Abstract
BACKGROUND
Comorbidity, disability, and polypharmacy commonly complicate the care of patients with heart failure. These factors can change biological response to therapy, reduce patient ability to adhere to recommendations, and alter patient preference for treatment and outcome. Yet, a comprehensive understanding of the complexity of patients with heart failure is lacking. Our objective was to assess trends in demographics, comorbidity, physical function, and medication use in a nationally representative, community-based heart failure population.
METHODS
Using data from the National Health and Nutrition Examination Survey, we analyzed trends across 3 survey periods (1988–1994, 1999–2002, 2003–2008).
RESULTS
We identified 1395 participants with self-reported heart failure (n = 581 in 1988–1994, n = 280 in 1999–2002, n = 534 in 2003–2008). The proportion of patients with heart failure who were ≥80 years old increased from 13.3% in 1988–1994 to 22.4% in 2003–2008 (P <.01). The proportion of patients with heart failure who had 5 or more comorbid chronic conditions increased from 42.1% to 58.0% (P <.01). The mean number of prescription medications increased from 4.1 to 6.4 prescriptions (P <.01). The prevalence of disability did not increase but was substantial across all years.
CONCLUSION
The phenotype of patients with heart failure changed substantially over the last 2 decades. Most notably, more recent patients have a higher percentage of very old individuals, and the number of comorbidities and medications increased markedly. Functional disability is prevalent, although it has not changed. These changes suggest a need for new research and practice strategies that accommodate the increasing complexity of this population.
There is growing concern that clinical practice guidelines fail to adequately address the care of complex patients.1–3 Patients with heart failure are particularly complex. They are generally older,4,5 and their care is commonly complicated by comorbidity, disability, and polypharmacy. These factors can change biological response to therapy,6 reduce patient ability to adhere to recommendations,7 and alter patient preference for treatment and outcome. Yet, a comprehensive understanding of the complexity of patients with heart failure is lacking.
Existing studies characterizing patients with heart failure have had various limitations. Hospital-based studies examine patients in the acute setting and cannot be generalized to the community.8–14 Population-based studies have focused mainly on risk factors and survival.4,5,15–21 No national survey has comprehensively characterized the complexity of patients with heart failure or determined whether the complexity is changing over time.
To assess the complexity of patients with heart failure, we analyzed data from the National Health and Nutrition Examination Survey (NHANES). Our objective was to describe trends in demographics, comorbidity, physical function, and medication use among adults with heart failure in nationally representative, community-based samples over time. We hypothesize that the proportion of patients who are elderly is substantial and growing, and the prevalence of comorbidity, disability, and polypharmacy is large and increasing.
MATERIALS AND METHODS
Data Source
We used data from NHANES III (1988 to 1994) and NHANES 1999 to 2008. The plan and operation of NHANES has been described in detail elsewhere.22,23 Briefly, the NHANES are cross-sectional, multistage probability sample surveys conducted by the National Center for Health Statistics. Participants undergo both interview and clinical examination. With the use of sampling weights, each survey produces statistics that are representative of the noninstitutionalized, civilian US population.
Beginning in 1999, NHANES transitioned from being a periodic survey to a continuous survey with data release every 2 years. For our analysis we combined 1999–2000 and 2001–2002 data releases into a 1999–2002 cohort. We similarly combined data releases to create a 2003–2008 cohort.
Study Sample
From an overall 25,679 NHANES participants aged ≥40 years who underwent examination, we excluded 20 participants who were pregnant and 133 participants with missing data regarding the presence of heart failure. Participants were identified as having heart failure if they answered “yes” to the interview question “Has a doctor or other health professional ever told you that you had congestive heart failure?”
