Abstract
Background and Objective
Blood vessel invasion plays a very important role in the progression and metastasis of cancer. However, blood vessel invasion as a prognostic factor for survival in non-small cell lung cancer (NSCLC) remains controversial. The aim of this study is to explore the relationship between blood vessel invasion and outcome in patients with NSCLC using meta-analysis.
Methods
A meta-analysis of published studies was conducted to investigate the effects of blood vessel invasion on both relapse-free survival (RFS) and overall survival (OS) for patients with NSCLC. Hazard ratios (HRs) with 95% confidence intervals (95% CIs) were used to assess the strength of this association.
Results
A total of 16,535 patients from 52 eligible studies were included in the systematic review and meta-analysis. In total, blood vessel invasion was detected in 29.8% (median; range from 6.2% to 77.0%) of patients with NSCLC. The univariate and multivariate estimates for RFS were 3.28 (95% CI: 2.14–5.05; P<0.0001) and 3.98 (95% CI: 2.24–7.06; P<0.0001), respectively. For the analyses of blood vessel invasion and OS, the pooled HR estimate was 2.22 (95% CI: 1.93–2.56; P<0.0001) by univariate analysis and 1.90 (95% CI: 1.65–2.19; P<0.0001) by multivariate analysis. Furthermore, in stage I NSCLC patients, the meta-risk for recurrence (HR = 6.93, 95% CI: 4.23–11.37, P<0.0001) and death (HR = 2.15, 95% CI: 1.68–2.75; P<0.0001) remained highly significant by multivariate analysis.
Conclusions
This study shows that blood vessel invasion appears to be an independent negative prognosticator in surgically managed NSCLC. However, adequately designed large prospective studies and investigations are warranted to confirm the present findings.
Introduction
Non-small cell lung cancer (NSCLC) constitutes approximately 80% of all cases of primary lung cancers and is one of the most common tumors worldwide [1]. Although surgical resection is the current standard of treatment for early-stage patients, only 15% of patients diagnosed with NSCLC survive for 5 years [2]. Prognostic factors may identify subsets of patients with a worse prognosis and facilitate the selection of a more aggressive treatment strategy. The tumor-node-metastasis (TNM) staging system is the best prognostic index for operable NSCLC [2]. However, each patient's prognosis varies significantly within each TNM stage, which makes it difficult to predict accurately the outcome for each patient, especially for patients with early-stage lung cancer.
The degree of histological differentiation, extent of operation, visceral pleural invasion, and many biological factors involving in cancer development and progression are useful for predicting survival and aiding the management of patients with NSCLC [3]–[5]. Our previous meta-analysis concluded that the methylation of RASSF1A within its promoter region could serve as an independent prognostic marker for NSCLC [6]. In the latest edition (7th) of the TNM classification, tumor size is evaluated in detail, with visceral pleural invasion clearly defined; T1 tumors are still classified as T2 if the visceral pleura elastic layer is invaded [1]. In testicular germ cell tumors, cancer cell invasion of blood vessels qualifies as a local spread of tumors. Testicular tumors localized to the testis and epididymis are classified as T1 when blood vessel invasion (BVI) is absent, whereas they are upgraded to T2 if vascular invasion is present [7].
In 1992, Macchiarini et al. first demonstrated that NSCLC patients harboring BVI have a significant tendency to relapse in the first 5-year period after surgery and would be suitable for adjuvant chemotherapy [8]. In the last decade, BVI has also been reported to be a strong predictor of recurrence [9]–[19] or death for patients with NSCLC in many studies [8]–[10], [12], [16], [17], [20]–[47], but has not been confirmed in other studies [48]–[55]. Based on the discordant results obtained by a large number of studies on lung cancer, we performed a literature-based systematic review to better quantify the prognostic effects of BVI on both relapse-free survival (RFS) and overall survival (OS) in patients with NSCLC.
Materials and Methods
Publication selection, data extraction and methodological assessment
Studies were identified via a search of the electronic databases PubMed (National Library of Medicine, Bethesda, USA) and EMBASE (Elsevier, Amsterdam, the Netherlands) between 1980 and 2011 using the following key words: non-small cell lung cancer, NSCLC, vessel invasion, vascular invasion, relapse, recurrence, prognostic and prognosis (last search was updated on July 10, 2011). To be eligible for inclusion, studies had to meet the following criteria: (i) blood vessel invasion (not lymphovascular invasion) was measured in surgically resected primary tumor samples, and (ii) the relationship between BVI and survival was investigated, and the results were published as a full paper.
Abstracts, reviews and case reports were excluded because of insufficient data for meta-analysis. Non-English language papers were not included in the review. To avoid duplication of patient data, we carefully noted the author names and the research centers involved for all authors. If more than one publication reported the same population data, only the most recently reported data or complete data were used. We also performed a manual search from the references of relevant publications, including original articles and reviews, to identify additional studies. Three authors (J. Wang, J. Chen and X. Chen) did the search and identification independently using a standardized approach, and the selection of a study was reached by consensus. Information retrieved from the reports included author names, year of publication, patient resources, study size, methods, histology, and disease stage. Methodological assessment was also independently performed by three investigators (J. Wang, J. Chen and X. Chen). Disagreements were adjudicated by a third investigator (B. Wang) after referring to the original articles. Scoring for each study was conducted according to the European Lung Cancer Working Party scale by Steels et al [3]. Studies included in the systematic review were denoted ‘eligible’, and those providing sufficient data for the meta-analysis are denoted ‘evaluable’.
