Abstract
Following intracerebroventricular (i.c.v.) injection of ovine CRF (oCRF), an endogenous peptide agonist at both CRF1 and CRF2 receptors, defensive behaviors of CD-1 mice were evaluated in the Mouse Defensive Test Battery (MDTB). Behavioral measures taken before, during, and after predator (a hand-held anesthetized rat) confrontation included exploratory activity, risk assessment, avoidance, flight, freezing, defensive threat/attack, and residual emotional responses. Both low (0.1nmol) and high (0.2nmol) doses of oCRF robustly suppressed exploratory activities and increased risk assessment during the initial familiarization period. Flight speed and jump escapes when the mouse was chased were significantly elevated by the 0.2nmol dose. Both doses enhanced freezing and avoidance to a distant predator when the escape route was blocked. The 0.2nmol dose also potentiated flight responses to a contacting predator in a highly confined space. Both oCRF groups traveled shorter distances and exhibited less escape attempts following the removal of the threat stimulus. These findings indicate that non-selective activation of CRF receptors via ventricular infusion of oCRF potentiates defensive behaviors relevant to the demand of specific challenges, generally enhancing the predominant defensive behavior in each specific situation.
Keywords: oCRF, CRF agonist, defensive behaviors, mice
1. Introduction
Corticotropin-releasing factor (CRF), a 41 amino acid peptide, is a pituitary secretagogue for adrenocorticotropin hormone (ACTH) [49] that also functions as a neurotransmitter/neuromodulator in regulating endocrine, behavioral, autonomic, and immune response to stress [4],[24]. These actions of CRF and other related peptide family members are primarily mediated by two CRF receptor subtypes termed CRF1 and CRF2 [22],[47] which are distinct in terms of tissue distribution [15],[39],[41],[50]and pharmacological properties [35]. In rodents, dense CRF1 mRNA expression is found in the isocortex, olfactory bulb, medial septum, cerebellum [15],[26],[50], and brainstem sensory relay nuclei [50]. CRF1 expression is also evident in the basolateral nucleus of the amygdala [15],[50], hippocampus, and anterior pituitary [50]. In contrast, central CRF2 mRNA expression follows a pattern that is more restricted than, and distinct from, that of CRF1, with high densities found in the intermediate lateral septum, ventromedial hypothalamus (VMH), the bed nucleus of the stria terminalis (BNST), cortical nucleus of the amygdala, dorsal raphe, nucleus of the solitary tract, and non-neuronal elements of the choroids plexus [15], [44], [50].
Anatomical heterogeneity of CRF1 and CRF2 suggest the existence of functional differences between them. Overall, evidence accumulated so far has been relatively consistent for a role of CRF1 in mediating responsivities to threat but much less so for that of CRF2 [44]. Mice deficient for CRF1 exhibited reduced anxiety-like behaviors [46]. Moreover, mice with conditional inactivation of forebrain CRF1, but spared anterior pituitary CRF1, showed less anxiety-like behavior [31]. Pharmacological studies showed that activation of central CRF1 enhanced anxiety-like behaviors in both mice [45] and rats [48] and peripheral administration of CRF1 antagonists reduced stress-induced behavioral and physical responses in several paradigms [18],[21],[23]. Down-regulation of CRF1 by antisense oligodeoxynucleotide led to attenuated anxiety-like behaviors in rats [28],[34]. In contrast, the role of CRF2 remains controversial, with some groups reporting results that support an anxiolytic role of CRF2 [2], [16], [32], [38] and others reporting anxiolytic effects of CRF2 antagonism [1], [25], [29].
Unconditioned defensive behaviors, such as those elicited by encounters with predators, are increasingly used to provide measures of behavioral responses to threat and stress [9], [42]. The Mouse Defense Test Battery (MDTB) is comprised of six sub-tests that vary in threat intensity/proximity that can be used to measure novelty exploration, anti-predator defensive responses including flight, freezing, risk assessment, defensive threat and attack, and residual emotional responses (following the removal of the threat). By virtue of its ability to capture a spectrum of defensive behaviors in a single session, the MDTB has been used extensively as a pre-clinical model in testing various drugs, especially those that are potentially effective against anxiety disorders[9], [20].
The aim of the present study was to examine the effects of i.c.v. administration of ovine CRF (oCRF), an endogenous peptide agonist with a 6.7-fold higher affinity to CRF1 than CRF2 [30], on defensive behaviors in CD-1 mice in the MDTB.
2. Materials and methods
2.1. Animals
Adult 12-week old male CD-1 mice (Charles River Laboratories, St. Louis, MO), weighing 35–45 g at the time of surgery, were used for this study. Mice were individually-housed in standard polypropylene cages in a temperature controlled room (20±2 °C) with ad libitum access to food and water and were maintained on a 12 hr light/dark cycle (lights on at 06:00 AM). Mice were allowed 1 week to acclimate to the vivarium prior to the start of the surgery. Surgical and experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Hawaii.