Measurements
Trends were assessed for variables in 4 general domains: demographics, comorbidity, physical function, and medication use. Demographic variables included age, sex, race/ethnicity, education, and socioeconomic status. Participants that were eligible for federal food assistance programs, based on income and household size, were categorized as having low socioeconomic status.24,25
We evaluated trends in the prevalence of several comorbidities common in older patients and in patients with heart failure.26,27 The presence of myocardial infarction, angina, stroke, diabetes (excluding gestational diabetes only), asthma, chronic obstructive pulmonary disease, arthritis, cancer, thyroid disease, and osteoporosis were determined by self-report. Analysis of the subsample of participants who underwent fasting blood draw showed substantial agreement between self-reported diabetes and diabetes based on medication use and fasting blood glucose levels (κ = .79).28 Participants were considered to have high cholesterol if they self-reported that they were taking medication for cholesterol or if their total cholesterol was ≥240 mg/dL.29 Participants were considered to have hypertension if their mean systolic blood pressure was ≥140 mm Hg, their mean diastolic blood pressure was ≥90 mm Hg, or they self-reported that they were taking medication for high blood pressure. According to World Health Organization standards, anemia was defined as having a hemoglobin level of <12 g/dL for women or <13 g/dL for men,30 and obesity was defined as body mass index ≥30 kg/m2.31 Waist circumference was assessed to supplement body mass index as a measure of obesity. As recommended by the National Institutes of Heath, high-risk waist circumference was defined as ≥88 cm and ≥102 cm for women and men, respectively.32 Kidney function was determined using serum creatinine and the abbreviated Modification of Diet in Renal Disease formula to calculate glomerular filtration rates.33 Kidney disease was defined as a glomerular filtration rate <60 mL/min per 1.73 m2, which corresponds to clinically significant, stage 3 kidney disease as set forth by the National Kidney Foundation.34
A composite indicator of disease burden was calculated by tallying the number of chronic conditions for each patient. The following conditions were included in this composite measure: myocardial infarction, asthma, diabetes, chronic obstructive pulmonary disease, arthritis, cancer, thyroid disease, osteoporosis, hypercholesterolemia, hypertension, anemia, obesity, and kidney disease. Stroke, an acute event that may not result in chronic deficits, was not included. Angina was not included because data were unavailable for years 1988–1994. Although myocardial infarction is not a chronic condition, it was included as a proxy for coronary artery disease. In summing the number of chronic conditions, conditions with missing values were treated as if the condition were not present; 11% of participants had one or more chronic conditions with missing values.
Two measures of disability and one measure of impairment were used to assess function: activities of daily living disability, mobility disability, and vision impairment. Participants were considered to have activities of daily living disability if they reported having much difficulty with or being unable to perform any of the following 3 tasks: dressing, eating, or getting in and out of bed. Participants were considered to have mobility disability if they reported having much difficulty with or being unable to walk 2 to 3 blocks or walk up 10 steps. Participants were considered vision impaired if they were unable to read ordinary newspaper print even with the help of contacts/glasses. Disability measures were assessed only for participants aged ≥60 years.
Medication use was ascertained during home interviews. NHANES participants were asked to list names and dosages of all current medications and to show available medication containers for verification. NHANES classified medications according to Lexicon Plus, a proprietary database of Cerner Multum, Inc. (Denver, Colo).35 In calculating the total number of prescription medications, medications taken for <2 weeks were excluded.
Statistical Analysis
We analyzed trends across time periods (1988–1994, 1999–2002, 2003–2008). All analyses were performed using SAS-callable SUDAAN statistical software (Research Triangle Institute, Research Triangle Park, NC).36 Data were weighted according to NHANES analytical guidelines. The weighting methodology adjusts for bias that may arise from total nonresponse to examination.24 Prevalence and mean estimates were age-adjusted by the direct method to the 2003–2008 heart failure population based on 40–59, 60–69, 70–79, and 80+ years age groups. Linear trends across time periods were determined based on the method of Fisher and Yates;36 the test for linear trend is the same as the comparison of first and last survey periods. In accordance with NHANES analytical guidelines, estimates with a coefficient of variation >0.30 were considered unreliable.24 To account for multiple comparisons, we used a cutoff of α = 0.01.
RESULTS
Demographics
We identified 1395 participants with heart failure (n = 581 in 1988–1994, n = 280 in 1999–2002, n = 534 in 2003–2008). The mean age was 67.7 years. The proportion ≥80 years old significantly increased from 13% in 1988–1994 to 22% in 2003–2008 (Table 1). Overall, 49% of participants with heart failure were female and 76% were non-Hispanic white. These figures were consistent over time. A large proportion of patients with heart failure are poorly educated, living in poverty, or living alone. In 2003–2008, 36% had not completed high school, 23% had low socioeconomic status, and 26% were living alone.
Table 1.