Statistical methods
We performed separate meta-analyses using an adjusted or unadjusted hazard ratio (HR) for RFS and OS. Usually, HRs and 95% confidence intervals (CIs) were directly obtained from published literatures using univariate [8]–[11], [13]–[15], [18], [19] or multivariate survival analyses. In some studies, BVI was determined to be an independent prognostic indicator using multivariate analysis; HRs and 95% CIs were generally reported [9], [10], [16], [18], [20], [21], [23], [26]–[29], [31], [33]–[36], [38], [40]–[45], [47]–[49], [52], [56]–[59], [60]. Some studies reported the HR but did provide sufficient information on survival by BVI status; we thus estimated the HR and CIs according to the methods of Parmar et al [61]. As shown in Table S1, the total number of events [53], the log-rank statistic or its P value [9]–[12], [14], [17], [18], [20]–[22], [25], [27], [28], [34], [35], [39]–[42], [44], [46], [47], [50], [51], [54], [57], [58], or data from Kaplan-Meier survival curves [16]–[19], [59] were used to allow an approximate calculation of the HR. Heterogeneity was tested with the χ2-based Q test [62]. When the effects were assumed to be homogenous, the fixed-effect model was used; otherwise, the random-effect model based on Mantel–Haenszel method was applied [36] A funnel plot and Egger's linear regression test were used to investigate any possible publication bias [63]. The correlation between the score measurements was determined using the Spearman rank correlation coefficient. The score measurements involving the value of a discrete variable were calculated using the nonparametric Mann-Whitney U test. For all analyses, a two-sided P value of <0.05 was considered statistically significant. All analyses were performed using STATA version 11.0 software (Stata Corporation, College Station, TX, USA).
Results
Study selection and characteristics
In total, our electronic data search retrieved 206 references. Four studies were excluded because an identical patient cohort occurred within another selected cohort [64]–[67]. Eight studies were not included in the overall meta-analysis because they investigated lymphovascular invasion and outcome in NSCLC patients. The other excluded records include 19 reviews, 3 other diseases, 3 case reports, 13 non-English articles and 103 studies without available survival information (Table S2). Macchiarini et al. analyzed the correlation between BVI and outcome in 28 patients receiving induction therapy [68]. This study was also excluded from the systematic review because it did not meet the inclusion criteria. Finally, there were 52 eligible studies investigating the prognostic value of BVI in patients with NSCLC published from 1993 to 2011. The PRISMA Checklist and Flow Diagram for the studies is shown in Checklist S1 and Figure S1.
The individual characteristics of the 52 eligible studies are reported in Table S3. The total number of patients was 16,535 (range, 35–2295; median, 171). In all, according to the positivity for BVI as defined by the authors, 29.8% of tumors harbored BVI by cancer cells (median; range from 6.2% to 77.0%). A total of 47 studies dealt with all types of NSCLC, four with adenocarcinoma alone [14], [27], [38], [41] and one with squamous cell carcinoma alone [39]. A total of 23 studies dealt with only stage I patients. Twenty-eight studies investigated blood vascular invasion by using hematoxylin and eosin (H&E) stain alone. Three studies investigated blood vascular invasion by staining with H&E and for CD34 with or without CD31 immunochemistry [12], [50], [53]. Twenty studies evaluated the status of BVI in tumors in combination with elastic staining.
Of the 52 publications eligible for systematic review, four were not evaluable by meta-analysis owing to the lack of survival data even after writing to the authors for complementary information [30], [32], [53], [55]. Twelve of the these eligible studies identified BVI as a poor prognostic factor for RFS [8], [10]–[14], [16], [18], [19], [21], [60], [69], and three identified BVI as not significant for RFS [35], [53], [54] by univariate analysis. However, three studies were not included in final meta-analysis due to insufficient survival information [17], [53] or overlap between cohorts [10], [11]. Eight studies reported the significant prognostic role of BVI for RFS by multivariate analysis [8], [9]–[11], [13]–[15], [19], [60], and two identified BVI as not significant for RFS [18], [54].
In univariate analysis, 33 studies identified blood vessels as a significant prognostic factor for OS [8], [9], [12], [20]–[25], [27]–[34], [43]–[47], [58], [16], [17], [35], [37]–[42], [59], and seven identified it as not significant for survival [18], [50], [52]–[55], [70]. In multivariate analyses, there were 27 studies that identified BVI as a significant prognostic factor for OS [8]–[10], [16], [20], [23], [26], [27], [31]–[47], [58], [59] and 10 that identified blood vessel as a non-significant prognostic factor [18], [21], [28], [30], [48], [49], [52], [53], [56], [70]. Evaluability was not associated with positivity in the systematic review. The rate of significant results for OS was 62.7% for evaluable trials (33/52) compared with 60.0% (2/4) for non-evaluable trials (P = 0.626) irrespective of whether these studies used univariate or multivariate analyses.
Quality assessment of study
As shown in Table 1, the global quality assessment score, expressed as a percentage, ranged from 46.5% to 63.6%. There was no significant association between the global score and the number of patients in all eligible studies (P = 0.32). As for the global score, no significant difference was found between the evaluable and the non-evaluable trials (P = 0.18). Similarly, no statistically significant difference was shown between the significant trials and the non-significant trials in univariate (P = 0.86) or multivariate analysis for OS (P = 0.52) (Table 1).
Table 1. Quality scores analysis of the 52 eligible studies by the European Lung Cancer Working Party score according to studies characteristics.
| Studies | Design (/10) | Laboratory method (/10) | Generalizability (/10) | Results analysis | Global score (%) |
| All (52) | 4.0 | 5.6 | 6.4 | 6.0 | 55.0 |
| Patients number | |||||
| Spearman r | 0.17 | 0.14 | 0.10 | 0.25 | 0.14 |
| P | 0.22 | 0.32 | 0.47 | 0.08 | 0.32 |
| Evaluable for meta-analysis | |||||
| Yes (48) | 4.0 | 4.2 | 6.4 | 7.5 | 58.9 |
| No (4) | 4.0 | 5.6 | 6.4 | 5.0 | 52.5 |
| P | 0.38 | 0.15 | 0.13 | 0 .36 | 0.18 |
| Significant results for OS in univariate analysis | |||||
| Yes (33) | 4.0 | 4.9 | 5.6 | 7.5 | 59.5 |
| No (7) | 6.0 | 4.2 | 6.4 | 7.5 | 60.2 |
| P | 0.78 | 085 | 0.98 | 0.81 | 0.86 |
| Significant results for OS in multivariate analysis | |||||
| Yes (27) | 4.0 | 5.6 | 6.4 | 7.0 | 55.3 |
| No (10) | 4.0 | 5.6 | 6.4 | 6.3 | 53.6 |
| P | 0.36 | 0.91 | 0.91 | 0.63 | 0.52 |
Scores distributions are summarized by median values.