2.2. Surgery
Mice were anesthetized with sodium pentobarbitol (80 mg/kg). Glycopyrrolate (0.04 mg/mouse, Luitpold Pharmaceuticals, NY) was co-administered with pentobarbitol to prevent respiratory complications. A 26-gauge stainless steel guide cannula, 8.0 mm in length, was stereotaxically implanted in the right ventricle based on coordinates from the Mouse Brain Atlas (Paxinos and Franklin, 2001). The coordinates were AP 0.2mm, ML 1.0mm, DV 2.3mm (below the skull surface). The guide cannula was anchored with dental cement and two stainless still screws and a dummy stylet was used to maintain potency. The exact injection sites were verified histologically and data were analyzed only from those that had received injections in the correct target sites.
2.3. Peptide Infusion
oCRF used in the current study is the general gift from Dr. Joachim Spiess. The peptide was dissolved in highly purified acetic acid artificial cerebrospinal fluid (aCSF: 124 mM NaCl/26.4 mM NaHCO3/10 mM glucose/3.3 mM KCl/2.5 mM CaCl2/2.4 mM MgSO4/1.2 mM KH2PO4, pH=7.4). Mice were randomly assigned to one of the three groups: aCSF (vehicle control, n=9), 0.1nmol (n=8), and 0.2 nmol (n=10) of oCRF. Each mouse was gently restrained by hand and the stylet removed, and 1.0 μl of oCRF or aCSF was injected through the guide cannula over a time course of 30s. The injector was left in place for another 60 sec to allow for drug diffusion. The 32-gauge injector was made of stainless tubing (Small Parts Inc. FL) and connected to a 10-μl Hamilton microsyringe with polyethylene-20 tubing (Plastic One, VA). The injector extended 1.0 mm below the end of the guide cannula into the ventricle.
2.4. Behavioral Test Schedule
After a 1-week recovery period, mice were injected with oCRF or aCSF and tested in the MDTB 30 min post injection. The MDTB takes about 12 min to complete. Behavioral tests were performed during the light cycle, between 1 and 4 p.m. Experimenters and scorers were blind to treatment conditions.
2.5. Test Apparatus: MDTB
The MDTB test was conducted in an oval runway, 0.40m wide, 0.30m high, and 4.4 m in total length, consisting of two 2-m straight segments joined by two 0.4-m curved segments and separated by a median wall (2.0 m×0.30 m × 0.06m). The apparatus was elevated 0.8 m from the floor to enable the experimenter to hold the rat and move with ease while minimizing subjects’ view with her. All parts of the apparatus were constructed with black Plexiglas. The floor of the apparatus was marked every 20cm with white lines to facilitate distance measurement. Two ceiling-mounted video cameras were used to record the test and the room was illuminated with one 100-watt red light.
2.6. Behavioral paradigm: the Mouse Defense Test Battery (MDTB)
2.6.1. Pretest (familiarization period)
Each subject was placed in one end of the oval runway and allowed to freely explore the apparatus for the next the 3-min. Number of line-crossings and rears were counted. In addition, stretch attend posture (SAP) and the number of steps were measured for three 20-second sample periods (sample 1: 0~20 sec; sample 2: 60~80 sec; sample 3: 120~140 sec)
2.6.2. Predator avoidance test
Immediately after the pre-test, a hand-held anesthetized rat was brought up to the subject at an approximate speed of 0.5 m/s. Approach was initiated only if the subject was standing still with its head oriented toward the rat. Approach was terminated when contact with the subject occurred, or the subject fled from the approaching rat. If the subject fled, avoidance distance (distance between rat and subject at point of flight) and the distance the subject traveled before making the first stop (escape distance) were recorded. This procedure was repeated five times.
2.6.3. Chase/flight test
The hand-held rat was brought up to the subject at a speed of approx. 2.0 m/s. Chase was initiated only when the subject was at a standstill with its head oriented toward the rat, and stopped when the subject had traveled a distance that equals to three laps around the runway (~ 14.2m). Total time to complete the distance was recorded and overall flight speed (m/s) was calculated for each animal. Numbers of stops, reversals, and head orientations were also counted. The rat was removed upon chase completion.
2.6.4. Closed alley test
Following the chase test, the runway was converted to a 120-cm long (door-to-door) straight alley by closing a hinged hatch at one end and inserting a removable door at the other. As soon as the mouse moves to one end of the alley, the hand-held rat was introduced to the opposite end and gently shaken in place for 30 sec, during which time numbers of voluntary approaches and duration of freezing were measured.
2.6.5. Forced contact test
In this sub-test, the closed alley was shortened to 40cm long and the rat was quickly brought up to contact with the subject. For each such contact, occurrences of vocalization, upright posture, bite, jump attacks, and jump escapes were registered. Three trials, each consists of five forced contacts, were conducted.