Trends in Demographics among Patients with Heart Failure
| Variable | 1988–1994 (n = 581) | 1999–2002 (n = 280) | 2003–2008 (n = 534) | Change, 1988–2008 (95% CI) | P Value |
|---|---|---|---|---|---|
| Age-specific data | |||||
| Age (years) | 67.4 (0.7) | 67.3 (1.1) | 68.4 (0.6) | 1.0 (−0.7–2.8) | .24 |
| Age group | |||||
| 40–59 | 23.7 (3.4) | 31.3 (3.9) | 23.1 (2.3) | 0.5 (−8.8–7.8) | .90 |
| 60–69 | 28.9 (2.5) | 17.0 (2.7) | 28.2 (2.7) | 0.7 (−8.0–6.6) | .85 |
| 70–79 | 34.1 (2.7) | 30.9 (3.2) | 26.2 (2.1) | −7.9 (−14.5-−1.2) | .02 |
| 80+ | 13.3 (1.7) | 20.8 (3.6) | 22.4 (2.4) | 9.1 (3.4–14.8) | .002 |
| Age-adjusted data | |||||
| Demographics | |||||
| Female | 51.4 (3.2) | 48.8 (3.6) | 45.6 (2.5) | −5.7 (−14.3–2.8) | .18 |
| Non-Hispanic white | 73.5 (2.9) | 75.8 (4.2) | 76.6 (2.5) | 3.1 (−4.9–11.0) | .44 |
| < High school education | 53.8 (2.7) | 47.7 (3.4) | 36.1 (3.1) | −17.7 (−26.1-−9.3) | <.001 |
| Low socioeconomic statusa | 28.3 (2.9) | 38.9 (4.9) | 22.6 (2.3) | −5.7 (−13.0–1.6) | .12 |
| Lives alone | 25.4 (1.9) | 27.6 (2.5) | 25.8 (2.2) | 0.4 (−5.5–6.4) | .89 |
| Ever smoker | 62.2 (2.9) | 59.0 (2.8) | 63.0 (2.7) | 0.8 (−7.0–9.3) | .86 |
| Current smoker | 21.1 (2.6) | 18.5 (3.6) | 17.3 (1.6) | −3.7 (−9.5–2.1) | .20 |
CI = confidence interval.
All variables are in weighted % or mean (standard error).
See “Materials and Methods” for definition.
Comorbid Conditions
Comorbidity was common. In 2003–2008, more than half of patients with heart failure had hypertension (73%), hypercholesterolemia (54%), or arthritis (62%) (Table 2). Nearly half had a history of myocardial infarction (48%) or were obese (47%).
Table 2.
Trends in Comorbid Conditions among Patients with Heart Failure
| Variable | 1988–1994 (n = 581) | 1999–2002 (n = 280) | 2003–2008 (n = 534) | Change, 1988–2008 (95% CI) | P Value |
|---|---|---|---|---|---|
| MI | 58.8 (3.3) | 46.5 (3.3) | 47.7 (3.1) | −11.2 (−20.3-−2.0) | .02 |
| Angina | NAb | 38.7 (3.8) | 26.9 (2.7) | −11.9 (−12.1-−2.6)c | .01c |
| Stroke | 17.6 (2.1) | 16.6 (2.6) | 19.4 (2.4) | 1.8 (−4.8–8.4) | .59 |
| Diabetesa | 24.7 (2.7) | 29.3 (2.9) | 38.3 (2.3) | 13.6 (6.4–20.7) | <.001 |
| Blood pressure (BP) | |||||
| Hypertensiona | 67.8 (2.5) | 71.2 (3.7) | 73.3 (2.2) | 5.5 (−1.1–12.1) | .10 |
| Systolic BP (mm Hg) | 141.7 (1.1) | 131.7 (1.8) | 130.6 (1.3) | −11.2 (−14.5-−7.8) | <.001 |
| Diastolic BP (mm Hg) | 73.1 (1.1) | 66.9 (1.5) | 65.9 (0.7) | −7.2 (−9.9-−4.5) | <.001 |
| Cholesterol | |||||
| Hypercholesterolemiaa | 41.3 (3.0) | 49.3 (3.9) | 53.5 (2.8) | 12.2 (5.0–19.4) | .001 |
| LDL cholesterold (mg/dL) | 139.2 (3.1) | 116.7 (5.1) | 105.3 (4.6) | −33.9 (−44.9-−22.8) | <.001 |
| HDL cholesterol <40 mg/dL | 35.1 (2.9) | 34.0 (3.8) | 25.8 (2.2) | −9.2 (−16.6-−1.8) | .02 |
| Obesity | |||||
| Obesitya | 32.8 (2.5) | 40.3 (4.7) | 46.8 (2.5) | 13.9 (7.5–20.4) | <.001 |
| BMI (kg/m2) | 28.3 (0.3) | 30.0 (0.8) | 31.1 (0.4) | 2.8 (1.8–3.7) | <.001 |
| Waist circumference (cm) | |||||
| Women | 95.9 (1.2) | 102.6 (1.9) | 105.5 (1.2) | 9.6 (6.1–12.1) | <.001 |
| Men | 102.0 (1.0) | 105.1 (1.2) | 109.2 (1.4) | 7.2 (3.7–10.7) | <.001 |
