Abstract
Background
The highly prevalent parasite Toxoplasma gondii reportedly manipulates rodent behavior to enhance the likelihood of transmission to its definitive cat host. The proximate mechanisms underlying this adaptive manipulation remain largely unclear, though a growing body of evidence suggests that the parasite-entrained dysregulation of dopamine metabolism plays a central role. Paradoxically, the distribution of the parasite in the brain has received only scant attention.
Methodology/Principal Findings
The distributions of T. gondii cysts and histopathological lesions in the brains of CD1 mice with latent toxoplasmosis were analyzed using standard histological techniques. Mice were infected per orally with 10 tissue cysts of the avirulent HIF strain of T. gondii at six months of age and examined 18 weeks later. The cysts were distributed throughout the brain and selective tropism of the parasite toward a particular functional system was not observed. Importantly, the cysts were not preferentially associated with the dopaminergic system and absent from the hypothalamic defensive system. The striking interindividual differences in the total parasite load and cyst distribution indicate a probabilistic nature of brain infestation. Still, some brain regions were consistently more infected than others. These included the olfactory bulb, the entorhinal, somatosensory, motor and orbital, frontal association and visual cortices, and, importantly, the hippocampus and the amygdala. By contrast, a consistently low incidence of tissue cysts was recorded in the cerebellum, the pontine nuclei, the caudate putamen and virtually all compact masses of myelinated axons. Numerous perivascular and leptomeningeal infiltrations of inflammatory cells were observed, but they were not associated with intracellular cysts.
Conclusion/Significance
The observed pattern of T. gondii distribution stems from uneven brain colonization during acute infection and explains numerous behavioral abnormalities observed in the chronically infected rodents. Thus, the parasite can effectively change behavioral phenotype of infected hosts despite the absence of well targeted tropism.
Introduction
Toxoplasma gondii is a highly prevalent, intracellular protozoan parasite with an indirect life cycle. It infects a very broad spectrum of warm-blooded vertebrates, including humans, as intermediate hosts but can reproduce sexually only in the feline intestine [1]. The transmission of T. gondii is facilitated by its ability to modify the behavior of its intermediate hosts. Indeed, behavioral studies have gathered abundant evidence that latent toxoplasmosis is associated with impaired motor performance, deficits in spatial learning and memory, reduced anxiety, higher activity levels in both novel and familiar environments, sensory attention deficits, altered novelty seeking behavior, longer reaction times, and, most importantly, reduced avoidance of feline predators (for review, see [2]–[5]). These parasite-induced behavioral changes undoubtedly increase the predation risk in infected rodents and thereby enhance the likelihood of the parasite transmission to its definitive cat host. Moreover, the fact that infected rodents lose their innate fear of cat odor and develop a mild attraction to it, but retain the aversion to odors of other predators, which do not serve as definitive hosts of the parasite [6]–[9], cannot be explained as a by-product of parasitosis and clearly implies highly specific manipulation of the host behavior.
After acute infection characterized by fast asexual reproduction, T. gondii encysts in the brain (and other tissues) and remains and slowly asexually reproduces there for the host's lifetime. The parasite is thus well placed to affect the behavior of the infected individual. However, the proximate mechanisms by which T. gondii alters brain function remain largely unclear. The convergence of evidence, albeit at times circumstantial, suggests that synergic effects of three different mechanisms may underpin the T. gondii impact on local neural processing. First, chronic infection may result in a local degenerative cell loss. Parasites within neurons could directly cause the death of infected neurons or atrophy of their processes and inflammation may contribute, via the production of nitric oxide and other toxic oxygen products, to the death of neighboring neurons [10]. Because neurons greatly outnumber the T. gondii brain cysts, this phenomenon has been considered rather marginal until recently (e.g., [5]). However, recent magnetic resonance imaging studies reporting dilated ventricles in chronically infected mice [10] and reduced grey matter volume in T. gondii positive schizophrenia patients compared to that of T. gondii negative patients [11] are consistent with a significant loss of the brain parenchyma. Second, local immune responses required to keep T. gondii dormant may, through the production of proinflammatory cytokines, interferon-γ and indoleamine 2,3-dioxigenase, alter the levels, turnover and efficiency of many neuromodulators, including dopamine, glutamate and serotonin (for detailed discussion see [12], [13]). Finally, T. gondii may directly influence neurotransmitter levels. Whilst the association between increased dopamine levels and latent toxoplasmosis comes from the mid-eighties [14], recent studies have provided evidence strongly suggesting that the parasite directly increases local dopamine metabolism [15], [16]. More specifically, T. gondii contains genes encoding a tyrosine hydroxylase, a rate-liming enzyme of dopamine biosynthesis [15], and the encysted parasite expresses dopamine in vivo and enhances the K+-induced release of dopamine from dopaminergic neurons in vitro [16]. The implication of dopamine is supported by the hyperactivity of infected rodents [17]–[21], altered novelty seeking behavior in infected rodents [17], [18], [22], [23] and humans [12], [24,], and by the fact that dopamine agonists and antagonists disrupt the parasite-induced behavioral phenotypes [20], [25]. These findings reinforce the putative link between toxoplasmosis and schizophrenia (e.g., [26], [27]).
Paradoxically, the distribution of the parasite in the brain has received only scant attention (mouse: [21], [28]–[30]; immunocompromised mouse: [31]; rat: [7], [32]). Useful information also comes from neuroradiological and neuropathological examinations of human patients with AIDS (e.g., [33]–[36]). Most of the studies mentioned provide only a rough description of the parasite distribution and are based on arbitrarily chosen or equidistant sections. In this study, we therefore systematically mapped the distribution of T. gondii tissue cysts and histopathological lesions in the brains of mice with latent toxoplasmosis. Special attention was paid to the dopaminergic system and brain regions implicated in the modulation of defensive and aversive behaviors, spatial orientation and learning, and sensorimotor performance. The revealed distribution pattern elucidates mechanisms underlying parasite-induced behavioral changes.
Results
Clinical appearance
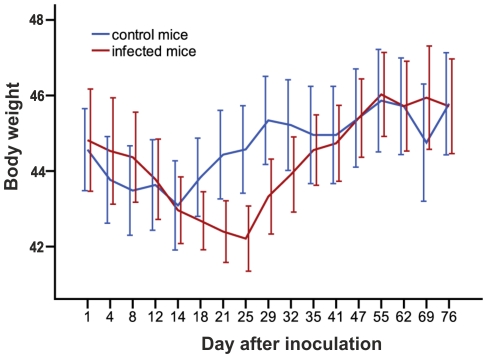
Typical symptoms of acute toxoplasmosis, i.e., lethargy, ruffled fur or hunched posture were not apparent after peroral infection with 10 tissue cysts of the avirulent HIF Toxoplasma strain. Nevertheless, we observed a significant transient reduction of body weight in the infected mice from 21st to 29th day p.i. (GLM repeated measures, p = 0.017; Fig. 1). After this period, which likely coincided with the acute phase of toxoplasmosis, the body weights were no longer different between the infected and control mice (p = 0.783).
Figure 1. Transient reduction of body weight in the infected mice.
The abscissa shows body weight, the ordinate shows time (in days) after inoculation. Error bars denote the 95% confidence interval.
Number and size of T. gondii cysts
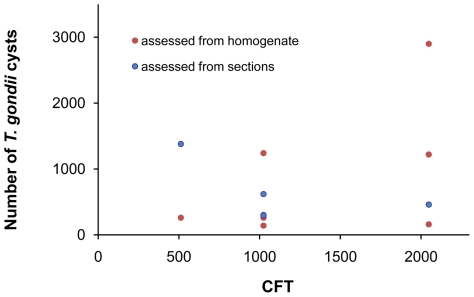
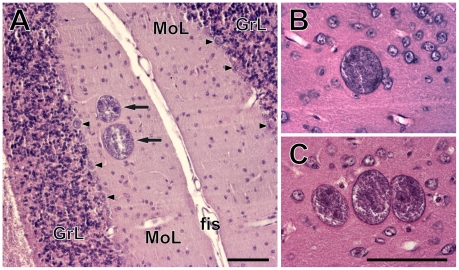
Tissue cysts of T. gondii were found in brains of all infected mice. In the histologically processed brains, the numbers of brain cysts observed ranged between 297 and 1380 (mean ± s. d. = 611±402). The admittedly less reliable estimates based on brain homogenates showed a wider range between 140 and 2900 (mean ± s. d. = 883±938). It is unclear whether these latter counts reflect inter-individual differences in cyst density or technical limitations of the method. There was no relation between the anti- T. gondii titer and the number of brain tissue cysts (Kendall's τ = 0.093, p = 0.705; Fig. 2). The size of the cysts ranged between 10 and 70 µm. They were found solitary or in groups of 2–10 in hematoxylin-eosin stained sections (Fig. 3). On average, about 80% of the cysts were solitary, ∼13% of the cysts were found in pairs, ∼4% in triads; larger groups were rare.
Figure 2. Numbers of brain tissue cysts and T. gondii antibody titers are not correlated.
The abscissa shows total numbers of brain cysts, the ordinate shows antibody titers determined by the complement fixation test.
Figure 3. Toxoplasma gondii cysts in the brains of chronically infected CD1 mice.