2.6.6. Posttest
Upon completion of the forced contact test, the subject was released from the enclosure and its activities were recorded for another 3 min during which time the experimenter and the rat stimulus were out of sight. Line-crossings and escapes attempts (rearing and jump escapes), were counted.
2.7. Statistical analysis
Data were analyzed with one-way ANOVA. Subsequent comparisons between
treatment groups and control were carried out using Dunnett’s t-test
3. Results
3.1. Pretest activity and risk assessment
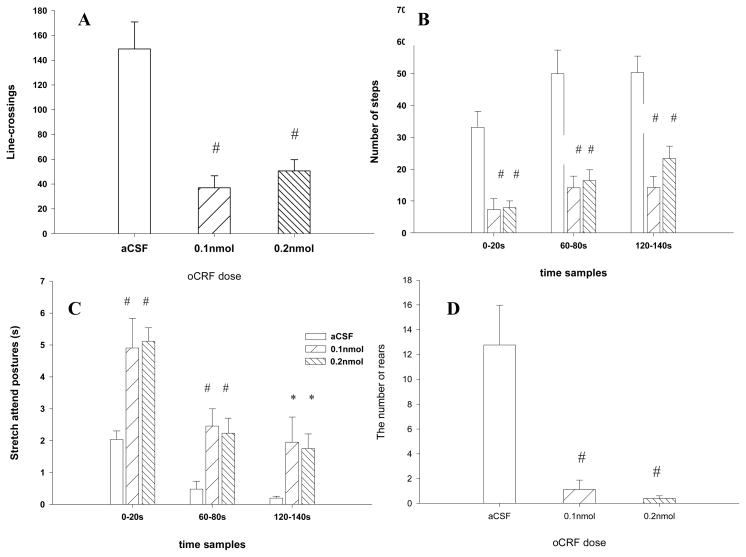
Fig. 2. presents runway activities during the 3-min pretest. One-way ANOVA revealed a significant drug effect on line-crossings [F (2, 24) =16.74, p<.001] (Fig. 2A). Subsequent Dunnett’s test showed that both 0.1nmol and 0.2nmol of oCRF significantly reduced this exploratory behavior (p<.001 for each). For a finer analysis, Dunnett’s test revealed that in each of the three 20-s periods sampled both oCRF groups made significantly fewer steps relative to aCSF treated mice (p<.001 for each comparison) (Fig. 2B). In addition, both oCRF treated groups exhibited higher levels of this risk assessment behavior than controls in each of the three sample periods (p<.01 for both doses for sample 1 and 2, p<.05 for both doses for sample 3) (Fig. 2C). Rearing (Fig. 2D) was also significantly affected by oCRF infusion [F (2, 24)=13.61, p<.001], with both dose groups showing significantly fewer rearing than controls (p<.001 for each: Dunnett’s comparison).
Fig. 2.
Effects of oCRF on activities in the 3-min pretest. (A) line-crossings (B) number of rears (C) number of steps in three 20-s sample periods (D) duration of stretch attend posture in three 20-s sample periods. Data represent mean ± SEM. *p < .05; #p < .01 (Dunnett t test).
3.2. Predator avoidance test
Table 1 presents data for the avoidance test. There were no significant main effects of oCRF injection on any measure: avoidance, avoidance distance, escape, or escape distance.
Table 1.
Effects of oCRF on avoidance and escape behaviors in the predator avoidance test
| Avoidance distance (cm) | Number of Avoidance | Escape distance (cm) | Number of Escape | |
|---|---|---|---|---|
| aCSF | 78.21±13.79 | 1.67±0.50 | 52.56±5.56 | 4.22±0.40 |
| 0.1nmol | 58.20±18.66 | 1.63±0.63 | 47.78±14.70 | 3.00±0.76 |
|
| ||||
| 0.2nmol | 75.37±12.18 | 3.00±0.47 | 37.90±5.61 | 4.50±0.34 |
3.3. Chase/flight test
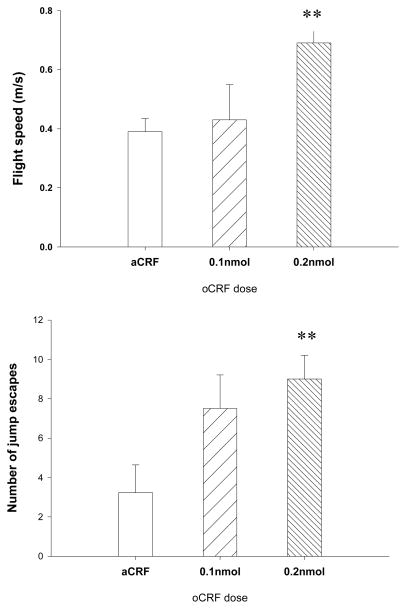
oCRF treatment had significant effect on overall flight speed [F (2, 24) =5.01, p<.05], with the 0.2 nmol group fleeing from the rat faster than aCSF controls (p<.01) (Fig. 3). A significant drug effect was also found for jump escapes [F (2, 24)=4.53, p<.05], and post-hoc Dunnett’s test indicated that the 0.2nmol, but not the 0.1nmol, group, exhibited more jump escapes relative to controls (p<.01)(Fig 3). oCRF had no reliable effect on the number of stops [F (2, 24)=1.80, ns] or reversals [F(2,24)=1.86, ns] (Table 2). The main effect of oCRF on head orientations is significant [F(2,24)=3.90, p<.05]. However, subsequent Dunnett’s test failed to reveal significant dose effect.