| High-risk waist circumferencea | 57.6 (3.4) | 73.0 (2.9) | 75.7 (2.7) | 18.0 (8.5–27.6) | <.001 |
| Kidney function | |||||
| Kidney diseasea | 34.7 (2.9) | 41.2 (3.6) | 45.9 (2.1) | 11.2 (4.2–18.3) | .002 |
| Creatinine (mg/dL) | 1.10 (0.06) | 1.29 (0.09) | 1.23 (0.04) | 0.13 (0.00–0.26) | .06 |
| Creatinine >2 mg/dL | 4.6 (1.8) | 6.9 (1.8) | 7.4 (1.0) | 2.8 (−1.2–6.7) | .17 |
| Anemiaa | 15.3 (2.7) | 15.0 (1.8) | 22.2 (2.7) | 6.9 (−0.6–14.5) | .07 |
| Asthma | 18.0 (2.5) | 16.8 (2.7) | 24.6 (1.9) | 6.6 (0.3–12.9) | .04 |
| Arthritis | 60.4 (2.9) | 68.5 (3.3) | 62.0 (2.4) | 1.6 (−5.4–8.6) | .65 |
| COPD | 26.2 (2.8) | 26.8 (2.9) | 30.9 (2.3) | 4.8 (−2.5–12.1) | .20 |
| Cancer | 21.5 (2.5) | 29.3 (2.8) | 24.4 (2.0) | 2.9 (−3.6–9.4) | .38 |
| Thyroid disease | 9.9 (1.5) | 15.2 (2.8) | 22.9 (1.9) | 13.0 (8.3–17.7) | .001 |
| Osteoporosis | 4.9 (1.2) | 13.6 (3.1) | 16.4 (2.5) | 11.5 (5.9–17.1) | .001 |
BMI = body mass index; CI = confidence interval; COPD = chronic obstructive pulmonary disease; HDL = high-density lipoprotein; LDL = low-density lipoprotein; MI = myocardial infarction.
All variables are in weighted % or mean (standard error).
See “Materials and Methods” for definitions.
Data not available because data collection methods differed substantially from methods in subsequent years.
Comparison is for 1999–2002 vs 2003–2008.
Calculated for fasting subsample only (n = 271 in 1988–1994, n = 105 in 1999–2002, n = 136 in 2003–2008).
Comorbidity was not only common, but also grew increasingly prevalent over the last decades. The age-adjusted proportion of patients with heart failure who had 5 or more comorbid chronic conditions increased from 42% in 1988–1994 to 58% in 2003–2008 (Figure). The main conditions driving this increase included hypercholesterolemia (41% to 54%), diabetes (25% to 38%), obesity (33% to 47%), kidney disease (35% to 46%), thyroid disease (10% to 23%), and osteoporosis (5% to 16%) (Table 2).
Figure.
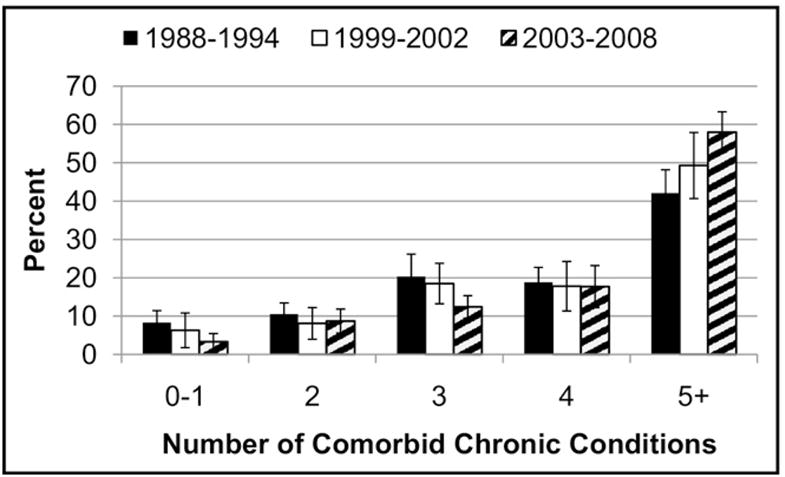
Trends in number of comorbid chronic conditions among patients with heart failure.
The prevalence of myocardial infarction and angina trended downward, but these changes were not statistically significant. There was improved control of blood pressure and lipid levels.
Disability and Impairment
Age-adjusted levels of disability did not change over time, but were high throughout all periods. In 2003–2008, 57% of patients ≥60 years old with heart failure were mobility disabled; 11% were activities of daily living disabled; and 12% were vision impaired (Table 3).
Table 3.
Trends in Disability and Impairment among Patients with Heart Failure
| Variable | 1988–1994 (n = 480) | 1999–2002 (n = 224) | 2003–2008 (n = 441) | Change, 1988–2008 (95% CI) | P Value |
|---|---|---|---|---|---|
| Mobility disableda | 52.6 (3.2) | 57.6 (4.2) | 56.9 (2.8) | 4.3 (−4.1–12.7) | .31 |
| Activities of daily living disableda | 12.5 (1.9) | 13.8 (2.1) | 11.1 (1.5) | −1.4 (−6.2–3.4) | .57 |
| Vision impaireda | 11.8 (1.7) | 11.1 (2.8) | 11.7 (1.8) | −0.1 (−4.9–4.7) | .97 |
CI = confidence interval.