OS = overall survival.
Meta-analysis of the impact of BVI on survival for all patients
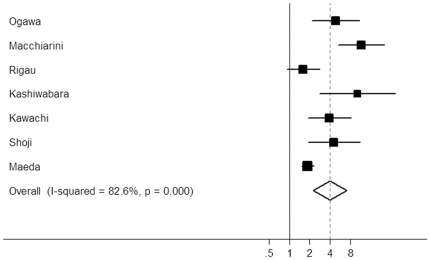
The results of meta-analysis of BVI and prognosis in NSCLC are shown in Table 2. BVI was significantly correlated with poor RFS according to univariate analysis, with a combined HR of 3.28 (95% CI: 2.14–5.05; P<0.0001) (11 studies, 4,498 patients). Significant heterogeneity was detected among these studies (P<0.001, Q = 90.00). The data of multivariate analyses for RFS were available in six studies including 3,088 patients. We obtained a combined HR value of 3.98 (95% CI: 2.24–7.06; P<0.0001) with significant heterogeneity (P<0.001, Q = 34.44) (Fig. 1).
Table 2. Results of meta-analysis of blood vessel invasion and prognosis in NSCLC.
| Groups | Estimate of relative hazard | Homogeneity test | |||
| HR | 95% CI | p | Q (df) | p | |
| All studies | |||||
| Unadjusted OS (31 studies, n = 8,528) | 2.22 | 1.93–2.56 | <0.0001 | 79.63 (33) | <0.001 |
| Adjusted OS (28 studies, n = 9,873) | 1.90 | 1.65–2.19 | <0.0001 | 84.40 (28) | <0.001 |
| Unadjusted RFS (11 studies, n = 4,498 ) | 3.28 | 2.14–5.05 | <0.0001 | 90.00 (10) | <0.001 |
| Adjusted RFS (7 studies, n = 3,088) | 3.98 | 2.24–7.06 | <0.0001 | 34.44 (6) | <0.001 |
| Stage I studies | |||||
| Unadjusted OS (14 studies, n = 2,908 ) | 2.94 | 2.28–3.80 | <0.0001 | 22.83 (13) | 0.044 |
| Adjusted OS (13 studies, n = 3,974) | 2.15 | 1.68–2.75 | <0.0001 | 26.70 (12) | 0.009 |
| Unadjusted RFS (5 studies, n = 647) | 5.89 | 3.98–8.71 | <0.0001 | 3.76 (4) | 0.440 |
| Adjusted RFS (4 studies, n = 576) | 6.93 | 4.23–11.37 | <0.0001 | 3.61 (3) | 0.306 |
NSCLC = non-small-cell lung cancer; HR = hazard ratio; CI = confidence interval; OS = overall survival; RFS = relapse-free survival.
Figure 1. Forest plot showing the combined relative hazard ratio for relapse-free survival in all patient populations by multivariate analysis.
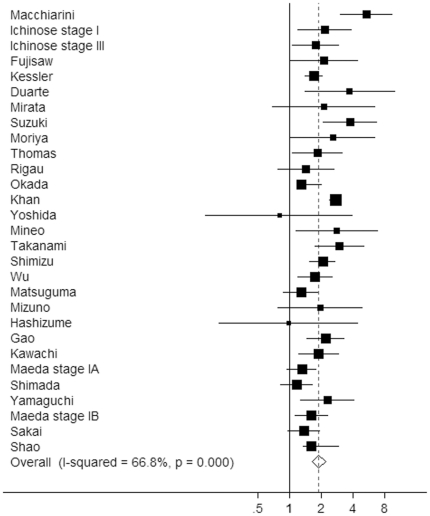
Thirty-one studies (comprising 8,528 cases) used for univariate analysis produced a pooled estimate of risk of 2.22 (95% CI: 1.93–2.56, P<0.0001). There was evidence of significant inter-study heterogeneity (P<0.001, Q = 79.63). Twenty-eight studies (comprising 9,873 cases) were used for the meta-analysis of BVI on OS by multivariate analysis (Fig. 2). The overall risk of death was 1.90 (95% CI: 1.65–2.19; P<0.0001) with a range of estimates from 0.80 to 3.79. Significant heterogeneity among studies was present (P<0.001, Q = 84.40).
Figure 2. Forest plot showing the combined relative hazard ratio for overall survival in all patient populations by multivariate analysis.
Meta-analysis of the impact of BVI on survival for stage I patients
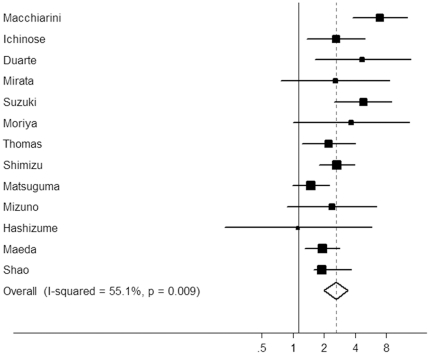
We also analyzed the association between the BVI and survival in early-stage cancer patients. As shown in Table 2, the summary HR estimates for stage I patients by univariate and multivariate analysis were 2.94 (95% CI: 2.28–3.80; P<0.0001) and 2.15 (95% CI: 1.68–2.75; P<0.0001) , respectively. BVI demonstrated a significant effect on RFS for stage I NSCLC according to univariate analysis (HR = 5.89, 95% CI: 3.98–8.71, P<0.0001) and multivariate analysis (HR = 6.93, 95% CI: 4.23–11.37, P<0.0001) (Fig. 3). These results suggest that NSCLC patients with BVI have a poor prognosis, irrespective of the tumor stage.
Figure 3. Forest plot showing the combined relative hazard ratio for overall survival of stage I patients by multivariate analysis.