Fig. 3.
Effects of oCRF in the Chase/flight test. Upper panel: overall flight speed (m/s); lower panel: number of jump escapes. Data represent mean ± SEM. *p < .05; **p < .01 (Dunnett t test).
Table 2.
Effects of oCRF on risk assessment behaviors in the chase/flight test
| Stops | Head orientations | Reversals | |
|---|---|---|---|
| aCSF | 3.67±0.75 | 1.44±0.48 | 1.56±0.60 |
| 0.1nmol | 5.88±1.08 | 2.00±0.57 | 3.38±0.94 |
|
| |||
| 0.2nmol | 3.80±0.87 | 0.30±0.30 | 3.20±0.66 |
3.4. Closed Alley test
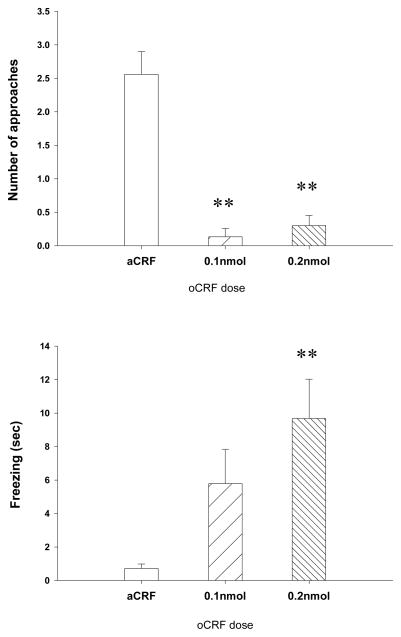
Behavioral responses in the straight alley test are summarized in Fig. 4. Approaches towards the threat stimulus were significantly affected by oCRF infusion [F (2, 24) =35.16, p<.001], with both oCRF groups showing fewer approaches than controls (p<.001 for each). A significant drug effect was also found for freezing [F (2, 24) =6.18, p<.01], with the 0.2nmol dose group exhibiting more robust freezing than controls (p<.01).
Fig. 4.
Effects of oCRF in the Closed Alley test. Data represent mean ± SEM. *p < .05; **p < .01. Upper panel: number of approaches towards the rat stimulus; lower panel: duration of freezing in 30-sec
3.5. Forced contact test
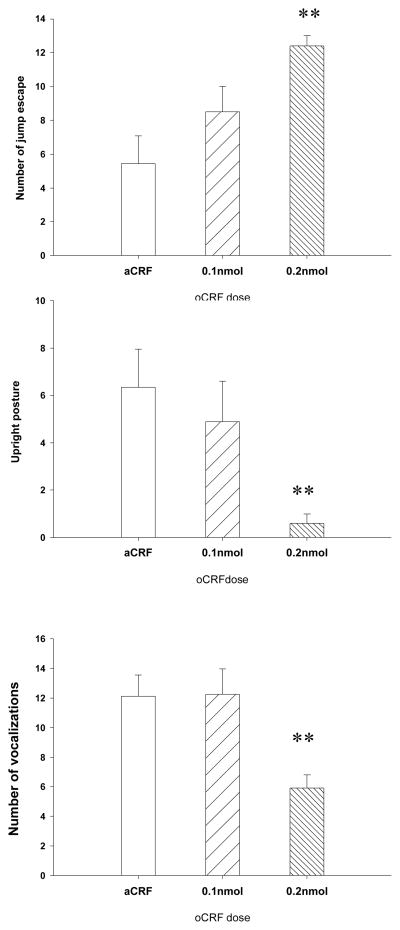
The main effect of drug treatment was significant for the number of jump escapes [F (2, 24) =7.78, p<.01], upright postures [F (2,24)=5.54, p<.01], and vocalizations [F (2, 24)=7.65, p<.01] (Fig. 5). Dunnett’s test detailed that while the 0.1nmol dose had no significant effect on any of these behaviors, the 0.2nmol dose reduced vocalizations (p<.01) and upright postures (p<.01) and increased jump escapes (p<.001). The number of jump attacks did not differ among groups [F (2, 24)=1.63, ns]. Bites were rarely seen and were not statistically analyzed.