All variables are in weighted % (standard error).
See “Materials and Methods” for definitions.
Medication Use
Table 4 shows trends in medication use, with drugs listed in order of decreasing frequency of use in 2003–2008. Prescription drug use in patients with heart failure increased from a mean of 4.1 prescriptions to 6.4 prescriptions. In 2003–2008, the highest decile managed more than 11 prescriptions. Although the use of digoxin and calcium channel blockers decreased, most medications experienced a sharp increase in use, including cardiovascular and noncardiovascular drugs. Potassium supplementation and aspirin use did not change significantly over time but were quite common, 20% and 28%, respectively, in the period 2003–2008.
Table 4.
Trends in Prescription Medication Use among Patients with Heart Failure
| Variable | 1988–1994 (n = 581) | 1999–2002 (n = 280) | 2003–2008 (n = 534) | Change, 1988–2008 (95% CI) | P Value |
|---|---|---|---|---|---|
| No. of prescription medications | 4.1 (0.2) | 5.1 (0.3) | 6.4 (0.2) | 2.4 (1.8–2.9) | <.001 |
| Cardiovascular medications | |||||
| Diuretic | 44.8 (2.9) | 51.2 (3.6) | 57.6 (2.3) | 12.8 (5.7–19.9) | <.001 |
| ACE or ARB | 24.0 (2.7) | 47.1 (3.5) | 55.0 (2.6) | 31.1 (23.9–38.3) | <.001 |
| β-blocker | 15.4 (2.2) | 36.0 (4.5) | 51.8 (2.5) | 36.3 (30.1–42.6) | <.001 |
| HMG-CoA reductase inhibitors | 6.4 (2.1) | 36.0 (3.8) | 45.5 (2.7) | 39.1 (32.3–45.9) | <.001 |
| Aspirin | 34.4 (2.8) | 36.5 (3.2) | 28.3 (3.1)a | −6.0 (−13.9–1.9) | .13 |
| Calcium channel blocker | 38.8 (3.3) | 28.1 (4.5) | 22.9 (2.0) | −15.9 (−23.3-−8.4) | <.001 |
| Potassium chloride | 15.9 (2.3) | 19.5 (2.7) | 19.5 (2.3) | 3.6 (−3.0–10.2) | .28 |
| Warfarin | 9.7 (1.7) | 20.6 (2.7) | 17.9 (2.1) | 8.2 (2.9–13.6) | .003 |
| Digoxin | 30.0 (2.9) | 20.0 (1.7) | 14.7 (2.1) | −15.3 (−22.2-−8.4) | <.001 |
| Antianginal agent | 30.7 (2.6) | 24.3 (2.4) | 12.5 (1.4) | −18.2 (−24.0-−12.3) | <.001 |
| Antiarrhythmic agent | 11.9 (2.1) | 2.5 (1.2)b | 5.7 (1.0) | −6.1 (−10.7-−1.6) | .009 |
| Noncardiovascular medications | |||||
| Antidiabetic agent | 18.2 (2.2) | 24.5 (3.1) | 31.1 (2.2) | 12.9 (6.6–19.3) | <.001 |
| PPI or H2 Antagonist | 13.8 (2.0) | 23.0 (2.7) | 32.3 (3.3) | 18.5 (10.7–26.2) | <.001 |
| Levothyroxine | 4.8 (1.4) | 10.8 (2.2) | 18.2 (1.8) | 13.5 (9.2–17.7) | <.001 |
| Bronchodilator | 11.1 (2.4) | 9.3 (2.2) | 14.2 (1.7) | 3.1 (−1.9–8.1) | .22 |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; CI = confidence interval; HMG-CoA = 3-hydroxy-3-methylglutaryl-coenzyme A; PPI = proton pump inhibitor.
All variables are in weighted % or mean (standard error).
Data available only for 2003–2004.
Statistically unreliable.
DISCUSSION
Patients with heart failure are remarkably complex. Specifically, in a nationally representative sample, patients with heart failure are very old with high rates of comorbidity, disability, and polypharmacy. Furthermore, not only are patients complex, but, even after adjusting for age, they have become increasingly complex over the last decades. In 2003–2008, nearly a quarter of community-dwelling patients with heart failure were ≥80 years old; more than half had 5 or more comorbid chronic conditions; more than half were mobility disabled; and on average, a patient with heart failure managed more than 6 prescription medications.
