Abstract
Amyloid β (Aβ) peptides are proteolytic products from amyloid precursor protein (APP) and are thought to play a role in Alzheimer disease (AD) pathogenesis. While much is known about molecular mechanisms underlying cerebral Aβ accumulation in familial AD, less is known about the cause(s) of brain amyloidosis in sporadic disease. Animal and postmortem studies suggest that Aβ secretion can be up-regulated in response to hypoxia. We employed a new technology (Single Molecule Arrays, SiMoA) capable of ultrasensitive protein measurements and developed a novel assay to look for changes in serum Aβ42 concentration in 25 resuscitated patients with severe hypoxia due to cardiac arrest. After a lag period of 10 or more hours, very clear serum Aβ42 elevations were observed in all patients. Elevations ranged from approximately 80% to over 70-fold, with most elevations in the range of 3–10-fold (average approximately 7-fold). The magnitude of the increase correlated with clinical outcome. These data provide the first direct evidence in living humans that ischemia acutely increases Aβ levels in blood. The results point to the possibility that hypoxia may play a role in the amyloidogenic process of AD.
Introduction
Amyloid β (Aβ) peptides are produced by many cell types in the body but the expression is particularly high in the brain. Accumulation of Aβ in the form of extracellular plaques is a neuropathological hallmark of Alzheimer disease (AD) and thought to play a central role in the neurodegenerative process [1]. In familial AD, brain amyloidosis is caused by mutations in the genes encoding amyloid precursor protein (APP) or γ-secretase; the enzyme complex that together with β-secretase is responsible for producing Aβ from APP [2]. However, very little is known about what drives Aβ accumulation in the brains of patients with sporadic AD.
Mounting evidence suggest that cerebral hypoxia may play a role in the pathogenesis of sporadic AD. Epidemiological data show that cerebrovascular risk factors, such as hypertension, hyperhomocysteinemia and hypercholesterolemia, increase the risk of AD [3], [4], [5], [6]. Mild transient brain hypoperfusion in AD transgenic mice results in an acute increase in Aβ secretion through induced β-secretase (BACE1) protein expression [7], which is regulated by hypoxia inducible factor 1-α (HIF1-α) [8]. In a similar manner, bilateral carotid occlusion in rats results in time-dependent accumulation of oligomeric Aβ in the hippocampus [9]. In humans, a marked increase in Aβ expression, peaking at 4 days post-stroke, is found in pyramidal neurons in the hippocampus of patients who died from ischemic stroke [10]. Finally, a neuropathological study shows that in patients resuscitated after cardiac arrest who died 3–36 days later, APP over-expression is found in cortical and subcortical neurons together with non-fibrillar Aβ plaques in the neuropil [11].
To further elucidate the link between hypoxia and Aβ production in living humans, we employed a new technology (Single Molecule Arrays, SiMoA) capable of ultrasensitive protein measurements [12], [13] to look for changes in serum Aβ42 concentration in serial blood samples from patients following cardiac arrest and resuscitation.
Methods
Ethics statement
The study was performed at the general intensive care unit at Uppsala University Hospital, Sweden, and approved by the Human Ethics Committee of Uppsala, Sweden. Written consent for participation was obtained from a legal next of kin, and later from survivors, when considered competent.
Patients
Twenty-five unconscious patients (8 women, 17 men, age range 25–85 years, mean 62 years) with cardiac arrest were resuscitated with restoration of spontaneous circulation (ROSC). Patients exhibited systolic blood pressure >80 mmHg after ROSC and a Glasgow Coma Scale ≤7. Hypothermia treatment to a body temperature of 32–34°C for 24 hours, ventilation, and pharmacologic support were administered immediately after resuscitation as described [14]. All patients received an arterial line in the radial or femoral artery for blood sampling. Serial blood samples were collected, starting as soon as possible in the emergency phase (within 6 h after cardiac arrest), and continuing at 1, 2, 6, 12, 24, 48, 72, 96, and 108 h after cardiac arrest. Serum aliquots were frozen at −70°C until analysis.