Fig. 5.
Effects of oCRF in the Forced Contact test. Data represent mean ± SEM. *p < .05; **p < .01. Upper panel: number of jump escapes; middle panel: number of upright postures; lower panel: number of sonic vocalizations
3.6. Post test
One-way ANOVA revealed a significant main effect of oCRF on posttest line-crossings [F (2, 24)=34.0, p<.001] (Table 3). Subsequent Dunnett’s tests indicated that line-crossings were significantly reduced by both doses of oCRF (p<.001 for each comparison). Escape attempts, a combined measure of rears and jumps, was significantly influenced by oCRF [F (2, 24)=33.6, p<.001], with both oCRF dose groups scoring lower than controls (p<.01 for each).
Table 3.
Effects of oCRF on activities in the posttest
| Line-crossings | Escape attempts | |
|---|---|---|
| aCSF | 161.67±12.88 | 24.90±2.24 |
| 0.1nmol | 38.00±12.12** | 1.63±0.98** |
|
| ||
| 0.2nmol | 44.40±10.73** | 3.10±2.10** |
4. Discussion
4.1. Specific behavioral effects of oCRF differed between subtests of the MDTB, in accord with variations in defensive behaviors elicited in the different subtests
In this study we have evaluated behavioral effects of centrally administered oCRF on various defensive behaviors in CD-1 mice. Behavioral analyses suggested that the relative dominance of specific unconditioned defensive responses of rodents depends on features of the threat and the situation in which it is presented. Clearly manifested and tangible threat stimuli tend to elicit flight when an escape route is available; hiding if a refuge is accessible; and freezing if neither of the above choices is present. As the threat moves closer and contacts the animal, defensive threat (e.g. upright posture, sonic vocalizations) and defensive attack are elicited. These are often followed by abrupt escape attempts. Potential threat stimuli, e.g. predator odor/sound, novel and/or open place, tend to elicit risk assessment behaviors while suppressing locomotor activities [6], [19], and [36]. Besides the commonly recognized stretch attend posture/movement, risk assessment behaviors may also manifest as crouch sniffing [7], vigilant sensory scanning, and approach/investigation [8]. These morphs of risk assessment behaviors permit information-gathering while minimizing the danger of being detected [11], [37]. Finally, stimuli or situations associated with previous encounters with a threat can elicit defensive responses [9]. Relevant to the present study, the subtests of the MDTB are designed to capture responses to (1) novel environment (the pretest), (2) looming/approaching predator (the predator avoidance test), (3) contacting predator when escape is possible, (4) distant threat when escape is blocked and no shelter is available, (5) immediate predator threat in a tightly confined space, and (6) residual emotional responses post threat-removal.
With a few exceptions, oCRF potentiated the dominant defensive behaviors in most subtests of the MDTB. In the pretest, both doses of oCRF markedly suppressed exploratory activity measures and increased risk assessment. It is interesting to find that although oCRF groups mice showed marked inhibition of locomotion (Fig 2A) and enhanced risk assessment relative to controls (Fig 2C), they did exhibited gradual habituation of these behaviors (Fig. 2B and 2C), suggesting that oCRF treated animals are cable of processing sensory inputs efficiently and adjusting behavioral responses accordingly. In the chase/flight test, 0.2nmol of CRF potentiated two focal responses, flight speed and jump escapes, indicating that oCRF enhances predominant defenses without causing motor impairment. In the closed alley test, freezing was reduced by 0.2nmol of oCRF and the number of approaches was lowered by both doses. While control mice spontaneously approached the rat for an average of 2.5 times in the 30 sec trial, oCRF (0.2nmol) mice mostly remained in the far corner and exhibited fairly robust freezing---a pattern similar to that of a wild-derived strain of mice known to be more defensive than laboratory strains [12]. In the forced contact test, the 0.2nmol dose of oCRF produced opposite effects on escape attempts and defensive threats (sonic vocalization and upright posture), i.e. potentiating the former while reducing the latter, suggesting a shift of terminal defense in a way that favors flight over confrontation. Finally, despite having just displayed robust fleeing and jumping, oCRF mice showed strong behavioral inhibition in the posttest, as indicated by 75% fewer line-crossings than controls. Escape attempts, a combined count of rearing and jump escapes, was potently suppressed by oCRF, suggesting a shift to passive coping strategies in the absence of the specific threat object
4.2. Relationship to previous findings
The pretest activity data are in good agreement with the finding that CRF inhibits locomotor activity in novel surroundings [27] and that oCRF suppressed pre-CS baseline activity in a conditioned freezing test [43]. The finding that rearing was also suppressed in the pretest was in accord with a study showing that the same behavior was reduced by i.c.v. infusion of CRF in rats tested in an open field [13]. In the predator avoidance test, oCRF treatment did not have appreciable effects. Previous studies using several peripherally administered CRF1 antagonists failed to reveal consistent effects of CRF1 antagonism on avoidance response in this subtest of the MDTB [9]. It is possible that the avoidance response to slowly approaching predator threat is not particularly sensitive to acute challenge of the CRF system. Flight and jump escapes were potentiated by 0.2nmol of oCRF. Interestingly, a recent study [14] showed that local injection of oCRF into the dorsal periaqueductal gray, at doses up to 0.1 μg, failed to affect flight, suggesting involvement of upper stream neural mechanisms. The oCRF-potentiated freezing observed in the closed alley test was in accord with the finding that i.c.v. injection of this peptide potentiated conditioned freezing in mice [40], [43]. Note that the enhancement of freezing paralleled a clear reduction of approaches towards the rat stimulus. Previously studies reported that benzodiazepine full agonists diazepam and clobazam increase the threat-approaching behavior whereas CRF1 antagonists are ineffective. As such, our finding of reduced approaches could have resulted from enhanced freezing or reflects a heightened anxiety-like state induced by oCRF, or both. The finding that oCRF potently suppressed escape attempts after removal of the threat, together with data of the pretest and the closed alley test, prompts the speculation that passive–coping was the preferable defensive strategy in oCRF treated mice when the threat was not immediate.