(A) A pair of cysts (arrows) in the molecular layer of the cerebellar cortex. Arrowheads point to the Purkinje cells. GrL, granular layer; fis, cerebellar fissure; MoL, molecular layer. (B–C) A single cyst (B) and a cyst triad (C) in the isocortex. Scales, 100 µm (in C for B, C).
Neither T. gondii cysts in the brain nor anti- T. gondii antibodies in the blood were detected in the control mice.
Distribution of T. gondii cysts in the CNS
Tissue cysts of the parasite were found in 54 anatomically defined brain regions, which altogether occupied 92% of the brain (Table 1). Locations of all cysts recorded within five brains examined in detail are shown in Figures 4, 5. The cysts were bilaterally distributed; no discernible lateralization was observed. However, the cyst density was not homogeneous, i.e., some brain regions were infected significantly more than others (GLMM, χ2 = 1086.4, p<10−6) and, importantly, brain region typical infestation levels featured a significant concordance among the infected mice (Kendall's W = 0.419, p<0.001). Among five fundamental brain parts, the telencephalon exhibited the highest cyst density. Comprising ∼56% of the mouse brain volume it contained ∼75% of cysts. Indeed, with very few exceptions, all consistently highly infected brain regions were located within the telencephalon (see below). In contrast, the metencephalon was much less infected than expected from its volume. Comprising ∼12% of the brain volume it contained only ∼5% of cysts. The remaining major brain parts (the diencephalon, mesencephalon and myelencephalon) contained numbers of cyst that corresponded well with their volumes.
Table 1. Quantitative data on Toxoplasma gondii cysts distribution.
| Brain region | Volume (mm3) | Mean numbers of cysts ± SD | Mean cyst density ± SD (cysts/mm3) | Tropism indexa | GLMMb |
| Forebrain cortex | |||||
| Olfactory bulb | 11.09 | 38.8±23.76 | 3.50±2.14 | 5 | *** |
| Olfactory nucleus | 2.19 | 6.8±6.62 | 3.11±3.03 | 3 | n.s. |
| Frontal association cortex | 1.31 | 7±4.69 | 5.33±3.57 | 4 | *** |
| Orbital cortex | 4.24 | 13.8±7.98 | 3.26±1.88 | 5 | *** |
| Olfactory tubercle | 1.62 | 3.4±4.92 | 2.10±3.05 | 2 | n.s. |
| Piriform cortex | 15.02 | 21.8±19.28 | 1.45±1.28 | 1 | n.s. |
| Tenia tecta | 1.14 | 6.6±4.45 | 5.79±3.91 | 4 | *** |
| Dorsal peduncular cortex | 0.61 | 0.6±0.80 | 0.99±1.32 | 2 | n.s. |
| Limbic cortex | 1.93 | 2±1.79 | 1.04±0.93 | 2 | n.s. |
| Cingulate cortex | 4.45 | 9.6±7.68 | 2.16±1.73 | 3 | n.s. |
| Motor cortex | 16.14 | 51.4±45.94 | 3.17±2.85 | 5 | *** |
| Somatosensory cortex | 28.45 | 78.6±70.31 | 2.76±2.47 | 5 | *** |
| Insular cortex | 6.76 | 13.6±16,41 | 2.01±2.43 | 2 | n.s. |
| Retrosplenial cortex | 5.82 | 5.8±1.47 | 1.00±0.25 | 1 | n.s. |
| Parietal cortex | 1.61 | 3.8±2.93 | 2.36±1.82 | 3 | n.s. |
| Visual cortex | 11.24 | 25.8±21.83 | 2.30±1.94 | 4 | n.s. (*) |
| Auditory cortex | 5.22 | 8.4±7.17 | 1.61±1.38 | 3 | n.s. |
| Temporal cortex | 1.12 | 2.4±4.32 | 2.15±3.86 | 1 | n.s. |
| Entorhinal cortex | 12.65 | 39.4±24.51 | 3.12±1.94 | 5 | *** |
| Hippocampus | 27.02 | 64.4±59.92 | 2.38±2.22 | 4 | *** |
| Forebrain subcortical regions | |||||
| Amygdala | 9.59 | 20.6±15.33 | 2.15±1.60 | 4 | n.s. |
| Septal nuclei | 4.46 | 5.6±4.76 | 1.25±1.07 | 2 | n.s. |
| Caudate putamen | 20.92 | 17.4±6.09 | 0.83±0.29 | 0 | *** |
| Globus Pallidus | 2.30 | 1±1.26 | 0.44±0.55 | 2 | n.s. (*) |
| Nucleus accumbens | 3.28 | 4.4±3.20 | 1.34±0.98 | 1 | n.s. |
| Ventral pallidum | 1.66 | 5.6±1.85 | 3.38±1.12 | 5 | n.s. (**) |
| Substantia innominata | 0.89 | 0.2±0.40 | 0.22±0.45 | 1 | n.s. |
| Thalamus | 16.58 | 32.2±15.93 | 1.94±0.96 | 3 | n.s. |
| Hypothalamus | 11.86 | 10.4±6.22 | 0.88±0.52 | 1 | *** |
| Zona incerta | 1.56 | 3±1.41 | 1.93±0.91 | 3 | n.s. |
| Midbrain | |||||
| Superior colliculus | 4.59 | 7.6±9.46 | 1.66±2.06 | 2 | n.s. |
| Inferior colliculus | 6.26 | 6.4±6.05 | 1.02±0.97 | 1 | n.s. (**) |
| Tegmentum | 6.73 | 15±10.35 | 2.23±1.54 | 4 | n.s. |
| Periaqueductal gray | 2.83 | 3.6±5.24 | 1.27±1.85 | 1 | n.s. |
| Substantia nigra | 1.96 | 4.2±2.79 | 2.14±1.42 | 3 | n.s. |
| Paranigral nucleus | 0.07 | 0.6±1.20 | 8.11±16.22 | 1 | n.s. (*) |
| Ventral tegmental area | 0.72 | 3.2±3.66 | 4.45±5.08 | 3 | * |
| Deep mesencephalic nucleus | 4.49 | 5.2±4.12 | 1.16±0.92 | 2 | n.s. |
| Lateral lemniscus nuclei | 1.09 | 2.2±1.47 | 2.03±1.35 | 3 | n.s. |
| Hindbrain | |||||
| Cerebellum | 35.86 | 20.6±17.06 | 0.58±0.48 | 0 | *** |
| Pontine nuclei | 0.75 | 0±0 | 0.00±0 | 0 | *** |
| Vestibular nuclei | 2.29 | 3.8±2.04 | 1.66±0.89 | 3 | n.s. |
| Locus coeruleus | 0.08 | 0.2±0.40 | 2.59±5.18 | 1 | n.s. |
| Periolivary region | 1.19 | 1.8±1.33 | 1.51±1.12 | 3 | n.s. |
| Raphe nuclei | 1.87 | 2±1.26 | 1.07±0.68 | 2 | n.s. |
| Intermediate reticular nucleus | 1.89 | 4.2±4.40 | 2.23±2.33 | 3 | n.s. |
| Parvicellular reticular nucleus | 4.58 | 2.6±3.32 | 0.57±0.73 | 1 | ** |
| Gigantocellular reticular nucleus | 3.34 | 7.2±4.62 | 2.16±1.39 | 4 | n.s. |
| Spinal trigeminal nucleus | 4.58 | 4±3.03 | 0.87±0.66 | 2 | n.s. |
| Medullary reticular nucleus | 0.59 | 5±4.90 | 8.48±8.30 | 4 | *** |
| Hypoglossal nucleus | 0.45 | 2.6±3.38 | 5.83±7.58 | 3 | ** |
| Facial nucleus | 0.76 | 3.4±4.92 | 4.50±6.51 | 2 | * |
| Fiber tracts & commissures | |||||
| Corpus callosum | 10.29 | 0.2±0.40 | 0.02±0.04 | 0 | *** |
| Cerebral peduncle | 1.54 | 1.2±1.17 | 0.78±0.76 | 1 | n.s. (*) |
| Anterior commissure | 1.05 | 0±0 | 0.00±0 | 0 | *** |
| Capsula interna | 2.43 | 0±0 | 0.00±0 | 0 | *** |
Tropism index indicates the number of animals in which infestation was higher than expected from the total parasite load and volume of a particular structure.
The GLMM (generalized linear mixed model) tests positive or negative tropism toward a particular structure. Significance after Šidák correction is indicated by asterisks; significance before the Šidák correction is given in parentheses. Significance levels:
***, P<0.001;
**, P<0.01;
*, P<0.05; n.s., not significant. See Methods for details.