Test of heterogeneity and subgroup analyses
There was large heterogeneity in this meta-analysis. Firstly, we performed the subgroup analyses stratified by ethnicity. BVI status did show a significant effect on RFS according to multivariate analysis in Asians (HR = 3.87, 95% CI: 2.07–7.22, P<0.001; heterogeneity test, P = 0.006) and in Caucasians (HR = 4.25, 95% CI: 0.61–29.5, P<0.001; heterogeneity test, P = 0.28). There was still evidence of statistical heterogeneity in Asians that was largely due to the contribution of the report by Maeda et al. which included the largest patient population. Furthermore, the heterogeneity disappeared when excluding this study and the value of pooled HR was not significantly altered (HR = 4.82, 95% CI: 3.12–7.46, P<0.001; heterogeneity test, P = 0.65). Subgroup analyses by methods of BVI evaluation showed that the pooled HRs for RFS by multivariate analysis were 4.91 (95% CI: 1.82–13.24, P<0.001; heterogeneity test, P = 0.06) in studies evaluating BVI with H&E method and 3.16 (95% CI: 1.51–6.63, P<0.001; heterogeneity test, P = 0.02) in those evaluating BVI by a combination of H&E method and immunochemistry or elastic staining.
In the subgroup analysis by ethnicity, statistically significantly increased risks for death were found among Asians (HR = 1.68, 95% CI: 1.53–1.85, P<0.001; heterogeneity test, P = 0.06) and among Caucasians (HR = 1.74, 95% CI: 1.46–2.09, P<0.001; heterogeneity test, P = 0.45). In addition, BVI was significantly correlated with poor OS according to multivariate analysis in studies evaluating BVI with H&E method (HR = 2.04, 95% CI: 1.68–2.47, P<0.001; heterogeneity test, P = 0.03) and in those evaluating BVI by a combination of H&E method and immunochemistry or elastic staining (HR = 1.60, 95% CI: 1.39–1.84, P<0.001; heterogeneity test, P = 0.28). These results showed that the heterogeneities were effectively decreased or removed in the subgroups by ethnicity and methods for BVI evaluation.
Publication bias statistics were determined using the methods of Egger et al [63]. No publication bias was found for the studies used for univariate analysis (P = 0.596) or for multivariate analysis (P = 0.24).
Discussion
Microscopic metastasis begins with the local invasion by tumor cells into host stroma within or surrounding the primary tumor. When tumor cells penetrate a blood vessel or a peripheral lymphatic, they can detach, disseminate and arrest in the microvasculature through the circulation [70]. In fact, the presence of vascular invasion by neoplastic cells indicates that the cancers are in a metastatic phase. BVI was found to correlate with disease progress in many types of cancers [71]–[73]. Our meta-analysis provides strong evidence that pathologic BVI is a prognostic factor for survival in patients with NSCLC by univariate analysis. More importantly, this effect remained significant according to multivariate analysis, showing that BVI is an independent prognostic factor for poor survival regardless of tumor size or lymph node status. In addition, vascular invasion is a strong predictor of survival in early stage tumors when adjusted for other prognostic factors.
Cancer metastasis and progression is a complex and multistep process. BVI appears to be a fundamental step in cancer metastasis and spread, leading to unfavorable prognosis for patients with NSCLC. In general, BVI was defined as tumor cell embolization in the vascular lumen on routine H&E and elastic lamina stain. Ichinose et al. reported that venous invasion correlated with poor prognosis among patients with completely resected NSCLC but that arterial invasion did not [47]. However, in most of the studies, arterial and venous invasion has not been separately studied pathologically due to the inability in some cases to discriminate between arterial and venous invasion. In addition, most of the studies included in this meta-analysis investigated the correlation between intratumoral BVI and prognosis, but some studies demonstrated the prognostic role of extratumoral vascular invasion. Shimada et al. reported that both intratumoral and extratumoral vascular invasion were significant prognostic factors, but only the extratumoral vascular invasion group was associated with advanced pathologic staging, lymph node metastasis, and lymphatic permeation [21]. Our meta-analysis focused on the effect of tumor vascular invasion on the survival of NSCLC patients irrespective of whether these studies detected intratumoral or extratumoral vascular invasion or whether they determined arterial and venous invasion. Although almost all intratumoral blood vessels are occluded by the surrounding tumor cells and stromal cells, intratumoral and extratumoral blood vessels are thought to be functional blood vessels. In fact, Shimada et al., found that all extratumoral vascular invasion cases also had intratumoral vascular invasion [21].
Our meta-analysis had some limitations. First, the level of evidence obtained by retrospective studies is lower than that provided by randomized controlled trials. Second, data from published trials rather than individual patient data were used in the systematic review. Third, in most of meta-analyses, there was evidence of significant heterogeneity although the random-effect model based on Mantel–Haenszel method rather than the fixed-effect model was applied. Studies may have differed with regard to the baseline characteristics of the patients included, the adjuvant treatment they might have received, the duration of follow-up, and adjustments for other cofactors. For example, some studies included a small number of stage IV or IIIB patients, which accounted for the heterogeneity. These studies were finally maintained in the meta-analyses because the overall designs of studies were similar to those used in the other studies. Many variations to the method of BVI assessment exist, although in most studies BVI was defined as the presence of neoplastic structures inside the lumen of a vessel. Some studies detected BVI by staining with hematoxylin and eosin alone or in combination with elastic-van Gieson stain or by staining with Victoria blue hematoxylin and eosin, which can lead to significant heterogeneity. Subgroup analyses indicated that methods for BVI evaluation contributed largely to heterogeneity. Thus, standardization of evaluating vascular invasion and quality control is needed. In addition, an important issue that needs to be taken into account is the type of adjuvant therapy that each patient received because chemotherapy and/or therapies that target the epidermal growth factor receptor can change the outcome for NSCLC patients. However, the majority of published studies lacked detailed information regarding patient treatment. In this study, we used a methodology assessment on the treatment of lung cancer reported by Steels et al [3]. However, this approach does not fully protect from potential bias because we could not take all the studies into account. Therefore, our results need to be substantiated by further prospective studies.