Our study furthers previous research by comprehensively and representatively describing the complexity of patients with heart failure. In a nationally representative sample, we report on demographics, comorbidity, disability, and polypharmacy. In describing comorbidity and polypharmacy, we included a broad range of cardiac and noncardiac conditions. Prior studies have focused primarily on cardiovascular conditions.
We found similar or higher rates of comorbidity than previously have been reported. Compared with a study by Braunstein et al,27 which used inpatient and outpatient Medicare data from 1999, we found (in 1999–2002) higher rates of hypercholesterolemia (49% vs 21%), kidney disease (38% vs 11%), arthritis (68% vs 16%), and osteoporosis (14% vs 5%). Compared with 1997–2002 data from a population-based study from Olmsted County,16 we found (in 1999–2002) higher rates of diabetes (29% vs 19%) and obesity (40% vs 30%). Differences in data type and patient demographics may explain some of these differences. Whereas Braunstein et al27 and the Olmsted County study16 used administrative data (medical records or medical claims) to determine comorbidity, we used a combination of self-report and standardized examination. Administrative data may underreport comorbidity as not all patient conditions are recorded at each encounter. Differences in patient demographics also could explain our higher estimates of comorbidity. Our study population was comparatively young. Whereas most prior studies have been limited to patients ≥65 years old and have included patients in nursing homes, our study looked at noninstitutionalized subjects ≥40 years old. Younger patients with heart failure have higher rates of coronary artery disease, hypercholesterolemia, diabetes, and chronic obstructive pulmonary disease.13,37
Our findings corroborate prior studies, both community-based and hospital-based, which have shown that rates of diabetes and obesity have increased in patients with heart failure.9,12,16,18 A strength of NHANES is that it allowed trending of various other complicating conditions. We found significant increases in the rates of hypercholesterolemia, kidney disease, thyroid disease, and osteoporosis. To our knowledge, we are the first to clearly examine long-term trends in noncardiac conditions in patients with heart failure.
To our knowledge, we also are the first to examine long-term trends in the use of noncardiovascular medications in patients with heart failure. Over the last decades, the use of antidiabetic agents, levothyroxine, and proton pump inhibitors has significantly increased. Regarding trends in the use of cardiovascular medications, our findings are in line with previous studies.8,11,14,38 There has been increased use of drugs with demonstrated survival advantage (angiotensin-converting enzyme inhibitors and beta-blockers) and decreased use of drugs with demonstrated lack of survival advantage (digoxin, calcium channel blockers, and anti-arrhythmics). Previously, Masoudi et al14 described an increase in overall prescription medication use from 6.8 prescriptions in 1998–1999 to 7.5 in 2000–2001 in recently hospitalized Medicare beneficiaries with heart failure. Current findings show that this trend holds true over a longer time period and in the outpatient setting.
In addition to polypharmacy and comorbidity, we were interested in describing the prevalence of disability in patients with heart failure. In the community setting, rates of disability did not change over time, but were nevertheless substantial throughout all study periods. Our ability to detect change in disability may have been limited because our measurement only captured severe disability in noninstitutionalized subjects. It is difficult to compare our findings with prior studies39,40 because of differences in measure definitions.
The growing complexity that we have demonstrated suggests a need to fundamentally change the way we research and care for patients with heart failure. The proportion of patients with heart failure who are very elderly is increasing. This figure will likely continue to increase as the elderly are expected to comprise a growing and substantial portion of the US population.41 Yet, the very elderly are inadequately studied in clinical trials. They are often under-enrolled, and those that are enrolled tend to have less comorbidity.42 Furthermore, analyses rarely evaluate safety and effectiveness in the very elderly separately, although evidence suggests that they experience higher rates of adverse drug events and may have distinct pathophysiology.43,44 Our study emphasizes the need for future clinical trials to evaluate safety and effectiveness in older patients and in patients with multiple comorbidities.
Our study also emphasizes the need for physicians to be careful and judicious when caring for patients with heart failure. With such high prevalence of comorbidity, disability, and polypharmacy, physicians need to be mindful of possible adverse drug events and nonadherence. Nonadherence could be a particularly salient issue given that a large proportion of patients with heart failure are poorly educated, have low socioeconomic status, or live alone. As patients with heart failure grow increasingly complex, physicians will need to better their ability to prioritize treatment recommendations based on relative benefits and harms and in the context of patient preferences.