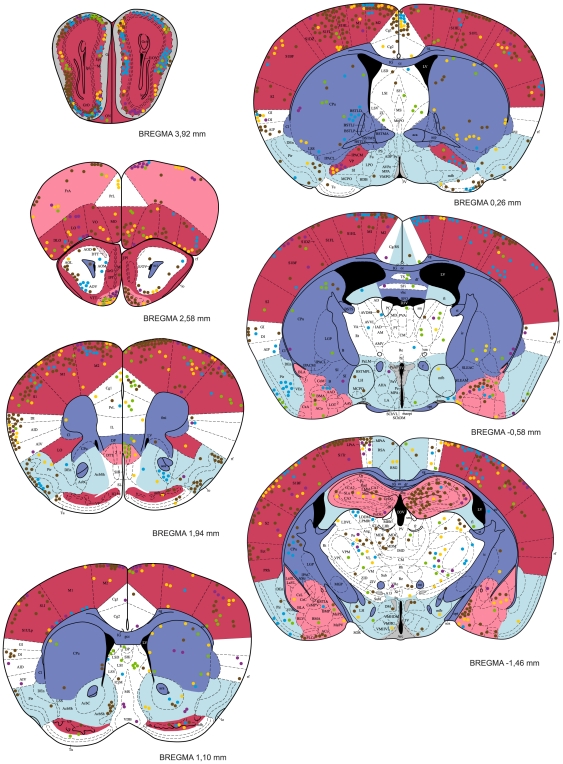
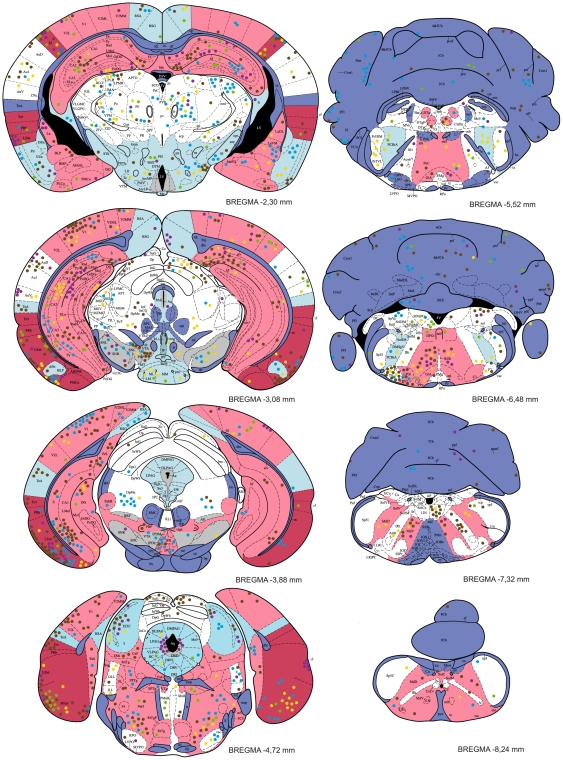
Figure 4. The distribution of T. gondii cysts in the forebrain of a mouse with latent toxoplasmosis.
Coronal diagrams compile results from five infected CD1 mice. Tissue cyst locations are indicated by color circles (each circle represents a single cyst); colors refer to the individual animals investigated. The cyst density within brain regions is classified into five classes – very high, high, medium, low, and very low, which are colored red, pink, white, blue, and dark blue, respectively. Grey indicates the dopaminergic system. The antero-posterior positions of the sections are determined by Bregma coordinates. See Abbreviations S1 for abbreviations.
Figure 5. The distribution of T. gondii cysts in the caudal forebrain, the cerebellum and the brainstem of a mouse with latent toxoplasmosis.
See caption to Fig. 4 for explanation.
Within the telencephalon, a consistently high cyst density (i.e., seen in all five brains examined) was observed in the external plexiform layer of olfactory bulb, the ventral pallidum and the entorhinal, somatosensory, motor and orbital cortices. Moreover, in four out of five brains examined, a high cyst density was observed in the frontal association and visual cortices, the tenia tecta, and, importantly, the hippocampus and the amygdala. Among non-telencephalic brain regions, the tegmentum, the gigantocellular and medullary reticular nuclei exhibited a high cyst density in four out of five brains examined. By contrast, a consistently low incidence of tissue cysts was recorded in the cerebellum, the pontine nuclei, the caudate putamen, the accessory olfactory bulb, and virtually all compact masses of myelinated axons. For instance, the corpus callosum, the anterior and posterior commissures, the capsula interna, and the cerebellar peduncles were devoid of cysts; the cerebral peduncle, the fimbria of the hippocampus and the hippocampal commissures featured a low incidence of cysts. On average, the cyst density was ∼12 times lower in the white matter than that in the grey matter. In four out of five brains examined, a low cyst density was also observed in the piriform, retrosplenial and temporal cortices, the nucleus accumbens, the substantia innominata, the hypothalamus, the periaqueductal grey, the inferior colliculus and the parvicellular reticular nucleus.
It is notable that, in the isocortex and the cerebellar cortex, cysts were most abundant within the molecular and/or external granular layers and within the molecular layer, respectively.
No evidence for tropism toward the dopaminergic system
None of the structures containing dopaminergic neurons exhibited a consistently high incidence of T. gondii cysts (Table 1, Figs. 4, 5). The substantia nigra (A 9) and the ventral tegmental area (A 10) were highly infected in three and two animals, respectively. In the remaining animals, these structures exhibited a relatively low incidence of cysts. In the zona incerta, a high incidence of cysts was observed in a single animal, a low incidence in two animals. Moreover, cysts were not localized within its ventromedial part that contains dopaminergic neurons (A 13, cf. Fig. 4). Likewise, the mesencephalic periaqueductal grey (A 11) was highly infected only in a single animal. Hypothalamus and, in particular, all hypothalamic regions containing dopaminergic neurons (i.e., dorsal and posterior hypothalamus, A 11; arcuate nucleus, A 12; periventricular hypothalamus, A 14; the ventral surface of hypothalamus and ventral part of the bed nucleus of stria terminalis, A 15) featured a low incidence of cysts (Figs. 4, 5). Finally, within the olfactory bulb, cysts were preferentially located in the external plexiform layer and only seldom occurred in the glomerular layer. Thus, the cysts were in close vicinity but not in contact with the dopaminergic periglomerular cells (note that both dendrites and axons of these interneurons are confined within the glomerular layer).
No evidence for tropism toward the hypothalamic defensive system (HDS)
The HDS (for review see [37]) plays a pivotal role in eliciting innate defensive behavior after exposure to a natural predator. It consists of three highly interconnected hypothalamic medial zone nuclei, namely, the anterior hypothalamic nucleus, the dorsomedial part of the ventromedial nucleus and the dorsal premammillary nucleus. These nuclei were devoid of cysts.
In addition, except for the amygdala (see below) none of the brain regions that provide major neural inputs to and/or outputs from the HDS featured a consistently high incidence of T. gondii cysts. For instance, the bed nucleus of the stria terminalis, the infralimbic cortex and the lateral preoptic area were virtually devoid of cysts; the lateral septal nucleus and the periaqueductal grey was highly infected only in a single animal; the prelimbic cortex and the ventral tegmental area in two animals. Amygdaloid nuclei that project to the HDS, namely the posteroventral part of the medial amygdaloid nucleus and the posterior part of the basomedial amygdaloid nucleus were infected in three animals; the lateral amygdaloid nucleus that receives input from the HDS was infected in four animals.
Besides the aforementioned structures, numerous other brain regions have been reported to be activated by exposure to a live cat or cat odor [38]–[43]. None of these brain regions but the motor cortex featured a consistently high incidence of cysts. A few other regions also exhibited a high cyst density, although not in all animals. The anterior olfactory nucleus, the cingulate cortex, and the ventral orbital cortex were highly infected in three animals. The remaining telencephalic “defense-related” regions, including the infralimbic, piriform and retrosplenial agranular cortices, the septum, the shell of the nucleus accumbens and the caudate putamen were less infected than expected from their volumes. The lateral hypothalamus, the perifornical region and the reuniens thalamic nucleus were infected in three animals, the dorsomedial hypothalamic nucleus and the anteromedial thalamic nucleus in one animal. Other hypothalamic and thalamic “defense-related” regions, including the medial preoptic, lateral preoptic and retrochiasmatic areas, and the paraventricular hypothalamic and paraventricular thalamic, intralaminar thalamic and lateral habenular nuclei were devoid of cysts. Finally, the cuneiform nucleus was highly infected in one animal and a single cyst was observed in the locus coeruleus.
No evidence for tropism toward specific amygdaloid compartments
As noted above, the amygdaloid complex was highly infected in four out of five brains examined. It is to be noted, however, that cysts were not preferentially associated with a particular amygdaloid compartment and the cyst distribution differed significantly between individuals. Nevertheless, if data compiled from five brains are considered, some amygdaloid divisions were more infected than others. A high cyst density was observed in the anterior amygdaloid area, the medial and cortical amygdaloid nuclei; a moderate cyst density in the lateral, basolateral and basomedial amygdaloid nuclei and in the amygdalohippocampal area. Importantly, a single cyst was observed in the central amygdaloid nucleus.
Distribution of T. gondii cysts in the hippocampus and parahippocampal region
The entorhinal cortex was highly infected in all brains examined. Both the medial and lateral entorhinal cortices were highly infested with cysts. A much lower cyst density was observed in the ectorhinal and perirhinal cortices, which were infected in one and three animals, respectively. Similarly, the presubiculum and parasubiculum were infected only in two animals. The hippocampus was highly infected in four out of five brains examined. But again, distribution differed significantly between individuals and no tropism toward a particular hippocampal compartment was observed. The cysts were abundant within all cytoarchitectonically distinct regions: the dentate gyrus, the CA1–CA3 fields and the subiculum. Topographically, both the dorsal (septal) and the ventral (temporal) hippocampus were highly infected.