Patient outcome was assessed using the Glasgow-Pittsburgh cerebral performance category (CPC) scale at discharge from the intensive care unit and 6 months later [15]. The CPC scale ranges from 1 to 5, with 1 representing mildest possible neurological deficit (patient is able to return to work), and 4–5 representing the most severe deficit (vegetative) and death. A CPC of 1 or 2 was considered a good outcome and a CPC score of 3–5 a poor outcome, as described [14]. For patients who died after discharge from the ICU, the better of the two scores was used, as recommended by the Utstein templates [16].
Arrays
Patient serum samples were measured in triplicate by a novel SiMoA Aβ42 assay. The technique involves performing a paramagnetic bead-based ELISA, followed by isolation of individual capture beads in arrays of femtoliter-sized reaction wells [13]. Fifty femtoliter reaction wells were prepared as described [12]. The ends of bundles of 50,000 optical fibers were polished, and one end of each bundle was etched in mild acid solution. Differential etch rates of the optical fiber core and cladding glass of the bundles caused 4.5 µm diameter, 3.5 µm deep wells to be formed, giving arrays of microwells across the bundles. Arrays were mounted in linear groups of eight within glass holders, spaced to correspond with microtiter plate well columns that served as rinse troughs following bead loading.
Reagents
Paramagnetic carboxy beads (2.7 µm, Varian) were coated with a commercially available monoclonal (Covance 6E10) directed to the N-terminus of Aβ42, and diluted to 5×106 beads/mL in Tris-BSA. Biotinylated detector was a commercially available monoclonal (Invitrogen H31L21) directed to the C-terminus, diluted to 0.1 µg/ml in PBS with newborn calf serum, NCS (PBS/NCS). Reporter streptavidin:β-galactosidase (SβG) was a conjugate of purified streptavidin (Thermo Scientific) and βG (Sigma), diluted to 25 pM in PBS/NCS with 1 mM MgCl2.
ELISA and imaging
Bead-sample incubations and immunocomplex labeling were conducted in conical 96 well plates (Axygen) in three steps, starting with analyte capture, incubation with biotinylated detector, and labeling with SβG. Beads from the ELISA were loaded into microwells by centrifugation at 1,300 g for 10 minutes. Wells containing beads with labeled Aβ42 were visualized by the hydrolysis of enzyme substrate (resorufin β-D-galactopyranoside, RGP, Invitrogen) by βG into fluorescent product. RGP was introduced to the wells during array sealing just prior to imaging. Enzyme-containing wells were imaged by fluorescence microscope fitted with a CCD camera. The images were analyzed to determine the average number of label enzymes/bead (AEB) [13].
Statistics
Aβ42 levels and changes were compared between outcome groups using unpaired t-tests in GraphPad Prism 5 (GraphPad Software Inc., La Jolla, USA).
Results
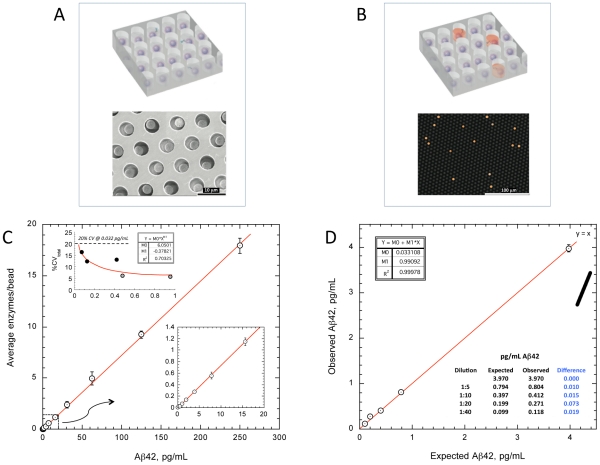
Singulation of capture beads within microwells permits buildup of fluorescent product from a single enzyme label, making the signal from a single immunocomplex readily detectable (Figure 1A–B). The novel Aβ42 assay has a dynamic range to 250 pg/mL (Figure 1C), with limit of quantification of less than 0.04 pg/mL, enabling precise measurement of low abundance serum Aβ42 in all samples. Precise quantification of peripheral Aβ42 to subpicogram levels has not been previously possible with conventional assay methods. The extreme sensitivity of the assay also permits pre-dilution of the samples, reducing endogenous interferences that have been problematic with assays for this peptide (Figure 1D).