Publication and reporting bias also has to be considered. We did not look for unpublished papers, reviews or abstracts because the required data were usually available only in full publications. Another potential source of bias is related to the method used to extrapolate the HR. If the HR was not reported by author, it was calculated from the data included in the article or extrapolated from the survival curves, which involves making assumptions. In addition, each study adjusted for different covariates and only the studies that found significant results in univariate analysis performed multivariate analysis; thus, pooling the results may have produced bias. Nevertheless, no publication bias was detected using Egger's test, suggesting that the statistics obtained approximate the actual results.
In conclusion, the relative risk of recurrence and death for an individual patient whose tumor showed BVI by neoplastic cells was nearly 4 and 2 times higher, respectively, than that of a patient whose tumor did not show BVI by neoplastic cells. We have demonstrated the prognostic power of a single independent pathologic marker for NSCLC. We suggest that NSCLC patients with BVI receive adjunct systematic chemotherapy and that BVI should be incorporated in the new edition of the TNM classification. However, large, properly designed studies that employ standard methodology to assess BVI are needed to demonstrate whether BVI can provide prognostic information in addition to the currently used TNM staging system. Moreover, according to the recent report by Kato et al., the significance of a combination of microvessel count and BVI could be more important than that of either BVI or microvessel count alone [74].
Supporting Information
The flow of the included studies.
(DOC)
PRISMA checklist.
(DOC)
Data source for the estimating of HR form included studies evaluating blood vessel invasion and prognosis.
(DOC)
Characteristics of literatures excluded in this systematic review.
(DOC)
Main characteristics and results of eligible studies evaluating blood vessel invasion and RFS or OS in patients with NSCLC.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded in part by the National Nature Science Foundation of China (No. 30901788) and the Shandong Provincial Nature Science Foundation (No. ZR2010HQ038 and No. ZR2010HM059). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, et al. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 3.Steels E, Paesmans M, Berghmans T, Branle F, Lemaitre F, et al. Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. Eur Respir J. 2001;18:705–719. doi: 10.1183/09031936.01.00062201. [DOI] [PubMed] [Google Scholar]
- 4.Meert AP, Martin B, Delmotte P, Berghmans T, Lafitte JJ, et al. The role of EGF-R expression on patient survival in lung cancer: a systematic review with meta-analysis. Eur Respir J. 2002;20:975–981. doi: 10.1183/09031936.02.00296502. [DOI] [PubMed] [Google Scholar]
- 5.Mascaux C, Martin B, Paesmans M, Berghmans T, Dusart M, et al. Has Cox-2 a prognostic role in non-small-cell lung cancer? A systematic review of the literature with meta-analysis of the survival results. Br J Cancer. 2006;95:139–145. doi: 10.1038/sj.bjc.6603226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Wang B, Chen X, Bi J. The prognostic value of RASSF1A promoter hypermethylation in non-small cell lung carcinoma: a systematic review and meta-analysis. Carcinogenesis. 2011;32:411–416. doi: 10.1093/carcin/bgq266. [DOI] [PubMed] [Google Scholar]
- 7.Greene FL, Sobin LH. A worldwide approach to the TNM staging system: collaborative efforts of the AJCC and UICC. J Surg Oncol. 2009;99:269–272. doi: 10.1002/jso.21237. [DOI] [PubMed] [Google Scholar]
- 8.Macchiarini P, Fontanini G, Hardin MJ, Chuanchieh H, Bigini D, et al. Blood vessel invasion by tumor cells predicts recurrence in completely resected T1 N0 M0 non-small-cell lung cancer. J Thorac Cardiovasc Surg. 1993;106:80–89. [PubMed] [Google Scholar]
- 9.Maeda R, Yoshida J, Ishii G, Hishida T, Nishimura M, et al. Poor prognostic factors in patients with stage IB non-small cell lung cancer according to the seventh edition TNM classification. Chest. 2011;139:855–861. doi: 10.1378/chest.10-1535. [DOI] [PubMed] [Google Scholar]
- 10.Maeda R, Yoshida J, Hishida T, Aokage K, Nishimura M, et al. Late recurrence of non-small cell lung cancer more than 5 years after complete resection: incidence and clinical implications in patient follow-up. Chest. 2010;138:145–150. doi: 10.1378/chest.09-2361. [DOI] [PubMed] [Google Scholar]
- 11.Maeda R, Yoshida J, Ishii G, Aokage K, Hishida T, et al. Long-term outcome and late recurrence in patients with completely resected stage IA non-small cell lung cancer. J Thorac Oncol. 2010;5:1246–1250. doi: 10.1097/JTO.0b013e3181e2f247. [DOI] [PubMed] [Google Scholar]
- 12.Turhan K, Samancilar O, Cagirici U, Goksel T, Nart D, et al. The effect of blood vessel invasion on prognosis of operated stage I non-small cell lung cancer patients. Thorac Cardiovasc Surg. 2010;58:28–31. doi: 10.1055/s-0029-1185881. [DOI] [PubMed] [Google Scholar]
- 13.Shoji F, Haro A, Yoshida T, Ito K, Morodomi Y, et al. Prognostic significance of intratumoral blood vessel invasion in pathologic stage IA non-small cell lung cancer. Ann Thorac Surg. 2010;89:864–869. doi: 10.1016/j.athoracsur.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 14.Kashiwabara K, Saeki S, Sasaki J, Nomura M, Kohrogi H. Combined evaluation of postoperative serum levels of carcinoembryonic antigen less than or equal to 2.5 ng/ml and absence of vascular invasion may predict no recurrence of stage I adenocarcinoma lung cancer. J Thorac Oncol. 2008;3:1416–1420. doi: 10.1097/JTO.0b013e31818dda85. [DOI] [PubMed] [Google Scholar]