Histopathological features of latent toxoplasmosis
Macroscopically, brains of the infected mice appeared normal in volume, shape and coloring. Nevertheless, a microscopic analysis revealed various histopathological lesions. Numerous perivascular and leptomeningeal infiltrations of inflammatory cells were observed, most often on the surface of the cerebral cortex and in the interhemispheric region (Figs. 6A–E); occasional occurrence of necrosis of the brain parenchyma was also noted (Figs. 6F, G). The motor, somatosensory, entorhinal, frontal association and orbital cortices were most affected, but the histopathological lesions were also seen in the olfactory bulb, the hippocampus, the thalamus, the hypothalamus, the caudate putamen and the cerebellum. Importantly, these lesions were not located in the close vicinity of T. gondii cysts.
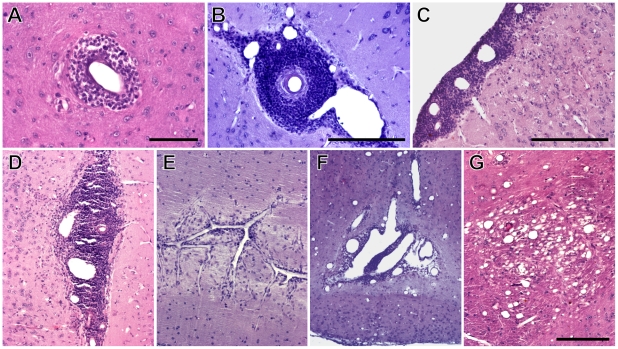
Figure 6. Histopathological lesions associated with latent toxoplasmosis.
(A–E) Perivascular and leptomeningeal infiltrations of inflammatory cells. (A, B) Detail of perivascular cuffing. (C–E) Leptomeningeal and perivascular cuffing on the surface of cerebral cortex (C), in the interhemispheric region (D) and in the cerebellum (E). (F) Extensive necrosis in the hypothalamus (observed in one mouse). (G) Vacuolisation of brain parenchyma in the thalamus. Note that vessels were dilated post mortem by pressure of the perfusion liquids. Scales, 100 µm (A), 200 µm (B, C, G, in G for D–G).
The cerebral microvasculature exhibited strong dilatation. However, the omnipresence and the extent of vasodilatation strongly suggest that vessels were dilated post mortem by pressure of the perfusion liquids.
None of the above described lesions but vasodilatation were observed in the control mice.
Discussion
Cysts containing T. gondii were found in brains of all the infected mice, but the total parasite load differed starkly between experimental animals. Remarkably, the most infested brain exhibited a more than one order of magnitude higher cyst density than the least infested one. The cysts were distributed in a wide variety of brain regions. Indeed, as much as 92% of the brain regions examined contained tissue cysts. Hence, T. gondii might affect the information processing within a wide variety of brain functional systems, provided that the parasite is able to alter brain function locally (no matter by which proximate mechanism). In spite of a significant inter-individual variability, four general properties of T. gondii distribution stand out from the scrutiny performed here. First, the cerebral cortical areas almost always exhibited higher cyst density than subcortical regions. Notable exceptions comprised of the poorly infested piriform, retrosplenial and temporal association cortices on one hand and the highly infested amygdala and tegmentum on the other hand. Second, the cerebellum featured a consistently low incidence of cysts. Third, compact myelinated fiber tracts and commissures were virtually devoid of cysts. Moreover, subcortical structures bounded by compact masses of myelinated axons (e.g., the caudate putamen and the pontine nuclei) were ranked among the least infected structures, implying that compact myelinated bodies constitute a barrier for the parasite. Finally, a selective tropism of T. gondii toward a particular functional system was not observed. In the context of the recently discussed hypotheses (see Introduction) it is important to note that the parasite is guided neither toward the dopaminergic system nor toward the hypothalamic defensive system.
Health status and total parasite load in the brain
The clinical appearance of acute acquired toxoplasmosis depends on the virulence of the T. gondii strain used, the initial dose and the port of entry of the parasite, the host species and/or strain, and the immunological and genetic status of the host [44]. To mimic natural conditions, peroral inoculation and a low challenge dose were used in the current study. We observed no apparent symptoms of the acute toxoplasmosis except for transient reduction of the body weight, which is in agreement with the previously reported asymptomatic course of the HIF strain infection [20], [45]. Weight reduction during the fourth week p.i. probably coincided with the onset of cellular and antibody immune responses and formation of intracellular cysts in the brain [46], [47]. A mild loss of weight in control mice immediately after the inoculation could be caused by irritation of the digestive tract with the stomach tube.
The aforementioned factors are likely also decisive for the parasite load in the brain, which, in turn, determines behavioral phenotype of the host with latent toxoplasmosis. Studies addressing behavioral and/or neurological abnormalities induced by T. gondii infection utilized various experimental paradigms, including intraperitoneal (e.g., [7], [9], [10], [21], [31], [32], [48], [49]), subcutaneous (e.g., [17], [29], [30]) or peroral (present study, [6], [19], [20], [25], [45], [50]) administration of various parasite doses and congenital T. gondii infection [18], [23], [51], [52].
Not unexpectedly, the different paradigms yielded ample variation in brain infestation (cf. e.g., current study, [7], [17], [20], [51]). More surprisingly, stark differences in brain cyst numbers were observed even when the same T. gondii strain and the administration protocol were utilized. For instance, the peroral inoculation of 10 tissue cysts of the HIF strain resulted in high (∼770 cysts per brain), mid (∼225 cysts per brain) and low (∼46 cysts per brain) brain infestation in CD1 outbred mice, CBA/J mice and F1 crosses between BALB/c and B10A mice, respectively (present study, [20], [45]). These observations are in line with strain-dependent differences in the murine susceptibility to toxoplasmosis [53], [54]. A rather low brain infestation has been reported in the rat [7], [32].
Lack of tropism and probabilistic nature of T. gondii distribution
The present study and earlier reports on the parasite distribution in the brain ([7], [21], [28]–[32], see Introduction) point to some general features but also to a marked variability in the parasite distribution patterns. First, and foremost, a very selective tropism of T. gondii toward a specific brain region and/or functional system is not indicated. All the aforementioned studies describe a rather wide T. gondii distribution. Moreover, the observed distribution patterns differ between experiments. For instance, the highest cyst density was reported for the olfactory bulb [29], the olfactory bulb and the cerebral cortex (present study), the cerebral cortex [31], the amygdala [7], the nucleus accumbens and the ventromedial hypothalamus [32] and the medulla oblongata [30]. In rat, the parasite seems to be much more confined to the medial brain regions than in the mouse (cf., [32]). Likewise, the olfactory bulb seems to be much less infected in the rat [7] when compared to the mouse. In AIDS patients with toxoplasma encephalitis, the lesions were found mainly in hemispheres (often in the fronto-parietal cortex), thalamus and basal ganglia [33]–[35], the parasites were scattered throughout the different brain parts [36]. These discrepancies may hypothetically be attributed to the differences in host species and/or strain, the T. gondii strain used, the inoculation paradigm and the post-infection time. One may also speculate that at least some of the aforementioned findings are inaccurate since prior studies are either based on qualitative analyses or semi-quantitative analyses of arbitrarily chosen sections or restricted to brain regions of interest. In any case, this limited data set suggests an unforeseen variability in the parasite distribution.
Second, a low incidence of the parasite in the cerebellum has been reported repeatedly (present study, [21], [30], [31]). The low level of infection might be caused by the cerebellar cytoarchitecture. The cerebellum features an extremely high cellular density, very small neurons and a low glia to neuron ratio [55], [56]. Given that neurons are much less efficiently infected by T. gondii than are astrocytes, and that cerebellar granular neurons are still less efficiently infected in vitro than hippocampal neurons (for review, see [57]), it is tempting to speculate that a high proportion of small, tightly packed granular neurons constitutes a limiting factor for T. gondii zoites proliferation and subsequent cyst formation.
Third and finally, a low incidence of cysts is also typical for the white matter ([31], present study). The present study highlights a virtual absence of cysts in compact myelinated fiber tracts and commissures. This observation is in line with the fact that toxoplasma encephalitis is, in contrast to encephalitis of other etiology (e.g. primary brain lymphoma), rarely associated with lesions in the corpus callosum [58].