Table 4 summarizes effects of the two doses of oCRF used in this study. While the 0.2nmol dose affected behaviors in all but one subtest, the 0.1nmol dose only affected behaviors in the pretest, the closed alley test, and the posttest. This pattern suggests that defensive behaviors in response to low intensity threat are more sensitive to oCRF than those triggered by high intensity threats (i.e. chase/flight, forced contact).
Table 4. Summary of effects of oCRF on defensive behaviors in the MDTB.
Note that while the 0.2nmol dose had significant effects in all but one subtest, the 0.1nmol dose only affected behaviors in those subtests in which the threat intensity is low.
| Subtest | Behaviors | 0.1nmol | 0.2nmol |
|---|---|---|---|
| Pretest | Line crossings | ** | ** |
|
| |||
| Risk assessment | ** | ** | |
|
| |||
| Predator Avoidance | Avoidance | 0 | 0 |
| Escape | 0 | 0 | |
|
| |||
| Chase/Flight | Flight speed | 0 | ** |
| Jump escapes | 0 | ** | |
|
| |||
| Closed Alley | Approaches | ** | ** |
|
| |||
| Freezing | 0 | ** | |
|
| |||
| Forced Contact | Jump escapes | 0 | ** |
|
| |||
| Upright postures | 0 | ** | |
|
| |||
| Vocalizations | 0 | ** | |
|
| |||
| Posttest | Line crossings | ** | ** |
|
| |||
| Escape attempts | ** | ** | |
p<.05;
p<.01; 0 — effect not significant
Finally, it is important to point out that while oCRF has often been considered as a highly selective CRF1 agonist [22], this view has been called into question in a recent study [30] demonstrating that oCRF is only 6.7-fold more selective for CRF1 than CRF2 endogenously expressed in rat olfactory bulb. Functional assays indicated that oCRF is 19-fold [45] to 57-fold [30] more selective to CRF1 in stimulating cAMP accumulation, but nevertheless less selective for CRF1 than the most recently developed CRF1 agonist cortagine [45]. These findings suggest that oCRF does not effectively discriminate CRF1 effects from CRF2 effects in vivo. In particular, i.c.v. injections of oCRF are likely to produce a mixed CRF1/2 activation in the septal region as this area is in close proximity to the ventricular system and exhibits anatomical heterogeneity of CRF receptor expression [17].
5. Conclusion
Effects of centrally administered oCRF on various defensive behaviors in CD-1 mice were evaluated in the present study. Our findings demonstrated that oCRF potentiated defensive behaviors in a situation-specific, dose-related manner, suggesting a general enhancement of defensiveness, rather than effects on specific behaviors.
Fig. 1.
The overhead view of the MDTB apparatus. The two 2-m straight segments of the oval runway are joined by two 0.4-m curved segments and separated by a median wall. The apparatus was elevated 0.8 m.
Acknowledgments
The authors gratefully acknowledge Dr. J. Spiess for his guidance and generous supply of the peptide. This work was supported by NIH grant U5 NS039406.