- 15.Kawachi R, Tsukada H, Nakazato Y, Takei H, Furuyashiki G, et al. Early recurrence after surgical resection in patients with pathological stage I non-small cell lung cancer. Thorac Cardiovasc Surg. 2009;57:472–475. doi: 10.1055/s-0029-1185734. [DOI] [PubMed] [Google Scholar]
- 16.Okada M, Sakamoto T, Nishio W, Uchino K, Tsubota N. Characteristics and prognosis of patients after resection of nonsmall cell lung carcinoma measuring 2 cm or less in greatest dimension. Cancer. 2003;98:535–541. doi: 10.1002/cncr.11530. [DOI] [PubMed] [Google Scholar]
- 17.Gabor S, Renner H, Popper H, Anegg U, Sankin O, et al. Invasion of blood vessels as significant prognostic factor in radically resected T1-3N0M0 non-small-cell lung cancer. Eur J Cardiothorac Surg. 2004;25:439–442. doi: 10.1016/j.ejcts.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 18.Rigau V, Molina TJ, Chaffaud C, Huchon G, Audouin J, et al. Blood vessel invasion in resected non small cell lung carcinomas is predictive of metastatic occurrence. Lung Cancer. 2002;38:169–176. doi: 10.1016/s0169-5002(02)00213-1. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa J, Tsurumi T, Yamada S, Koide S, Shohtsu A. Blood vessel invasion and expression of sialyl Lewisx and proliferating cell nuclear antigen in stage I non-small cell lung cancer. Relation to postoperative recurrence. Cancer. 1994;73:1177–1183. doi: 10.1002/1097-0142(19940215)73:4<1177::aid-cncr2820730409>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 20.Shao W, Wang W, Xiong XG, Cao C, Yan TD, et al. Prognostic impact of MMP-2 and MMP-9 expression in pathologic stage IA non-small cell lung cancer. J Surg Oncol. 2011;104:841–846. doi: 10.1002/jso.22001. [DOI] [PubMed] [Google Scholar]
- 21.Shimada Y, Ishii G, Hishida T, Yoshida J, Nishimura M, et al. Extratumoral vascular invasion is a significant prognostic indicator and a predicting factor of distant metastasis in non-small cell lung cancer. J Thorac Oncol. 2010;5:970–975. doi: 10.1097/JTO.0b013e3181dd1803. [DOI] [PubMed] [Google Scholar]
- 22.Naito Y, Goto K, Nagai K, Ishii G, Nishimura M, et al. Vascular invasion is a strong prognostic factor after complete resection of node-negative non-small cell lung cancer. Chest. 2010;138:1411–1417. doi: 10.1378/chest.10-0185. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi Y, Ishii G, Kojima M, Yoh K, Otsuka H, et al. Histopathologic features of the tumor budding in adenocarcinoma of the lung: tumor budding as an index to predict the potential aggressiveness. J Thorac Oncol. 2010;5:1361–1368. doi: 10.1097/JTO.0b013e3181eaf2f3. [DOI] [PubMed] [Google Scholar]
- 24.Ryuge S, Sato Y, Wang GQ, Matsumoto T, Jiang SX, et al. Prognostic significance of nestin expression in resected non-small cell lung cancer. Chest. 2011;139:862–869. doi: 10.1378/chest.10-1121. [DOI] [PubMed] [Google Scholar]
- 25.Bodendorf MO, Haas V, Laberke HG, Blumenstock G, Wex P, et al. Prognostic value and therapeutic consequences of vascular invasion in non-small cell lung carcinoma. Lung Cancer. 2009;64:71–78. doi: 10.1016/j.lungcan.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Kawachi R, Nakazato Y, Masui K, Takei H, Koshi-ishi Y, et al. Clinical significance of pleural lavage cytology for non-small cell lung cancer: is surgical resection valid for patients with positive pleural lavage cytology? Interact Cardiovasc Thorac Surg. 9:265–268. doi: 10.1510/icvts.2009.202010. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno T, Ishii G, Nagai K, Yoshida J, Nishimura M, et al. Identification of a low risk subgroup of stage IB lung adenocarcinoma patients. Lung Cancer. 2008;62:302–308. doi: 10.1016/j.lungcan.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Hashizume S, Nagayasu T, Hayashi T, Hidaka S, Tsuchiya T, et al. Accuracy and prognostic impact of a vessel invasion grading system for stage IA non-small cell lung cancer. Lung Cancer. 2009;65:363–370. doi: 10.1016/j.lungcan.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Rao J, Sayeed RA, Tomaszek S, Fischer S, Keshavjee S, et al. Prognostic factors in resected satellite-nodule T4 non-small cell lung cancer. Ann Thorac Surg. 2007;84:934–938. doi: 10.1016/j.athoracsur.2007.04.097. [DOI] [PubMed] [Google Scholar]
- 30.Ayed AK, Adesina A. Prognostic significance of cyclin D1 expression in resected stage I, II non-small cell lung cancer in Arabs. Interact Cardiovasc Thorac Surg. 2006;5:47–51. doi: 10.1510/icvts.2005.120030. [DOI] [PubMed] [Google Scholar]
- 31.Takanami I. Increased expression of integrin-linked kinase is associated with shorter survival in non-small cell lung cancer. BMC Cancer. 2005;5:1. doi: 10.1186/1471-2407-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlési F, Doddoli C, Torre JP, Giudicelli R, Fuentes P, et al. Comparative prognostic features of stage IIIAN2 and IIIB non-small-cell lung cancer patients treated with surgery after induction therapy. Eur J Cardiothorac Surg. 2005;28:629–634. doi: 10.1016/j.ejcts.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Wu CT, Chang YL, Shih JY, Lee YC. The significance of estrogen receptor beta in 301 surgically treated non-small cell lung cancers. J Thorac Cardiovasc Surg. 2005;130:979–986. doi: 10.1016/j.jtcvs.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Mineo TC, Ambrogi V, Baldi A, Rabitti C, Bollero P, et al. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumour vessel invasion after radical surgery for IB-IIA non-small cell lung cancer. J Clin Pathol. 2004;57:591–597. doi: 10.1136/jcp.2003.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto S, Tomita Y, Hoshida Y, Iizuka N, Monden M, et al. Expression level of valosin-containing protein (p97) is correlated with progression and prognosis of non-small-cell lung carcinoma. Ann Surg Oncol. 2004;11:697–704. doi: 10.1245/ASO.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 36.Okada M, Sakamoto T, Nishio W, Uchino K, Tsuboshima K, et al. Pleural lavage cytology in non-small cell lung cancer: lessons from 1000 consecutive resections. J Thorac Cardiovasc Surg. 2003;126:1911–1915. doi: 10.1016/s0022-5223(03)00715-3. [DOI] [PubMed] [Google Scholar]