Figure 1. Assay characteristics.
Arrays of femtoliter-volume wells permit isolation of 2.7 mm capture beads from a standard bead-based ELISA (A), enabling exquisite sensitivity to enzyme label by preventing fluorescent product from diffusing away into a bulk solution. At very low concentrations of Aβ42, beads contain either a single labeled immunocomplex or no complexes, giving rise to digital signal output corresponding to single molecules (B). Simultaneous counting of active wells across an array statistically powers estimates of average enzymes/bead. (C) Dose-response of digital immunoassay for Aβ42 (n = 3). Y-axis refers to average number of enzyme labels per individual microbead captured in the array. The concentration of label is reduced relative to standard immunoassays, resulting in improved signal∶background at very low Aβ42 concentration. Assay calibrators were purified Aβ42 (Merck) in PBS/BSA. (C inset) Limit of quantification (LoQ) was estimated from total coefficients of variation (CV) from five low panel members (spiked PBS/BSA panels, grey circles, and immunodepleted plasma, black circles) assayed repeatedly across five days. The Aβ42 concentration at which total assay imprecision reached 20% (LoQ) was 0.032 pg/mL. (D) Recovery at extremely low Aβ42 concentrations was tested by diluting spiked immunodepleted plasma with PBS/BSA zero calibrator (n = 3). Because samples are pre-diluted 4-fold prior to assay to reduce matrix effects, dilutions include an initial 4-fold dilution.
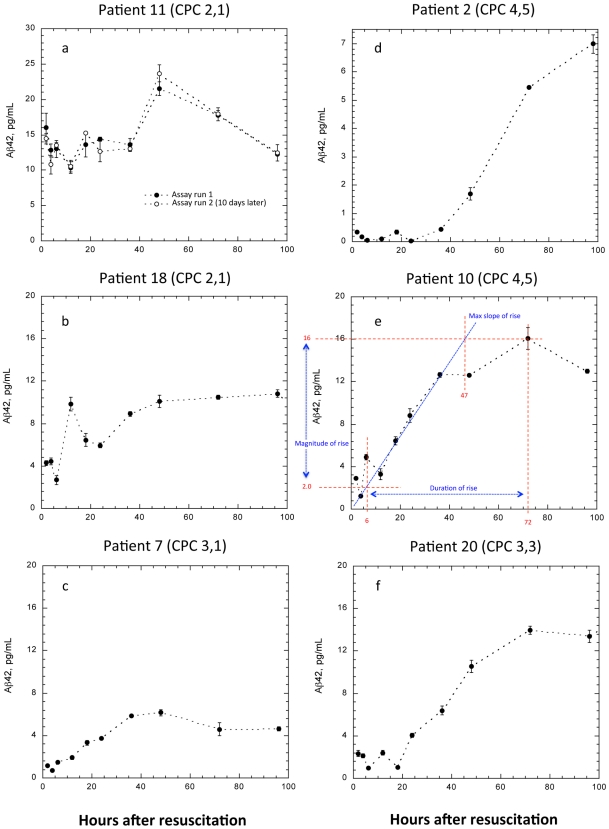
Figure 2 depicts representative elevation profiles for patients with good and poor 6-month outcomes. Profile parameters were: magnitude of Aβ42 rise, ratio of the peak to baseline Aβ42, slope of Aβ42 rise, and the duration over which the Aβ42 exhibited rising values (Figure 2E). The sum (Aβ42 score) of these parameters was also examined (Table 1). All patients were found to exhibit a significant time-dependent elevation of serum Aβ42. Generally, after a lag period of 10 or more hours, very clear Aβ42 elevations were observed. Elevations ranged from approximately 80% to over 70-fold, with most elevations in the range of 3–10-fold (average approximately 7-fold).