References
- 1.Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin-releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J Neurosci. 2002;22(7):2926–35. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE, Koob GF, Vale WW, Lee KF. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24(4):410–4. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 3.Bale TL, Vale WW. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. J Neurosci. 2003;23(12):5295–301. doi: 10.1523/JNEUROSCI.23-12-05295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 5.Bittencourt JC, Sawchenko PE. Do centrally administered neuropeptides access cognate receptors?: an analysis in the central corticotropin-releasing factor system. J Neurosci. 2000;20(3):1142–56. doi: 10.1523/JNEUROSCI.20-03-01142.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchard DC, Blanchard RJ, Rodgers RJ. Risk assessment and animal models of anxiety. In: Olivier B, Mos J, Slangen JL, editors. Animal Models in Psychopharmacology. Birkhauser Verlag; Basel: 1991. pp. 117–134. [Google Scholar]
- 7.Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29(8):1243–53. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Blanchard DC, Griebel G, Blanchard RJ. Mouse defensive behaviors: pharmacological and behavioral assays for anxiety and panic. Neurosci Biobehav Rev. 2001;25(3):205–18. doi: 10.1016/s0149-7634(01)00009-4. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard DC, Griebel G, Blanchard RJ. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27(8):1177–1185. doi: 10.1016/j.pnpbp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard DC, Griebel G, Blanchard RJ. The Mouse Defense Test Battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003;463(1–3):97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- 11.Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard RJ, Hebert MA, Ferrari P, Palanza P, Figueira R, Blanchard DC, Parmigiani S. Defensive behaviors in wild and laboratory (Swiss) mice: the mouse defense test battery. Physiol Behav. 1998;65(2):201–9. doi: 10.1016/s0031-9384(98)00012-2. [DOI] [PubMed] [Google Scholar]
- 13.Campbell BM, Morrison JL, Walker EL, Merchant KM. Differential regulation of behavioral, genomic, and neuroendocrine responses by CRF infusions in rats. Pharmacol Biochem Behav. 2004;77(3):447–55. doi: 10.1016/j.pbb.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho-Netto EF, Litvin Y, Blanchard RJ, Blanchard DC. Effects of intra-PAG infusion of ovine CRF (oCRF) on defensive behaviors in Swiss-Webster mice. doi: 10.1016/j.bbr.2006.10.003. in prep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995;15(10):6340–50. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, Hatton DC, Phillips TJ, Finn DA, Low MJ, Rittenberg MB, Stenzel P, Stenzel-Poore MP. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24(4):403–9. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 17.Dautzenberg FM, Py-Lang G, Higelin J, Fischer C, Wright MB, Huber G. Different binding modes of amphibian and human corticotropin-releasing factor type 1 and type 2 receptors: evidence for evolutionary differences. J Pharmacol Exp Ther. 2001;296(1):113–20. [PubMed] [Google Scholar]
- 18.Ducottet C, Griebel G, Belzung C. Effects of the selective nonpeptide corticotropin-releasing factor receptor 1 antagonist antalarmin in the chronic mild stress model of depression in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(4):625–31. doi: 10.1016/S0278-5846(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 19.Griebel G, Belzung C, Misslin R, Vogel E. The free-exploratory paradigm: an effective method for measuring neophobic behaviour in mice and testing potential neophobia-reducing drugs. Behav Pharmacol. 1993;4(6):637–644. [PubMed] [Google Scholar]
- 20.Griebel G, Moindrot N, Aliaga C, Simiand J, Soubrie P. Characterization of the profile of neurokinin-2 and neurotensin receptor antagonists in the mouse defense test battery. Neurosci Biobehav Rev. 2001;25(7–8):619–26. doi: 10.1016/s0149-7634(01)00045-8. [DOI] [PubMed] [Google Scholar]
- 21.Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, Geslin M, Scatton B, Maffrand JP, Soubrie P. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylphenyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301(1):333–45. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- 22.Grigoriadis DE, Liu XJ, Vaughn J, Palmer SF, True CD, Vale WW, Ling N, De Souza EB. 125I-Tyro-sauvagine: a novel high affinity radioligand for the pharmacological and biochemical study of human corticotropin-releasing factor 2 alpha receptors. Mol Pharmacol. 1996;50(3):679–86. [PubMed] [Google Scholar]
- 23.Gutman DA, Owens MJ, Skelton KH, Thrivikraman KV, Nemeroff CB. The corticotropin-releasing factor1 receptor antagonist R121919 attenuates the behavioral and endocrine responses to stress. J Pharmacol Exp Ther. 2003;304(2):874–80. doi: 10.1124/jpet.102.042788. [DOI] [PubMed] [Google Scholar]
- 24.Habib KE, Gold PW, Chrousos GP. Neuroendocrinology of stress. Endocrinol Metab Clin North Am. 2001;30(3):695–728. vii–viii. doi: 10.1016/s0889-8529(05)70208-5. [DOI] [PubMed] [Google Scholar]