- 37.Khan OA, Fitzgerald JJ, Field ML, Soomro I, Beggs FD, et al. Histological determinants of survival in completely resected T1-2N1M0 nonsmall cell cancer of the lung. Ann Thorac Surg. 2004;77:1173–1178. doi: 10.1016/j.athoracsur.2003.08.080. [DOI] [PubMed] [Google Scholar]
- 38.Moriya Y, Niki T, Yamada T, Matsuno Y, Kondo H, et al. Increased expression of laminin-5 and its prognostic significance in lung adenocarcinomas of small size. An immunohistochemical analysis of 102 cases. Cancer. 2001;91:1129–1141. doi: 10.1002/1097-0142(20010315)91:6<1129::aid-cncr1109>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 39.Tamura A, Hebisawa A, Hayashi K, Sagara Y, Fukushima K, et al. Prognostic significance of thrombomodulin expression and vascular invasion in stage I squamous cell carcinoma of the lung. Lung Cancer. 2001;34:375–382. doi: 10.1016/s0169-5002(01)00261-6. [DOI] [PubMed] [Google Scholar]
- 40.Thomas P, Doddoli C, Thirion X, Ghez O, Payan-Defais MJ, et al. Stage I non-small cell lung cancer: a pragmatic approach to prognosis after complete resection. Ann Thorac Surg. 2002;73:1065–1070. doi: 10.1016/s0003-4975(01)03595-0. [DOI] [PubMed] [Google Scholar]
- 41.Yokose T, Suzuki K, Nagai K, Nishiwaki Y, Sasaki S, et al. Favorable and unfavorable morphological prognostic factors in peripheral adenocarcinoma of the lung 3 cm or less in diameter. Lung Cancer. 2000;29:179–188. doi: 10.1016/s0169-5002(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki K, Nagai K, Yoshida J, Nishimura M, Takahashi K, et al. Conventional clinicopathologic prognostic factors in surgically resected nonsmall cell lung carcinoma. A comparison of prognostic factors for each pathologic TNM stage based on multivariate analyses. Cancer. 1999;86:1976–1984. doi: 10.1002/(sici)1097-0142(19991115)86:10<1976::aid-cncr14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 43.Kessler R, Gasser B, Massard G, Roeslin N, Meyer P, et al. Blood vessel invasion is a major prognostic factor in resected non-small cell lung cancer. Ann Thorac Surg. 1996;62:1489–1493. doi: 10.1016/0003-4975(96)00540-1. [DOI] [PubMed] [Google Scholar]
- 44.Duarte IG, Bufkin BL, Pennington MF, Gal AA, Cohen C, et al. Angiogenesis as a predictor of survival after surgical resection for stage I non-small-cell lung cancer. J Thorac Cardiovasc Surg. 1998;115:652–658. doi: 10.1016/S0022-5223(98)70331-9. [DOI] [PubMed] [Google Scholar]
- 45.Fujisawa T, Yamaguchi Y, Saitoh Y, Hiroshima K, Ohwada H. Blood and lymphatic vessel invasion as prognostic factors for patients with primary resected nonsmall cell carcinoma of the lung with intrapulmonary metastases. Cancer. 1995;76:2464–2470. doi: 10.1002/1097-0142(19951215)76:12<2464::aid-cncr2820761210>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 46.Harpole DH, Jr, Herndon JE, 2nd, Young WG, Jr, Wolfe WG, Sabiston DC., Jr Stage I nonsmall cell lung cancer. A multivariate analysis of treatment methods and patterns of recurrence. Cancer. 1995;76:787–796. doi: 10.1002/1097-0142(19950901)76:5<787::aid-cncr2820760512>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 47.Ichinose Y, Yano T, Asoh H, Yokoyama H, Yoshino I, et al. Prognostic factors obtained by a pathologic examination in completely resected non-small-cell lung cancer. An analysis in each pathologic stage. J Thorac Cardiovasc Surg. 1995;110:601–605. doi: 10.1016/S0022-5223(95)70090-0. [DOI] [PubMed] [Google Scholar]
- 48.Matsuguma H, Nakahara R, Igarashi S, Ishikawa Y, Suzuki H, et al. Pathologic stage I non-small cell lung cancer with high levels of preoperative serum carcinoembryonic antigen: clinicopathologic characteristics and prognosis. J Thorac Cardiovasc Surg. 2008;135:44–49. doi: 10.1016/j.jtcvs.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 49.Yoshida T, Tanaka S, Mogi A, Shitara Y, Kuwano H. The clinical significance of Cyclin B1 and Wee1 expression in non-small-cell lung cancer. Ann Oncol. 2004;15:252–256. doi: 10.1093/annonc/mdh073. [DOI] [PubMed] [Google Scholar]
- 50.Cagini L, Monacelli M, Giustozzi G, Moggi L, Bellezza G, et al. Biological prognostic factors for early stage completely resected non-small cell lung cancer. J Surg Oncol. 2000;74:53–60. doi: 10.1002/1096-9098(200005)74:1<53::aid-jso13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 51.Fu XL, Zhu XZ, Shi DR, Xiu LZ, Wang LJ, et al. Study of prognostic predictors for non-small cell lung cancer. Lung Cancer. 1999;23:143–152. doi: 10.1016/s0169-5002(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 52.Hirata T, Fukuse T, Naiki H, Hitomi S, Wada H. Expression of CD44 variant exon 6 in stage I non-small cell lung carcinoma as a prognostic factor. Cancer Res. 1998;58:1108–1110. [PubMed] [Google Scholar]