Taken together, the T. gondii distribution in the brain seems to be of a probabilistic nature. Factors determining the parasite distribution remain largely unclear. As noted above, we suggest that compact myelinated bodies may act as natural barriers for the parasite and that cytoarchitectural features such as the neuron size and glia to neuron ratio may influence the local parasite load. However, it seems likely that the mechanism underlying brain colonization during the acute infection is most decisive for the parasite distribution. T. gondii tachyzoites disseminate by the bloodstream using dendritic cells and monocytes/macrophages as transporters (Trojan horses) to the organ tissues, including the brain extravascular space ([59], [60], and citations therein). Abundant presence of T. gondii in the brain parenchyma and its scarcity in the plexus choroideus and circumventricular regions ([31], [61], present study, but see [30]) suggest that the parasite crosses the blood-brain barrier via the cerebral microvasculature rather than the blood-cerebrospinal fluid barrier via the plexus choroideus. Furthermore, it has been suggested that a higher number of parasites may reach brain regions that are characterized by higher blood flow during the acute phase of infection [31]. Although the hemodynamic response to neural activity is proximately driven by neuronal signalling, the local blood flow generally correlates with metabolic activity [62]. Consistently with this “high blood flow – high parasite density” hypothesis, we observed a high incidence of the parasite in the olfactory bulb and primary visual, somatosensory and motor cortices that feature a high level of oxidative metabolism, but a rather low incidence of parasite in the piriform cortex and some association cortices that feature a moderate level of oxidative metabolism (c.f. [63]). On the other hand, we observed accumulation of the parasite in neither the retrosplenial and auditory cortices nor the subcortical regions that feature a high metabolic activity. So this hypothesis is not tenable. Consequently, other factors, e.g. local properties of the blood-brain barrier, seem to be at work. A firm understanding of T. gondii distribution in the brain will require further mechanistic studies.
Effective manipulation of host behavior without well targeted tropism of parasite?
Recent literature suggests that T. gondii highly specifically decreases the vigilance of rats and mice for feline predators [6]–[9]. Infected rodents lose their innate fear and in fact are mildly attracted to cat odor, but retain the aversion to odors of other predators like mink or dog. This ‘suicidal’ attraction thus appears to be restricted to the feline predators, which serve as definitive hosts of the parasite. Such an intriguingly specific manipulation of the host behavior clearly calls for a highly specific mechanistic explanation [5]. A selective impairment of olfaction and an alteration in the emotional valence of cat odor achieved by the T. gondii-induced modification of neural processing within the amygdala and/or the nucleus accumbens are most discussed in this context (for review see [5], [13]). While all these hypotheses are highly relevant, their general validity is undermined by the lack of well targeted tropism of T. gondii. Indeed, the consistency in the behavioral responses starkly contrasts with the differences in the total parasite load and distribution between individuals, species and, in particular, between different studies (see discussion above). For instance, a high parasite density in the olfactory bulb has been reported in the mouse (∼3.5 cyst/mm3, present study) but not in the rat (<0.1 cyst/mm3, [7]). Furthermore, the distribution of the parasite does not suggest that T. gondii targets specifically the olfactory glomeruli important for processing innate responses to the predator odor. Innate responses to aversive odorants, including the avoidance of predators' smells, are mediated by receptors located in a dorsal zone of the olfactory epithelium, which project their axons to the anterodorsal domain of the olfactory bulb [64]. In addition, at least some aspects of feline odor are perceived and processed as pheromone-like stimuli that activate the accessory olfactory bulb and its projection areas [41], [43]. One would thus expect tropism towards the dorsal domain of the main accessory olfactory bulb and towards the accessory olfactory bulb. However, we have observed T. gondii cysts throughout the olfactory bulb, with the highest density within its lateral portion and almost no cysts in the accessory olfactory bulb.
Likewise, subtle tropism towards the amygdala has been reported in both rat and mouse, but the absolute density is much higher in the mouse (rat ∼0.15 cyst/mm3, [7]; mouse ∼2.1 cyst/mm3, present study); the parasite is not associated with particular, functionally well-defined amygdaloid nuclei (present study). The amygdala is not only essential for Pavlovian fear conditioning (for review, see e.g. [65], [66]) but via projection to the HDS also modulates unconditioned (innate) defensive responses [37]. Receiving inputs form thalamic and cortical sensory processing regions [67] it seems to be important for detecting and assessing danger in the sensory world. It is notable in this context that we have observed a rather high parasite density within the medial amygdaloid nuclei, which receive a robust input from the main and accessory olfactory systems [67] and express c-Fos after cat-odor exposure [39]. Lesions of the medial amygdala reduce freezing and risk assessment during cat-odor exposure [68]. It is also important to note that we have observed a high parasite density (∼2.4 cyst/mm3) in the ventral hippocampus. The ventral hippocampus modulates unconditioned defensive behaviors through its connections with the amygdala and hypothalamus (e.g., [69]–[71]). It sends projections to the HDS via the amygdala and bed nucleus the stria terminalis, and via the lateral septum. Lesions of the ventral hippocampus reduce anxiety-like behaviors in various experimental paradigms (e.g., [72]–[74]), importantly including cat-odor exposure [75]. But again, the ventral hippocampus was poorly infested in rat (<0.1 cyst/mm3, [7]). Finally, a high relative parasite density in the nucleus accumbens has been reported in the rat [32] but not in the mouse (∼1.3 cyst/mm3, present study). Indeed, this structure was less infected than expected from its volume in the mouse. Besides a pivotal role in encoding reward and aversion [76], the nucleus accumbens seems to be also involved in fear conditioning [77]. The activity of its principal neurons – medium spiny GABAergic neurons – is modulated by dopamine input from the ventral tegmental area and glutamate input from regions including the prefrontal cortex, amygdala and hippocampus (for review, see [76]), i.e., regions all reported to be infested in rodents with latent toxoplasmosis ([7], [32]; present study). Moreover, recent work suggests that changes in the activity of the dopaminergic neurons in the ventral tegmental area can also encode both rewarding and aversive states [78].
Taken together, the present data suggest that the attenuated defensive behavior of infected rats and mice may stem from serendipitous infestation of various brain regions implicated in modulation of defensive and aversive behaviors. These regions include the amygdala, the ventral hippocampus, the nucleus accumbens, the ventral tegmental area and the medial prefrontal cortex. The probability that at least some of these regions will be infected by chance seems to be quite high. This is consonant with reports that T. gondii reduces anxiety (the elevated plus-maze and the social interaction tests – [32]; the hole-board test – [20]) and affects learned fear response in chronically infected rodents [48]. Furthermore, it is also conceivable that T. gondii alters the olfactory processing of cat odor through serendipitous infestation of the olfactory glomeruli processing this information. Nevertheless, the predator-specific reduction of vigilance (i.e., fatal attraction to cat odor) is difficult to explain. While a possible existence of neural mechanisms or substrates that are dedicated only to the processing of cat odors (c.f., [5]) clearly deserves further research, the above reviewed observations are inconsistent with a notion that the parasite specifically targets the neural substrate of innate aversion to felines.
A potential effect of T. gondii on motor coordination, sensory processing and spatial navigation
A disruption of the defensive behavior clearly is not the only mechanism by which T. gondii can increase its transmission to the definitive feline host. First and foremost, infestation of various stages of the somatosensory and motor systems may compromise motor performance, sensorimotor integration and motor coordination. Indeed, we have observed a high parasite density in the somatosensory and motor cortices and in the ventrolateral thalamic nucleus. The latter is the main motor thalamic relay conveying cerebellar input to the motor and premotor cortical areas; the cerebello-thalamo-cortical pathway is most likely involved in the coordination of multi-joint movements (for review, see [79]). T. gondii infestation of the spinal cord has also been reported recently [28]. These observations are in line with motor coordination deficits reported in infected rodents [10], [21], [22], [80] and humans [81]–[83].
Second, a high to medium infestation of the olfactory bulb, the anterior olfactory nucleus, the visual cortex and the barrel field of the primary somatosensory cortex (i.e., the region that processes tactile information from the whiskers) may affect processing of sensory information. While nothing is known about the sensory capacities of chronically infected rodents, deficits in sensory driven reflexes such as the visual placing response (an extension of the paws on approach to a visual surface) or orientation response to whisker stimulation [21] clearly demonstrate impairment of sensorimotor integration. In addition, reduced sniffing, rearing and whisking during the first exposure to an open field [10], [20], [21], [84] indicate sensory attention deficits. A prolonged time devoted to sniffing and head dipping in holes in the hole-board test [20] seems to contradict these results. However, it is likely the anxiolytic effect of toxoplasmosis what accounts for high scores in this exploration-based anxiety test (c.f., [85]). Moreover, it is tempting to speculate that longer sniffing at holes is required to assess olfactory information due to T. gondii-induced impairment of olfaction. But even if true, the impairment clearly is not an absolute all-or-nothing phenomenon since infected rodents reportedly retain an aversion to food with a novel odor [7] and aversion to odors of predators that do not serve as a definitive host of T. gondii [8], [9].