- 25.Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003 Feb 1;23(3):1019–25. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current status of the nomenclature for receptors for corticotropin-releasing factor and their ligands. Pharmacol Rev. 2003;55(1):21–6. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 27.Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004;311(2):427–40. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 28.Heinrichs SC, Lapsansky J, Lovenberg TW, De Souza EB, Chalmers DT. Corticotropin-releasing factor CRF1, but not CRF2, receptors mediate anxiogenic-like behavior. Regul Pept. 1997;71(1):15–21. doi: 10.1016/s0167-0115(97)01005-7. [DOI] [PubMed] [Google Scholar]
- 29.Ho SP, Takahashi LK, Livanov V, Spencer K, Lesher T, Maciag C, Smith MA, Rohrbach KW, Hartig PR, Arneric SP. Attenuation of fear conditioning by antisense inhibition of brain corticotropin releasing factor-2 receptor. Brain Res Mol Brain Res. 2001;89(1–2):29–40. doi: 10.1016/s0169-328x(01)00050-x. [DOI] [PubMed] [Google Scholar]
- 30.Hoare SR, Sullivan SK, Fan J, Khongsaly K, Grigoriadis DE. Peptide ligand binding properties of the corticotropin-releasing factor (CRF) type 2 receptor: pharmacology of endogenously expressed receptors, G-protein-coupling sensitivity and determinants of CRF2 receptor selectivity. Peptides. 2005;26(3):457–70. doi: 10.1016/j.peptides.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Keck ME, Ohl F, Holsboer F, Muller MB. Listening to mutant mice: a spotlight on the role of CRF/CRF receptor systems in affective disorders. Neurosci Biobehav Rev. 2005;29(4–5):867–89. doi: 10.1016/j.neubiorev.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F, Hermanson O, Rosenfeld MG, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24(4):415–9. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J Neurosci. 2002;22(3):991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M, Holsboer F, Montkowski A. Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regul Pept. 1995;59(2):229–39. doi: 10.1016/0167-0115(95)00099-w. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Yu B, Neugebauer V, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J Neurosci. 2004;24(16):4020–9. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parmigiani S, Palanza P, Rogers J, Ferrari PF. Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci Biobehav Rev. 1999;23(7):957–69. doi: 10.1016/s0149-7634(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 37.Pinel JPJ, Mana MJ. Adaptive interactions of rats with dangerous inanimate objects: support for a cognitive theory of defensive behavior. In: Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S, editors. Ethoexperimental approaches to the study of behavior. Kluwer Academic Publishing; Dordrecht: 1989. pp. 137–151. [Google Scholar]
- 38.Preil J, Muller MB, Gesing A, Reul JM, Sillaber I, van Gaalen MM, Landgrebe J, Holsboer F, Stenzel-Poore M, Wurst W. Regulation of the hypothalamic-pituitary-adrenocortical system in mice deficient for CRH receptors 1 and 2. Endocrinology. 2001 Nov;142(11):4946–55. doi: 10.1210/endo.142.11.8507. [DOI] [PubMed] [Google Scholar]
- 39.Primus RJ, Yevich E, Baltazar C, Gallager DW. Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology. 1997;17(5):308–16. doi: 10.1016/S0893-133X(97)00071-7. [DOI] [PubMed] [Google Scholar]
- 40.Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19(12):5016–25. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radulovic J, Sydow S, Spiess J. Characterization of native corticotropin-releasing factor receptor type 1 (CRFR1) in the rat and mouse central nervous system. J Neurosci Res. 1998;54(4):507–21. doi: 10.1002/(SICI)1097-4547(19981115)54:4<507::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro-Barbosa ER, Canteras NS, Cezario AF, Blanchard RJ, Blanchard DC. An alternative experimental procedure for studying predator-related defensive responses. Neurosci Biobehav Rev. 2005;29(8):1255–63. doi: 10.1016/j.neubiorev.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Stiedl O, Meyer M, Jahn O, Ogren SO, Spiess J. Corticotropin-releasing factor receptor 1 and central heart rate regulation in mice during expression of conditioned fear. J Pharmacol Exp Ther. 2005;312(3):905–16. doi: 10.1124/jpet.104.075820. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi LK. Role of CRF(1) and CRF(2) receptors in fear and anxiety. Neurosci Biobehav Rev. 2001;25(7–8):627–36. doi: 10.1016/s0149-7634(01)00046-x. [DOI] [PubMed] [Google Scholar]
- 45.Tezval H, Jahn O, Todorovic C, Sasse A, Eckart K, Spiess J. Cortagine, a specific agonist of corticotropin-releasing factor receptor subtype 1, is anxiogenic and antidepressive in the mouse model. Proc Natl Acad Sci U S A. 2004;101(25):9468–73. doi: 10.1073/pnas.0403159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19(2):162–6. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 47.Todorovic C, Jahn O, Tezval H, Hippel C, Spiess J. The role of CRF receptors in anxiety and depression: Implications of the novel CRF(1) agonist cortagine. Neurosci Biobehav Rev. 2005 doi: 10.1016/j.neubiorev.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003;980(2):206–12. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- 49.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–7. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 50.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428(2):191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]