Figure 2. Serum Aβ42 following resuscitation from cardiac arrest.
CPC scores depicted are after discharge from the ICU and 6 months later. Panels on left (A–C) are profiles from patients exhibiting good outcomes, panels on the right (D–F) are from patients with poor outcome. (E) Illustration of Aβ42 profile analysis. Baseline Aβ42 was defined as the mean of the two lowest values in the initial 12 hours. The time of initial elevation was defined as the intersection between the baseline Aβ42 and the line of maximum ascension of the major elevation peak. The duration of the Aβ42 increase was defined as the difference between time of initial elevation and time beyond which no significant further rise was observed. The magnitude and maximum slope of rise are also indicated. (A) Patient exhibiting smallest relative increase in Aβ42 among all patients. For confirmation, the sample set was re-assayed on a different day. (D) Patient with poor outcome exhibiting the largest relative increase from baseline. (C and F) Patients with similar baseline Aβ42 and poor cerebral outcome upon discharge from the ICU. Six months later, patient BE had recovered good cerebral function, while patient LP had not. Error bars: standard deviation of triplicate measurements.
Table 1. Characteristics of serum Aβ42 elevation profiles from resuscitated survivors of cardiac arrest during the first 96–108 hours following admission to the intensive care unit.
| Patient | Magnitude of rise (pg/mL) | Duration of rise (hr) | Rise ratios (fold increase) | Max slope (pg/mL/hr) | Aβ42 score | |||||
| Good | Poor | Good | Poor | Good | Poor | Good | Poor | Good | Poor | |
| 1 | 17.4 | 4.10 | 0.621 | |||||||
| 2 | 6.90 | 60 | 70.0 | 0.150 | 137 | |||||
| 3 | 11.3 | 28 | 4.10 | 0.315 | 43.7 | |||||
| 4 | 11.9 | 25 | 4.30 | 0.466 | 41.7 | |||||
| 5 | 4.60 | 63 | 5.60 | 0.242 | 73.4 | |||||
| 6 | 8.60 | 78 | 9.60 | 0.110 | 96.3 | |||||
| 7 | 5.07 | 32 | 6.45 | 0.159 | 43.7 | |||||
| 8 | 7.10 | 21 | 2.45 | 0.592 | 31.1 | |||||
| 9 | 9.20 | 14 | 6.75 | 0.657 | 30.6 | |||||
| 10 | 14.0 | 66 | 8.00 | 0.340 | 88.3 | |||||
| 11 | 9.50 | 15 | 1.81 | 0.633 | 26.9 | |||||
| 12 | 26.7 | 18 | 5.45 | 1.48 | 51.6 | |||||
| 13 | 6.50 | 28 | 14.0 | 0.232 | 48.7 | |||||
| 14 | 11.0 | 55 | 6.50 | 0.202 | 72.7 | |||||
| 15 | 11.0 | 31 | 4.90 | 0.360 | 47.3 | |||||
| 16 | 13.3 | 3.00 | 0.887 | |||||||
| 17 | 7.20 | 13 | 3.13 | 0.554 | 23.9 | |||||
| 18 | 6.90 | 31 | 2.97 | 0.276 | 41.1 | |||||
| 19 | 8.80 | 3.20 | 0.587 | |||||||
| 20 | 12.3 | 56 | 8.70 | 0.273 | 77.3 | |||||
| 21 | 9.50 | 72 | 16.8 | 0.132 | 98.4 | |||||
| 22 | 7.10 | 31.7 | 0.372 | |||||||
| 23 | 14.7 | 33 | 8.00 | 0.445 | 56.1 | |||||
| 24 | 7.90 | 61 | 4.30 | 0.282 | 73.5 | |||||
| 25 | 7.20 | 72 | 15.4 | 0.141 | 94.7 | |||||
| Mean | 8.96 | 11.1 | 27.2 | 54.6 | 5.49 | 13.1 | 0.385 | 0.444 | 42.0 | 79.8 |
| P value | 0.137 | 0.00091 | 0.095 | 0.322 | 0.00056 | |||||
Parameters were sorted on the basis of good or poor 6-month outcome. Blood sampling was terminated early for patients 1, 16, 19 and 22 for medical reasons, and rise durations could not be estimated for these patients. The Aβ42 score equals the sum of magnitude of rise, duration of rise, rise ratio and max slope.