- 53.Lucchi M, Fontanini G, Mussi A, Vignati S, Ribechini A, et al. Tumor angiogenesis and biologic markers in resected stage I NSCLC. Eur J Cardiothorac Surg. 1997;12:535–541. doi: 10.1016/s1010-7940(97)00218-2. [DOI] [PubMed] [Google Scholar]
- 54.Bréchot JM, Chevret S, Charpentier MC, Appere de Vecchi C, Capron F, et al. Blood vessel and lymphatic vessel invasion in resected nonsmall cell lung carcinoma. Correlation with TNM stage and disease free and overall survival. Cancer. 1996;78:2111–2118. [PubMed] [Google Scholar]
- 55.Roberts TE, Hasleton PS, Musgrove C, Swindell R, Lawson RA. Vascular invasion in non-small cell lung carcinoma. J Clin Pathol. 1992;45:591–593. doi: 10.1136/jcp.45.7.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakai Y, Ohbayashi C, Kanomata N, Kajimoto K, Sakuma T, et al. Significance of microscopic invasion into hilar peribronchovascular soft tissue in resection specimens of primary non-small cell lung cancer. Lung Cancer. 2011;73:89–95. doi: 10.1016/j.lungcan.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Ruffini E, Asioli S, Filosso PL, Buffoni L, Bruna MC, et al. Significance of the presence of microscopic vascular invasion after complete resection of Stage I-II pT1-T2N0 non-small cell lung cancer and its relation with T-Size categories: did the 2009 7th edition of the TNM staging system miss something? J Thorac Oncol. 2011;6:319–326. doi: 10.1097/JTO.0b013e3182011f70. [DOI] [PubMed] [Google Scholar]
- 58.Shimizu K, Yoshida J, Nagai K, Nishimura M, Ishii G, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2005;130:160–165. doi: 10.1016/j.jtcvs.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 59.Gao Y, Zhang C, Li N, Zhou F, Shi S, He J. Vascular invasion as an independent prognostic indicator in radically resected non-small cell lung cancer. Chin J Cancer Res. 2008;20:33–38. [Google Scholar]
- 60.Maeda R, Yoshida J, Ishii G, Hishida T, Nishimura M, et al. Prognostic impact of intratumoral vascular invasion in non-small cell lung cancer patients.Thorax. 2010;65:1092–1098. doi: 10.1136/thx.2010.141861. [DOI] [PubMed] [Google Scholar]
- 61.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 62.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 63.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takanami I. The prognostic value of overexpression of Skp2 mRNA in non-small cell lung cancer. Oncol Rep. 2005;13:727–731. [PubMed] [Google Scholar]
- 65.Harpole DH, Jr, Herndon JE, 2nd, Wolfe WG, Iglehart JD, Marks JR. A prognostic model of recurrence and death in stage I non-small cell lung cancer utilizing presentation, histopathology, and oncoprotein expression. Cancer Res. 1995;55:51–56. [PubMed] [Google Scholar]
- 66.Fontanini G, Macchiarini P, Pepe S, Ruggiero A, Hardin M, et al. The expression of proliferating cell nuclear antigen in paraffin sections of peripheral, node-negative non-small cell lung cancer. Cancer. 1992;70:1520–1527. doi: 10.1002/1097-0142(19920915)70:6<1520::aid-cncr2820700613>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 67.Macchiarini P, Fontanini G, Hardin JM, Pingitore R, Angeletti CA. Most peripheral, node-negative, non-small-cell lung cancers have low proliferative rates and no intratumoral and peritumoral blood and lymphatic vessel invasion. Rationale for treatment with wedge resection alone. J Thorac Cardiovasc Surg. 1992;104:892–899. [PubMed] [Google Scholar]
- 68.Macchiarini P, Dulmet E, De Montpreville V, Chapelier A, Cerrina J, et al. Prognostic significance of peritumoural blood and lymphatic vessel invasion by tumour cells in T4 non-small cell lung cancer following induction therapy. Surg Oncol. 1995;4:91–99. doi: 10.1016/s0960-7404(10)80012-x. [DOI] [PubMed] [Google Scholar]
- 69.Anami Y, Iijima T, Suzuki K, Yokota J, Minami Y, et al. Bronchioloalveolar carcinoma (lepidic growth) component is a more useful prognostic factor than lymph node metastasis. J Thorac Oncol. 2009;4:951–958. doi: 10.1097/JTO.0b013e3181ad8631. [DOI] [PubMed] [Google Scholar]
- 70.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 71.Roxburgh CS, McMillan DC, Anderson JH, McKee RF, Horgan PG, et al. Elastica staining for venous invasion results in superior prediction of cancer-specific survival in colorectal cancer. Ann Surg. 2010;252:989–997. doi: 10.1097/SLA.0b013e3181f1c60d. [DOI] [PubMed] [Google Scholar]
- 72.Mohammed RA, Ellis IO, Mahmmod AM, Hawkes EC, Green AR, et al. Lymphatic and blood vessels in basal and triple-negative breast cancers: characteristics and prognostic significance. Mod Pathol. 2011;24:774–785. doi: 10.1038/modpathol.2011.4. [DOI] [PubMed] [Google Scholar]
- 73.Kim JH, Park SS, Park SH, Kim SJ, Mok YJ, et al. Clinical significance of immunohistochemically-identified lymphatic and/or blood vessel tumor invasion in gastric cancer. J Surg Res. 2010;162:177–183. doi: 10.1016/j.jss.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 74.Kato T, Kameoka S, Kimura T, Nishikawa T, Kobayashi M. The combination of angiogenesis and blood vessel invasion as a prognostic indicator in primary breast cancer. Br J Cancer. 2003;88:1900–1908. doi: 10.1038/sj.bjc.6600921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The flow of the included studies.
(DOC)
PRISMA checklist.
(DOC)
Data source for the estimating of HR form included studies evaluating blood vessel invasion and prognosis.
(DOC)
Characteristics of literatures excluded in this systematic review.
(DOC)
Main characteristics and results of eligible studies evaluating blood vessel invasion and RFS or OS in patients with NSCLC.
(DOC)