Limitations
A significant limitation of our study is that the presence of heart failure and several comorbid conditions were determined by self-report. Self-report—a measure that reflects a combination of true prevalence, diagnosis, and patient awareness—can lead to both overestimation and underestimation. Prior studies have shown that agreement between self-report and clinical diagnosis varies by disease. Asthma and chronic obstructive pulmonary disease are commonly over-reported; thyroid disease and cancer are often under-reported; diabetes and heart disease are relatively accurately reported.45–47 A prior NHANES study showed that self-report underestimates the presence of heart failure as compared with identification by clinical characteristics.48
Our study has other limitations. First, even though our data come from large national surveys, our sample sizes are limited because the prevalence of heart failure is low. As a result, we had power to detect only large trends. Second, because our data come from cross-sectional surveys, we could not comment as to whether comorbid conditions preceded or followed the diagnosis of heart failure.
CONCLUSION
The phenotype of patients with heart failure has changed substantially over the last 2 decades. Most notably, more recent patients have a higher percentage of very old individuals, and the number of comorbidities and medications increased markedly. Severe functional disability is prevalent, although it has not changed. These changes suggest a need for new research and practice strategies that accommodate the increasing complexity of this population.
CLINICAL SIGNIFICANCE.
Patients with heart failure have grown substantially more complex over the last 2 decades.
In a nationally representative community-dwelling sample, the proportion that was very old (aged ≥80 years) increased 2-fold.
Several comorbid conditions— hypercholesterolemia, diabetes, obesity, kidney disease, thyroid disease, and osteoporosis— became significantly more prevalent.
Mean prescription drug use increased more than 50%.
Acknowledgments
Funding: Catherine Wong is a research fellow supported by Sarnoff Cardiovascular Research Foundation. Dr. Chaudhry is supported by a K23/Beeson Career Development Award from the National Institute on Aging and the American Federation for Aging Research. Dr. Krumholz is supported by grant 1R01 HL081153-03 from the National Heart, Lung, and Blood Institute, and grant 1R01 HS016929-02 from the Agency for Healthcare Research and Quality and the United Health Foundation. We did not receive any additional funding, including pharmaceutical industry funds, for the preparation of this manuscript or any related research.
The authors wish to thank Dr. Elena Kuklina from the Centers for Disease Control and Prevention for biostatistical guidance.
Footnotes
This manuscript was presented at The Sarnoff Cardiovascular Research Foundation Annual Scientific Meeting in Washington, DC on May 1, 2010.
Conflict of Interest: All authors report no conflicts of interest.
Authorship: Catherine Wong was affiliated with Yale University as a Sarnoff Cardiovascular Research Fellow during the time the work was conducted. All authors had access to the data and a role in writing the manuscript.
References
- 1.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351(27):2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 2.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor PJ. Adding value to evidence-based clinical guidelines. JAMA. 2005;294(6):741–743. doi: 10.1001/jama.294.6.741. [DOI] [PubMed] [Google Scholar]
- 4.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347(18):1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 6.Gurwitz JH, Field TS, Judge J, et al. The incidence of adverse drug events in two large academic long-term care facilities. Am J Med. 2005;118(3):251–258. doi: 10.1016/j.amjmed.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Briesacher BA, Gurwitz JH, Soumerai SB. Patients at-risk for cost-related medication nonadherence: a review of the literature. J Gen Intern Med. 2007;22(6):864–871. doi: 10.1007/s11606-007-0180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonarow GC, Heywood JT, Heidenreich PA, et al. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2007;153(6):1021–1028. doi: 10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Kosiborod M, Lichtman JH, Heidenreich PA, et al. National trends in outcomes among elderly patients with heart failure. Am J Med. 2006;119(7):616.e1–616.e7. doi: 10.1016/j.amjmed.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Baker DW, Einstadter D, Thomas C, Cebul RD. Mortality trends for 23,505 Medicare patients hospitalized with heart failure in Northeast Ohio, 1991 to 1997. Am Heart J. 2003;146(2):258–264. doi: 10.1016/S0002-8703(02)94784-8. [DOI] [PubMed] [Google Scholar]
- 11.Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970–1974 and 1990–1994. Circulation. 2006;113(6):799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 12.Polanczyk CA, Rohde LE, Dec GW, DiSalvo T. Ten-year trends in hospital care for congestive heart failure: improved outcomes and increased use of resources. Arch Intern Med. 2000;160(3):325–332. doi: 10.1001/archinte.160.3.325. [DOI] [PubMed] [Google Scholar]
- 13.Havranek EP, Masoudi FA, Westfall KA, et al. Spectrum of heart failure in older patients: results from the National Heart Failure project. Am Heart J. 2002;143(3):412–417. doi: 10.1067/mhj.2002.120773. [DOI] [PubMed] [Google Scholar]
- 14.Masoudi FA, Baillie CA, Wang Y, et al. The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998–2001. Arch Intern Med. 2005;165(18):2069–2076. doi: 10.1001/archinte.165.18.2069. [DOI] [PubMed] [Google Scholar]
- 15.McCullough PA, Philbin EF, Spertus JA, et al. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39(1):60–69. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 16.Dunlay SM, Weston SA, Jacobsen SJ, Roger VL. Risk factors for heart failure: a population-based case-control study. Am J Med. 2009;122(11):1023–1028. doi: 10.1016/j.amjmed.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunlay SM, Weston SA, Redfield MM, et al. Anemia and heart failure: a community study. Am J Med. 2008;121(8):726–732. doi: 10.1016/j.amjmed.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.From AM, Leibson CL, Bursi F, et al. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006;119(7):591–599. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 20.Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: trends in incidence and survival in a 10-year period. Arch Intern Med. 1999;159(1):29–34. doi: 10.1001/archinte.159.1.29. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Ho K, Thom T. Changing epidemiological features of cardiac failure. Br Heart J. 1994;72(2 Suppl):S3–S9. doi: 10.1136/hrt.72.2_suppl.s3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat. 1994;1(32):1–407. [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. [Accessed March 4, 2010.];National Health and Nutrition Examination Survey. Available at: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 24.Centers for Disease Control and Prevention. [Accessed March 12, 2010.];National Health and Nutrition Examination Survey Analytic Guidelines. Available at: http://www.cdc.gov/nchs/nhanes/nhanes3/nh3gui.pdf.