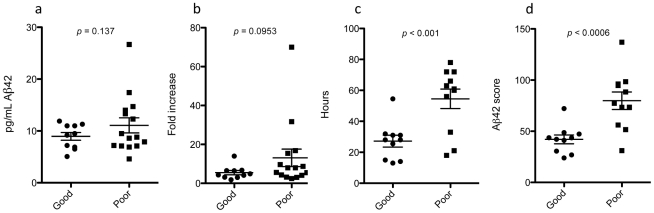
Aβ42 profile characteristics for each patient are given in Table 1, and graphed in Figure 3. There was no relationship between baseline Aβ42 and outcome (p = 0.53). Large increases in Aβ42, as reflected by rise magnitudes and ratios, exhibited marginal association with poor outcome (student t-test p = 0.137, 0.095 respectively), while a long duration of rise was associated with poor outcome (p<0.001). Slope and total area under the curve (AUC) were not significant (p = 0.32, 0.16 respectively, later not shown). Weak association between AUC and outcome could be due to incomplete expression of the major elevation peak over the sampling interval in many cases (e.g., Figure 2D). Arbitrarily summing the profile parameters further enhanced discrimination (p<0.0006), giving 90% positive and negative predictive values for 6-month outcome using a cut point of 50. Age and gender did not influence the results (p>0.35 for all comparisons).
Figure 3. Features of Aβ42 elevation profiles were compared with 6-month overall cerebral outcome.
(A) Magnitude of Aβ42 rise; (B) ratio of maximum to baseline Aβ42; (C) duration of major Aβ42 rise; (D) sum of A to C plus maximum slope of Aβ42 rise. Error bars are the standard error of the means.
Discussion
To our knowledge, these data are the first to link hypoxia with acute elevations in serum Aβ42 levels in living humans. These data corroborate earlier results from animal models, which all suggest a direct relationship between hypoxia and up-regulated amyloidogenic APP-processing [7], [8], [9]. While we make no claim of the observed changes being brain-specific, the data show that circulatory failure with hypoxia dramatically increases Aβ42 levels in blood. Induced Aβ42 production due to hypoxia appears to be the most likely explanation for the results, but increased release from cerebral tissues due to blood-brain barrier breakdown or reduced clearance of the peptide cannot be excluded. The magnitude of the increase is associated with clinical outcome, which in turn probably reflects the severity of the ischemic episode.
A limitation of the study is the lack of a control group. However, earlier results show that the day-to-day variability in Aβ42 levels normally is very low [17]. Moreover, the association of the SiMoA Aβ42 results with clinical outcome suggests a direct relationship between the ischemic episode and Aβ42 levels which is not secondary to the standardized treatment all patients received.