Finally, a high infestation of the entorhinal cortex (∼3.1 cyst/mm3) and the dorsal hippocampus (∼2.4 cyst/mm3) observed in this study suggests that T. gondii may reduce the efficiency of near space navigation (i.e., orientation within home range). These structures play pivotal roles in spatial navigation [86]–[88] and certain forms of learning and memory [71], [89], [90]. More specifically, the hippocampal place cells (pyramidal neurons) exhibit location-specific activity and their entire population likely generates an abstract map-like representation of the animal's spatial surroundings [86]. Besides encoding the animal's position, they are involved in the forming and consolidation of context-specific episodic memory (e.g., [91]–[93]). The entorhinal cortex harbors the grid cells [94], [95], which possess tessellating firing fields and encode environment-independent (path integration generated) neuronal map of self-location [88], and head direction cells [96], which have direction-specific activity and encode the animal's directional heading [97]. Thus, T. gondii is well placed to affect spatial orientation, learning and memory. Yet, behavioral evidence remains controversial. Infected mice exhibited diminished spatial learning and memory in double-training maze experiments [49], [98] and in the 8-arm radial maze test [50], but normal memory in object placement and object recognition tests [21]. Y-maze experiments suggested deficits in spatial working memory but not in recognition memory [9]. Infected rats showed impaired spatial learning but not memory in the same experiments [49], [98] and intact spatial learning in the Morris water maze [7]. It is likely, however, that various parasite loads and distributions in the brain account for most of these inconsistencies. Mice often feature a much higher brain infestation than rats (see discussion above) and hence more severe cognitive deficits. The fact that the learning performance of mice is negatively correlated with the number of brain cyst [49] further supports this assumption. Additionally, in stark contrast with the situation in mice, the hippocampus and parahippocampal region were reported to be poorly infested or devoid of cysts in rats [7], [32]. The histological findings thus seem to be largely consistent with the cognitive deficits.
Mechanisms underlying T. gondii-induced modification of brain function
As we have argued above, the pattern of T. gondii distribution in the brain may explain many of the behavioral abnormalities observed in the chronically infected rodents. But how does T. gondii alter brain function locally? The proximate mechanisms underpinning the T. gondii effect on local neural processing were not the subject of the present study. Nevertheless, two important inferences as to the mechanism of action can be drawn from the distribution data presented here. First, a remarkable interindividual variability in the distribution of the observed histopathological lesions suggests that T. gondii-induced local inflammatory immune responses and neurodegenerations cannot account for the highly consistent and specific behavioral changes associated with latent toxoplasmosis. However, it has to be noted for the sake of unbiased interpretation that frequent occurrence of perivascular inflammation contiguous to the hippocampus and the aqueduct of Sylvius has been reported recently [10]. In any case, altered neuromodulator levels secondary to inflammation may influence the behavioral phenotype of infected individuals. This phenomenon, together with the neurodegenerative loss of brain parenchyma, might significantly increase the predation risk in the infected rodents. Second, the lack of tropism towards the dopaminergic system clearly shows that the parasite is not specifically guided to dopaminergic neurons. On the other hand, the high average cyst densities in the ventral tegmental area (∼4.4 cyst/mm3) and the substantia nigra (∼2.1 cyst/mm3) suggest that the mere serendipitous infestation of the regions containing dopaminergic neurons may have a significant effect on dopamine metabolism. This is in line with the recent evidence for increased dopamine release from dopaminergic neurons infected with T. gondii [16]. Moreover, the fact that T. gondii cysts in the mouse brain co-express high levels of the parasite-encoded, dopamine synthesis enzyme tyrosine hydroxylase and dopamine itself raise an intriguing possibility that the parasite can synthesize and release dopamine and thereby increase the dopamine levels also outside of the dopaminergic neurons [15], [16]. Since dopamine receptors are rather ubiquitous (see e.g., [99]), the parasite-mediated increase of local dopamine levels may well provide a mechanism for altered information processing within many of the above discussed brain regions containing T. gondii cysts, including the olfactory bulb, the amygdala, the hippocampus, the nucleus accumbens and several cortical and thalamic regions.
Conclusion
The results of the present study show that T. gondii cysts are distributed throughout the brain and the parasite is not guided toward a particular functional system. The striking interindividual differences in the total parasite load and cyst distribution indicate a probabilistic nature of brain infestation. However, the cyst density is not homogeneous; some brain regions are consistently more infected than others. We argue that factors underlying brain colonization during acute infection, including local properties of the blood-brain barrier, regional cytoarchitectural features, the level of metabolism and blood flow, and the presence of compact myelinated bodies as natural barriers for the parasite, can contribute interactively to the increased infestation of certain cortical and subcortical regions. We further argue that subtle tropism stemming from uneven brain colonization suffices for the explanation of the numerous behavioral abnormalities observed in the chronically infected rodents, provided that the parasite is able to alter brain function locally. Specifically, the infestation of the somatosensory and motor cortices, and the ventrolateral thalamic nucleus may account for motor and coordination deficits; the infestation of the olfactory bulb, the visual cortex and the barrel field of the somatosensory cortex for sensory attention deficits and compromised sensorimotor integration; the infestation of the entorhinal cortex and the dorsal hippocampus for cognitive deficits and compromised spatial orientation; the infestation of the amygdala, the ventral hippocampus, the nucleus accumbens and the ventral tegmental area for the attenuated defensive behavior and decreased vigilance for predators, respectively. Thus, equipped with a capacity to modulate local dopamine metabolism, T. gondii can effectively change the behavioral phenotype of infected hosts despite the absence of well targeted tropism.
Materials and Methods
Ethics statement
Animal husbandry and all experimental procedures complied with the European Community regulations on the care and use of experimental animals, and were approved by the institutional animal care and use committee of the Faculty of Science, Charles University in Prague (2004/10).
Parasites and experimental animals
Fifty-eight male mice of the CD1 outbred strain, obtained from Anlab (Czech Republic), were used. The mice were housed in plastic cages with wood shaving bedding and nesting material and kept in a 12∶12 h light-dark cycle. Food and water were available ad libitum. At 6 months of age, twenty-nine mice were inoculated per orally with 0.5 ml of brain suspension in saline, which contained 10 tissue cysts of the avirulent cyst-forming HIF strain of Toxoplasma gondii, isolated in 1993 in the Czech Republic from the cerebrospinal fluid of a male HIV-positive patient with asymptomatic toxoplasmosis [45]. The brain suspension was prepared from the brain of a mouse infected previously and was administered to mice by a stomach tube. A control group of twenty-nine sham infected mice received 0.5 ml of saline. All mice were regularly observed for symptoms of acute toxoplasmosis and their weights were recorded.
Serological tests
Serological tests for toxoplasmosis were carried out in the National Reference Laboratory for Toxoplasmosis of the National Institute of Public Health, Prague. Blood for serological tests was taken from the orbital sinus of all experimental and control animals. Antibody titers for toxoplasmosis were determined by the complement fixation test (CFT) (SEVAC, Prague) according to standard procedure recommended by the manufacturer [100].
Histology
Eighteen weeks after inoculation, five infected and one control mouse were deeply anesthetized with intraperitoneal injection of ketamine (150 mg/kg) and xylazine (15 mg/kg), and perfused with 50 ml of warm heparinized saline, followed by 50 ml of 4% Bouin's fixative. Brains were immediately dissected, postfixed for 3 days in the same fixative, embedded in paraffin and sectioned in the coronal plane at a thickness of 8 µm. Sections were mounted on subbed slides, stained with hematoxylin-eosin and coverslipped with DPX (Fluka, Buchs, Switzerland). Brains of other 7 infected mice were homogenized and the number of brain cysts was determined in ten samples of 10 µl suspension per each brain homogenate at 200× magnification.
Data analysis
All sections (i.e., ∼1600 sections per animal) were systematically examined with an Olympus BX51 microscope at 200× magnification. The exact position of all tissue cysts and histopathological changes were noted; the cyst positions were drawn into coronal diagrams of a mouse brain [101] using CorelDraw 12 (Corel Corporation, Ottawa, Ontario, Canada). Cyst counts and densities were determined for 56 brain structures, total cyst counts were calculated for 5 fundamental brain parts.
Measurements of brain structure volumes were performed using a stereotaxic mouse brain atlas [101]. One hundred sixty one coronal diagrams were used for planimetry on a digitizer tablet; areas were measured using Scion Image for Windows (Scion Corporation, Frederick, Maryland, USA). The resulting area values were multiplied by the distance between the diagrams to arrive at the serial volumes. The sum of the volumes for a given structure was used to assess its relative size (see Table 1).
Statistical methods
Data were analyzed using SPSS for Windows (version 16). A general linear model (GLM) repeated measures procedure was used to compare the changes in body weight after inoculation between infected and sham infected animals. The Kendall tau rank correlation coefficient was used to assess association between the numbers of brain tissue cysts and T. gondii antibody titers. A generalized linear mixed model (GLMM) with Poisson error structure and a logarithmic link function was used to test the null hypothesis that the cyst density is determined solely by the total parasite load and independent of the brain region. After the rejection of this hypothesis, we used the Kendall's coefficient of concordance to arbitrate whether the probability of a high or low infestation of a particular brain region is consistent among the infected mice. The GLMM was also used to test whether a particular brain region is infested more or less than expected from its volume. The Šidák correction was used to counteract the problem of multiple comparisons. Because the GLMM is prone to produce false positive results in small structures (due to accidental focal infestation, see e.g., the facial and hypoglossal nuclei), we also calculated a tropism index indicating the number of animals, in which infestation was higher than expected.
Supporting Information
List of abbreviations.