- 25.United States Department of Agriculture. [Accessed March 12, 2010.];Food and Nutrition Service. Available at: http://www.fns.usda.gov/fsp/government/FY10_Income_Standards.htm.
- 26.Anderson G, Horvath J. The growing burden of chronic disease in America. Public Health Rep. 2004;119(3):263–270. doi: 10.1016/j.phr.2004.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42(7):1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 28.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 29.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 30.World Health Organization (WHO) Nutritional anemias: report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:1. [PubMed] [Google Scholar]
- 31.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 32.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 33.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139(2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 35.Cerner Multum, Inc. [Accessed March 12, 2010.];Lexicon. 2007 Available at: http://www.multum.com/Lexicon.htm.
- 36.Research Triangle Institute. SUDAAN Language Manual, Release 10.0. Research Triangle Park, NC: Research Triangle Institute; 2008. [Google Scholar]
- 37.Bouchard JL, Aurigemma GP, Goldberg RJ, et al. Heart failure in the “oldest old”: clinical and echocardiographic insights. Am J Geriatr Cardiol. 2007;16(4):236–242. doi: 10.1111/j.1076-7460.2007.06211.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee DS, Mamdani MM, Austin PC, et al. Trends in heart failure outcomes and pharmacotherapy: 1992 to 2000. Am J Med. 2004;116(9):581–589. doi: 10.1016/j.amjmed.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 39.Zhang JX, Rathouz PJ, Chin MH. Comorbidity and the concentration of healthcare expenditures in older patients with heart failure. J Am Geriatr Soc. 2003;51(4):476–482. doi: 10.1046/j.1532-5415.2003.51155.x. [DOI] [PubMed] [Google Scholar]
- 40.Gure TR, Kabeto MU, Blaum CS, Langa KM. Degree of disability and patterns of caregiving among older Americans with congestive heart failure. J Gen Intern Med. 2008;23(1):70–76. doi: 10.1007/s11606-007-0456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foot DK, Lewis RP, Pearson TA, Beller GA. Demographics and cardiology, 1950–2050. J Am Coll Cardiol. 2000;35(5 Suppl B):66B–80B. [PubMed] [Google Scholar]
- 42.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162(15):1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 43.Duncan AK, Vittone J, Fleming KC, Smith HC. Cardiovascular disease in elderly patients. Mayo Clin Proc. 1996;71(2):184–196. doi: 10.4065/71.2.184. [DOI] [PubMed] [Google Scholar]
- 44.Masoudi FA, Gross CP, Wang Y, et al. Adoption of spironolactone therapy for older patients with heart failure and left ventricular systolic dysfunction in the United States, 1998–2001. Circulation. 2005;112(1):39–47. doi: 10.1161/CIRCULATIONAHA.104.527549. [DOI] [PubMed] [Google Scholar]
- 45.Desai MM, Bruce ML, Desai RA, Druss BG. Validity of self-reported cancer history: a comparison of health interview data and cancer registry records. Am J Epidemiol. 2001;153(3):299–306. doi: 10.1093/aje/153.3.299. [DOI] [PubMed] [Google Scholar]
- 46.Harlow SD, Linet MS. Agreement between questionnaire data and medical records. The evidence for accuracy of recall. Am J Epidemiol. 1989;129(2):233–248. doi: 10.1093/oxfordjournals.aje.a115129. [DOI] [PubMed] [Google Scholar]
- 47.Okura Y, Urban LH, Mahoney DW, et al. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20(2):301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]