Although there are many differences between acute hypoxia due to cardiac arrest and age-related changes in the cerebrovasculature, it is tempting to speculate that hypoxia induced by arteriolosclerosis and/or lipohyalinosis may play a role in AD pathogenesis by a direct effect on Aβ levels in the brain. On top of this, lowering of pH in hypoxic tissues would further stimulate Aβ oligomerization and aggregation as readily observed in the test tube [18], [19], [20]. A direct effect of hypoxia on Aβ production may provide a putative mechanism for the mounting evidence from epidemiological studies linking cerebrovascular risk factors with AD [3], [4], [5], [6]. It may also help to explain the recently described association of sleep apnea-induced hypoxia with dementia [21].
The data indicate that the SiMoA Aβ42 assay represents a breakthrough in analytical capability for extremely sensitive and reproducible Aβ42 measurement in serum and plasma. The assay allows highly sensitive (limit of quantification 0.032 pg/mL) and precise quantification of Aβ42 in biological fluids. Current immunoassays for measurement of Aβ42 in plasma are based on the ELISA [22], [23], [24] or the Luminex [25] techniques. In addition to lower analytical sensitivity, these immunoassays are hampered by low recovery of spiked Aβ42 into plasma or serum, and as a result the measured concentration of Aβ42 increases when the plasma sample is diluted [25]. These phenomena are due to matrix effects, probably by interaction and binding of Aβ42 to plasma proteins. This means that unrelated plasma proteins contribute to the concentration interpreted to come from Aβ42 in this type of assay. The immunoassay presented here showed highly linear dilution characteristics, together with a recovery of around 100%, suggesting that it gives a very accurate measure of the Aβ42 concentration. This is likely because the interference from plasma proteins is lost when samples are pre-diluted 1∶4 prior to measurement. Because the assay has such high sensitivity, pre-diluting the samples does not compromise the capability of the method to precisely measure low abundance Aβ42. A highly accurate and specific assay for measurement of Aβ42 in blood will be of major importance both for pathogenic studies on AD disease mechanisms, and to monitor an effect on Aβ metabolism of novel therapeutic compounds in clinical trials, a hypothesis that will be tested in future studies.
Acknowledgments
We thank Rita Persson for skillful technical assistance.
Footnotes
Competing Interests: The authors have read the journal's policy and have the following conflicts: Drs. Song, Chang, Provuncher, Patel, Ferrell, Fournier, Kan, Campbell, Meyer, Rivnak, Pink, Minnehan, Piech, Rissin, Duffy and Wilson are employees at Quanterix Corporation. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials. There are no relevant patents and products in relation to this. The other authors have no competing interests.
Funding: This study was supported by grants from the Swedish Research Council (K2010-63P-21562-01-4, K2011-61X-20401-05-6), the Söderberg Foundation, Alzheimer's Association and Swedish State Support for Clinical Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Hooshmand B, Solomon A, Kareholt I, Leiviska J, Rusanen M, et al. Homocysteine and holotranscobalamin and the risk of Alzheimer disease: a longitudinal study. Neurology. 2010;75:1408–1414. doi: 10.1212/WNL.0b013e3181f88162. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Wang YJ, Zhang M, Xu ZQ, Gao CY, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76:1485–1491. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- 5.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer's and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo X, Pantoni L, Simoni M, Bengtsson C, Bjorkelund C, et al. Blood pressure components and changes in relation to white matter lesions: a 32-year prospective population study. Hypertension. 2009;54:57–62. doi: 10.1161/HYPERTENSIONAHA.109.129700. [DOI] [PubMed] [Google Scholar]