(DOCX)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Ministry of Education, Youth and Sport of the Czech Republic (Grant No. 0021620828) and Grand Agency of Czech Republic (Grant No. P303/11/1398). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hutchison WM, Work K. Toxoplasma – a versatile parasite. New Sci. 1969;29:464–466. [Google Scholar]
- 2.Webster JP. Rats, cats, people and parasites: the impact of latent toxoplasmosis on behaviour. Microbes Infect. 2001;3:1037–1045. doi: 10.1016/s1286-4579(01)01459-9. [DOI] [PubMed] [Google Scholar]
- 3.Webster JP. The effect of Toxoplasma gondii on animal behavior: Playing cat and mouse. Schizophr Bull. 2007;33:752–756. doi: 10.1093/schbul/sbl073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegr J. Influence of latent toxoplasmosis on the phenotype of intermediate hosts. Folia Parasitol (Praha) 2010;57:81–7. doi: 10.14411/fp.2010.010. [DOI] [PubMed] [Google Scholar]
- 5.Vyas A, Sapolsky R. Manipulation of host behaviour by Toxoplasma gondii: what is the minimum a proposed proximate mechanism should explain? Folia Parasitol (Praha) 2010;57:88–94. doi: 10.14411/fp.2010.011. [DOI] [PubMed] [Google Scholar]
- 6.Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci. 2000;267:1591–1594. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A. 2007;104:6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamberton PHL, Donnelly CA, Webster JP. Specificity of the Toxoplasma gondii-altered behaviour to definitive versus non-definitive host predation risk. Parasitology. 2008;135:1143–50. doi: 10.1017/S0031182008004666. [DOI] [PubMed] [Google Scholar]
- 9.Kannan G, Moldovan K, Xiao JC, Yolken RH, Jones-Brando L, et al. Toxoplasma gondii strain-dependent effects on mouse behaviour. Folia Parasitol (Praha) 2010;57:151–155. doi: 10.14411/fp.2010.019. [DOI] [PubMed] [Google Scholar]
- 10.Hermes G, Ajioka JW, Kelly KA, Mui E, Roberts F, et al. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation. 2008;5:48. doi: 10.1186/1742-2094-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horacek J, Flegr J, Tintera J, Verebova K, Spaniel F, et al. Latent toxoplasmosis reduces gray matter density in schizophrenia but not in controls: Voxel-based-morphometry (VBM) study. World J Biol Psychiatry. 2011 doi: 10.3109/15622975.2011.573809. doi: 10.3109/15622975.2011.573809. [DOI] [PubMed] [Google Scholar]
- 12.Skallová A, Novotná M, Kolbeková P, Gašová Z, Veselý V, et al. Decreased level of novelty seeking in blood donors infected with Toxoplasma. Neuro Endocrinol Lett. 2005;26:480–486. [PubMed] [Google Scholar]
- 13.Webster JP, McConkey GA. Toxoplasma gondii-altered host behaviour: clues as to mechanism of action. Folia Parasitol (Praha) 2010;57:95–104. doi: 10.14411/fp.2010.012. [DOI] [PubMed] [Google Scholar]
- 14.Stibbs HH. Changes in brain concentrations of catecholamines and indoleamines in Toxoplasma gondii infected mice. Ann Trop Med Parasitol. 1985;79:153–157. doi: 10.1080/00034983.1985.11811902. [DOI] [PubMed] [Google Scholar]
- 15.Gaskell EA, Smith JE, Pinney JW, Westhead DR, McConkey GA. A unique dual activity amino acid hydroxylase in Toxoplasma gondii. PLoS One. 2009;4:e4801. doi: 10.1371/journal.pone.0004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, et al. The neurotropic parasite Toxoplasma gondii increases dopamine metabolism. PLoS ONE. 2011;6(9):e23866. doi: 10.1371/journal.pone.0023866. doi: 10.1371/journal.pone.0023866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutchison WM, Aitken PP, Wells BWP. Chronic Toxoplasma infections and familiarity novelty discrimination in the mouse. Ann Trop Med Parasitol. 1980;74:145–150. doi: 10.1080/00034983.1980.11687324. [DOI] [PubMed] [Google Scholar]
- 18.Webster JP. The effect of Toxoplasma gondii and other parasites on activity levels in wild and hybrid Rattus norvegicus. Parasitology. 1994;109:583–589. doi: 10.1017/s0031182000076460. [DOI] [PubMed] [Google Scholar]
- 19.Hrdá S, Votýpka J, Kodym P, Flegr J. Transient nature of Toxoplasma gondii-induced behavioral changes in mice. J Parasitol. 2000;86:657–663. doi: 10.1645/0022-3395(2000)086[0657:TNOTGI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 20.Skallová A, Kodym P, Frynta D, Flegr J. The role of dopamine in Toxoplasma-induced behavioural alterations in mice: an ethological and ethopharmacological study. Parasitology. 2006;133:525–535. doi: 10.1017/S0031182006000886. [DOI] [PubMed] [Google Scholar]
- 21.Gulinello M, Acquarone M, Kim JH, Spray DC, Barbosa HS, et al. Acquired infection with Toxoplasma gondii in adult mice results in sensorimotor deficits but normal cognitive behavior despite widespread brain pathology. Microbes Infect. 2010;12:528–537. doi: 10.1016/j.micinf.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hay J, Hutchison WM, Aitken PP, Graham DI. The effect of congenital and adult-aquired Toxoplasma infections on activity and responsiveness to novel stimulation in mice. Ann Trop Med Parasitol. 1983;77:483–495. doi: 10.1080/00034983.1983.11811741. [DOI] [PubMed] [Google Scholar]
- 23.Berdoy M, Webster JP, Macdonald DW. Parasite-altered behaviour: is the effect of Toxoplasma gondii on Rattus norvegicus specific? Parasitology. 1995;111:403–409. doi: 10.1017/s0031182000065902. [DOI] [PubMed] [Google Scholar]
- 24.Flegr J, Preiss M, Klose J, Havlíček J, Vitáková M, et al. Decreased level of psychobiological factor novelty seeking and lower intelligence in men latently infected with the protozoan parasite Toxoplasma gondii. Dopamine, a missing link between schizophrenia and toxoplasmosis. Biol Psychol. 2003;63:253–268. doi: 10.1016/s0301-0511(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 25.Webster JP, Lamberton PH, Donnelly CA, Torrey EF. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii's ability to alter host behaviour. Proc Biol Sci. 2006;273:1023–30. doi: 10.1098/rspb.2005.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrey EF, Yolken RH. Could schizophrenia be a viral zoonosis transmitted from house cats? Schizophr Bull. 1995;21:167–171. doi: 10.1093/schbul/21.2.167. [DOI] [PubMed] [Google Scholar]
- 27.Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007;33:729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Cristina M, Marocco D, Galizi R, Proietti C, Spaccapelo R, et al. Temporal and spatial distribution of Toxoplasma gondii differentiation into bradyzoites and tissue cyst formation in vivo. Infect Immun. 2008;76:3491–3501. doi: 10.1128/IAI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson DJ, Graham DI, Hutchison WM. Pathological changes in the brains of mice infected with Toxoplasma gondii: a histological, immunocytochemical and ultrastructural study. Int J Exp Pathol. 1991;72:463–474. [PMC free article] [PubMed] [Google Scholar]
- 30.Kittas S, Kittas C, Paizi-Biza P, Henry L. A histological and immunohistochemical study of the changes induced in brains of white mice by infection with Toxoplasma gondii. Br J Exp Pathol. 1984;65:67–74. [PMC free article] [PubMed] [Google Scholar]
- 31.Dellacasa-Lindberg I, Hitziger N, Barragan A. Localized recrudescence of Toxoplasma infections in the central nervous system of immunocompromised mice assessed by in vivo bioluminescence imaging. Microbes Infect. 2007;9:1291–1298. doi: 10.1016/j.micinf.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez LE, Rojnik B, Urrea F, Urdaneta H, Petrosino P, et al. Toxoplasma gondii infection lower anxiety as measured in the plus-maze and social interaction tests in rats A behavioral analysis. Behav Brain Res. 2007;177:70–79. doi: 10.1016/j.bbr.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Post MJ, Chan JC, Hensley GT, Hoffman TA, Moskowitz LB, et al. Toxoplasma encephalitis in Haitian adults with acquired immunodeficiency syndrome: a clinical-pathologic-CT correlation. AJR Am J Roentgenol. 1983;140:861–868. doi: 10.2214/ajr.140.5.861. [DOI] [PubMed] [Google Scholar]
- 34.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 35.Arendt G, von Giesen HJ, Hefter H, Neuen-Jacob E, Roick H, et al. Long-term course and outcome in AIDS patients with cerebral toxoplasmosis. Acta Neurol Scand. 1999;100:178–184. doi: 10.1111/j.1600-0404.1999.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 36.Reiter-Owona I, Seitz H, Gross U, Sahm M, Rockstroh JK, et al. Is stage conversion the initiating event for reactivation of Toxoplasma gondii in brain tissue of AIDS patients? J Parasitol. 2000;86:531–536. doi: 10.1645/0022-3395(2000)086[0531:ISCTIE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 37.Canteras NS. The medial hypothalamic defensive system: Hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- 38.Canteras NS, Chiavegatto S, Ribeiro do Valle LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull. 1997;44:297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- 39.Dielenberg RA, McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25:597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- 40.Comoli E, Ribeiro-Barbosa ER, Canteras NS. Predatory hunting and exposure to a live predator induce opposite patterns of Fos immunoreactivity in the PAG. Behav Brain Res. 2003;138:17–28. doi: 10.1016/s0166-4328(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 41.McGregor IS, Hargreaves GA, Apfelbach R, Hunt GE. Neural correlates of cat odor-induced anxiety in rats: region-specific effects of the benzodiazepine midazolam. J Neurosci. 2004;24:4134–4144. doi: 10.1523/JNEUROSCI.0187-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beijamini V, Guimarães FS. c-Fos expression increase in NADPH-diaphorase positive neurons after exposure to a live cat. Behav Brain Res. 2006;170:52–61. doi: 10.1016/j.bbr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 43.Staples LG, McGregor IS, Apfelbach R, Hunt GE. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neuroscience. 2008;151:937–947. doi: 10.1016/j.neuroscience.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 44.Darcy F, Santoro F. Kierszenbaum F, editor. Toxoplasmosis. Parasitic infections and the immune system. 1994. pp. 163–201. Academic Press Inc., San Diego, Calif.
