), an inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling, in combination with irinotecan in a human colon cancer xenograft model. Clin Cancer Res. 2007;13:6450–6458. doi: 10.1158/1078-0432.CCR-07-1094. [DOI] [PubMed] [Google Scholar]
- 12.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 13.Hermiston ML, Zikherman J, Zhu JW. CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev. 2009;228:288–311. doi: 10.1111/j.1600-065X.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas ML, Lefrancois L. Differential expression of the leucocyte-common antigen family. Immunol Today. 1988;9:320–326. doi: 10.1016/0167-5699(88)91326-6. [DOI] [PubMed] [Google Scholar]
- 15.Wautier JL, Wautier MP. Blood cells and vascular cell interactions in diabetes. Clin Hemorheol Microcirc. 2001;25:49–53. [PubMed] [Google Scholar]
- 16.Jackson DE. The unfolding tale of PECAM-1. FEBS Lett. 2003;540:7–14. doi: 10.1016/s0014-5793(03)00224-2. [DOI] [PubMed] [Google Scholar]
- 17.Kim SJ, Kim JS, Papadopoulos J, et al. Circulating monocytes expressing CD31: implications for acute and chronic angiogenesis. Am J Pathol. 2009;174:1972–1980. doi: 10.2353/ajpath.2009.080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drevs J, Konerding MA, Wolloscheck T, et al. The VEGF receptor tyrosine kinase inhibitor, ZD6474, inhibits angiogenesis and affects microvascular architecture within an orthotopically implanted renal cell carcinoma. Angiogenesis. 2004;7:347–354. doi: 10.1007/s10456-005-1394-3. [DOI] [PubMed] [Google Scholar]
- 19.McCarty MF, Wey J, Stoeltzing O, et al. ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor with additional activity against epidermal growth factor receptor tyrosine kinase, inhibits orthotopic growth and angiogenesis of gastric cancer. Mol Cancer Ther. 2004;3:1041–1048. [PubMed] [Google Scholar]
- 20.Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62:4645–4655. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Syngeneic mice challenged with MCA-205 tumors (n = 3-4 mice per time point) were serially sacrificed and tumors collected for immunohistochemical determination of microvessel density. Analysis of MVD revealed similar dynamics of CD31+ cells to those observed by FNA (A). Representative immunohistochemical sections immunostained for CD31 are shown (B).