- 7.Koike MA, Green KN, Blurton-Jones M, Laferla FM. Oligemic hypoperfusion differentially affects tau and amyloid-{beta}. Am J Pathol. 2010;177:300–310. doi: 10.2353/ajpath.2010.090750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, et al. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: role of oxidative stress and HIF1alpha. J Neurochem. 2009;108:1045–1056. doi: 10.1111/j.1471-4159.2008.05858.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Xing A, Xu C, Cai Q, Liu H, et al. Cerebrovascular hypoperfusion induces spatial memory impairment, synaptic changes, and amyloid-beta oligomerization in rats. J Alzheimers Dis. 2010;21:813–822. doi: 10.3233/JAD-2010-100216. [DOI] [PubMed] [Google Scholar]
- 10.Qi JP, Wu H, Yang Y, Wang DD, Chen YX, et al. Cerebral ischemia and Alzheimer's disease: the expression of amyloid-beta and apolipoprotein E in human hippocampus. J Alzheimers Dis. 2007;12:335–341. doi: 10.3233/jad-2007-12406. [DOI] [PubMed] [Google Scholar]
- 11.Wisniewski HM, Maslinska D. Beta-protein immunoreactivity in the human brain after cardiac arrest. Folia Neuropathol. 1996;34:65–71. [PubMed] [Google Scholar]
- 12.Rissin DM, Fournier DR, Piech T, Kan CW, Campbell TG, et al. Simultaneous detection of single molecules and singulated ensembles of molecules enables immunoassays with broad dynamic range. Anal Chem. 2011;83:2279–2285. doi: 10.1021/ac103161b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rissin DM, Kan CW, Campbell TG, Howes SC, Fournier DR, et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010;28:595–599. doi: 10.1038/nbt.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortberg E, Zetterberg H, Nordmark J, Blennow K, Rosengren L, et al. S-100B is superior to NSE, BDNF and GFAP in predicting outcome of resuscitation from cardiac arrest with hypothermia treatment. Resuscitation. 2011;82:26–31. doi: 10.1016/j.resuscitation.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 16.Cummins RO, Chamberlain D, Hazinski MF, Nadkarni V, Kloeck W, et al. Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: the in-hospital ‘Utstein style’. A statement for healthcare professionals from the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Australian Resuscitation Council, and the Resuscitation Councils of Southern Africa. Resuscitation. 1997;34:151–183. doi: 10.1016/s0300-9572(97)01112-x. [DOI] [PubMed] [Google Scholar]
- 17.Bjerke M, Portelius E, Minthon L, Wallin A, Anckarsater H, et al. Confounding factors influencing amyloid Beta concentration in cerebrospinal fluid. Int J Alzheimers Dis 2010. 2010;pii:986310. doi: 10.4061/2010/986310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkitadze MD, Condron MM, Teplow DB. Identification and characterization of key kinetic intermediates in amyloid beta-protein fibrillogenesis. J Mol Biol. 2001;312:1103–1119. doi: 10.1006/jmbi.2001.4970. [DOI] [PubMed] [Google Scholar]
- 19.Guo M, Gorman PM, Rico M, Chakrabartty A, Laurents DV. Charge substitution shows that repulsive electrostatic interactions impede the oligomerization of Alzheimer amyloid peptides. FEBS Lett. 2005;579:3574–3578. doi: 10.1016/j.febslet.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 20.LeVine H., 3rd Thioflavine T interaction with synthetic Alzheimer's disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. Jama. 2011;306:613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okereke OI, Xia W, Irizarry MC, Sun X, Qiu WQ, et al. Performance characteristics of plasma amyloid-beta 40 and 42 assays. J Alzheimers Dis. 2009;16:277–285. doi: 10.3233/JAD-2009-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanderstichele H, Van Kerschaver E, Hesse C, Davidsson P, Buyse MA, et al. Standardization of measurement of beta-amyloid(1–42) in cerebrospinal fluid and plasma. Amyloid. 2000;7:245–258. doi: 10.3109/13506120009146438. [DOI] [PubMed] [Google Scholar]
- 24.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, et al. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 25.Hansson O, Zetterberg H, Vanmechelen E, Vanderstichele H, Andreasson U, et al. Evaluation of plasma Abeta(40) and Abeta(42) as predictors of conversion to Alzheimer's disease in patients with mild cognitive impairment. Neurobiol Aging. 2010;31:357–367. doi: 10.1016/j.neurobiolaging.2008.03.027. [DOI] [PubMed] [Google Scholar]