- 45.Kodym P, Blažek K, Malý M, Hrdá Š. Pathogenesis of experimental toxoplasmosis in mice with strains differing in virulence. Acta Parasitol. 2002;47:239–248. [Google Scholar]
- 46.Denkers EY, Gazzelini RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev. 1998;11:569–588. doi: 10.1128/cmr.11.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee YH, Channon JY, Matsuura T, Schwartzman JD, Shin DW, et al. Functional and quantitative analysis of splenic T cell immune responses following oral Toxoplasma gondii infection in mice. Exp Parasitol. 1999;91:212–221. doi: 10.1006/expr.1998.4359. [DOI] [PubMed] [Google Scholar]
- 48.Vyas A, Kim SK, Sapolsky RM. The effects of Toxoplasma infection on rodent behavior are dependent on dose of the stimulus. Neuroscience. 2007;148:342–348. doi: 10.1016/j.neuroscience.2007.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witting PA. Learning capacity and memory of normal and Toxoplasma-infected laboratory rats and mice. Z Parasitenkd. 1979;61:29–51. doi: 10.1007/BF00927085. [DOI] [PubMed] [Google Scholar]
- 50.Hodková H, Kodym P, Flegr J. Poorer results of mice with latent toxoplasmosis in learning tests: impaired learning processes or the novelty discrimination mechanism? Parasitology. 2007;134:1329–1337. doi: 10.1017/S0031182007002673. [DOI] [PubMed] [Google Scholar]
- 51.Hay J, Aitken PP, Hair DM, Hutchison WM, Graham DI. The effect of congenital Toxoplasma infection on mouse activity and relative preference for exposed areas over a series of trials. Ann Trop Med Parasitol. 1984;78:611–618. doi: 10.1080/00034983.1984.11811872. [DOI] [PubMed] [Google Scholar]
- 52.Hay J, Aitken PP, Arnott MA. The influence of congenital Toxoplasma infection on the spontaneous running activity of mice. Z Parasitenkd. 1985;71:459–462. doi: 10.1007/BF00928348. [DOI] [PubMed] [Google Scholar]
- 53.Araujo FG, Williams DM, Grumet FC, Remington JS. Strain-dependent differences in murine susceptibility to Toxoplasma. Infect Immun. 1976;13:1528–1530. doi: 10.1128/iai.13.5.1528-1530.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson AM. Strain-dependent, route of challenge-dependent, murine susceptibility to toxoplasmosis. Z Parasitenkd. 1984;70:303–309. doi: 10.1007/BF00927816. [DOI] [PubMed] [Google Scholar]
- 55.Herculano-Houzel S, Mota B, Lent R. Cellular scaling rules for rodent brains. Proc Natl Acad Sci U S A. 2006;103:12138–43. doi: 10.1073/pnas.0604911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 57.Fagard R, Van Tan H, Creuzet C, Pelloux H. Differential development of Toxoplasma gondii in neural cells. Parasitol Today. 1999;15:504–507. doi: 10.1016/s0169-4758(99)01568-9. [DOI] [PubMed] [Google Scholar]
- 58.Supiot F, Guillaume MP, Hermanus N, Telerman-Toppet N, Karmali R. Toxoplasma encefalitis in a HIV patient: unusual involvement of the corpus callosum. Clin Neurol Neurosurg. 1997;99:287–290. doi: 10.1016/s0303-8467(97)00101-7. [DOI] [PubMed] [Google Scholar]
- 59.Unno A, Suzuki K, Xuan X, Nishikawa Y, Kitoh K, et al. Dissemination of extracellular and intracellular Toxoplasma gondii tachyzoites in the blood flow. Parasitol Int. 57:515–518. doi: 10.1016/j.parint.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Lambert H, Dellacasa-Lindberg I, Barragan A. Migratory responses of leukocytes infected with Toxoplasma gondii. Microbes Infect. 2011;13:96–102. doi: 10.1016/j.micinf.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Conley FK, Jenkins KA. Immunohistological study of the anatomic relationship of Toxoplasma antigens to the inflammatory response in the brains of mice chronically infected with Toxoplasma gondii. Infect Immun. 1981;31:1184–1192. doi: 10.1128/iai.31.3.1184-1192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci. 2002;25:621–625. doi: 10.1016/s0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- 63.Hevner RF, Liu S, Wong-Riley MT. A metabolic map of cytochrome oxidase in the rat brain: histochemical, densitometric and biochemical studies. Neuroscience. 1995;65:313–342. doi: 10.1016/0306-4522(94)00514-6. [DOI] [PubMed] [Google Scholar]
- 64.Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:53–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- 65.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 66.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 67.De Olmos JS, Beltramino CA, Alheid G. Paxinos G, editor. Amygdala and extended amygdala of the rat: a cytoarchitectonical, fibroarchiotectonical, and chemoarchitectonical survey. The rat nervous system. 2004. pp. 509–603. Elsevier Academic Press, San Diego.
- 68.Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behav Neurosci. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- 69.Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- 70.Witter MP, Amaral DG. Paxinos G, editor. Hippocampal formation. The rat nervous system. 2004. pp. 635–704. Elsevier Academic Press, San Diego.
- 71.van Strien NM, Cappaert NL, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- 72.Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, et al. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- 74.Trivedi MA, Coover GD. Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol Learn Mem. 2004;81:172–184. doi: 10.1016/j.nlm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. Eur J Neurosci. 2006;23:2185–2196. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- 76.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwienbacher I, Fendt M, Richardson R, Schnitzler HU. Temporary inactivation of the nucleus accumbens disrupts acquisition and expression of fear-potentiated startle in rats. Brain Res. 2009;1027:87–93. doi: 10.1016/j.brainres.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 78.Liu ZH, Shin R, Ikemoto S. Dual role of medial A10 dopamine neurons in affective encoding. Neuropsychopharmacology. 2008;33:3010–3020. doi: 10.1038/npp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Groenewegen HJ, Witter MP. Paxinos G, editor. Thalamus. The rat nervous system. 2004. pp. 407–453. Elsevier Academic Press, San Diego.
- 80.Hutchison WM, Aitken PP, Wells BWP. Chronic Toxoplasma infections and motor performance in the mouse. Ann Trop Med Parasitol. 1980;74:507–510. doi: 10.1080/00034983.1980.11687376. [DOI] [PubMed] [Google Scholar]
- 81.Havlíček J, Gašová ZG, Smith AP, Zvára K, Flegr J. Decrease of psychomotor performance in subjects with latent ‘asymptomatic’ toxoplasmosis. Parasitology. 2001;122:515–520. doi: 10.1017/s0031182001007624. [DOI] [PubMed] [Google Scholar]
- 82.Yereli K, Balcioğlu IC, Ozbilgin A. Is Toxoplasma gondii a potential risk for traffic accidents in Turkey? Forensic Sci Int. 2006;163:34–37. doi: 10.1016/j.forsciint.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 83.Flegr J, Klose J, Novotná M, Berenreitterová M, Havlíček J. Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study. BMC Infect Dis. 2009;9:72. doi: 10.1186/1471-2334-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hutchison WM, Bradley M, Cheyne WM, Wells BWP, Hay J. Behavioural abnormalities in Toxoplasma-infected mice. Ann Trop Med Parasitol. 1980;74:337–345. doi: 10.1080/00034983.1980.11687350. [DOI] [PubMed] [Google Scholar]
- 85.Takeda H, Tsuji M, Matsumiya T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur J Pharmacol. 1998;350:21–29. doi: 10.1016/s0014-2999(98)00223-4. [DOI] [PubMed] [Google Scholar]
- 86.O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Claredon; 1978. [Google Scholar]
- 87.Best PJ, White AM, Minai A. Spatial progressing in the brain: the activity of hippocampal place cells. Annu Rev Neurosci. 2001;24:459–486. doi: 10.1146/annurev.neuro.24.1.459. [DOI] [PubMed] [Google Scholar]
- 88.Moser EI, Kropff E, Moser MB. Place cells, grid cells, ant the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- 89.Eichenbaum H. A cortical–hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 90.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 91.Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- 92.Ferbinteanu J, Shapiro ML. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- 93.Leutgeb S, Leutgeb JK, Barnes CA, Moser EI, McNaughton BL, et al. Independent codes for spatial and episodic memory in hippocampal neuronal ensembles. Science. 2005;309:619–623. doi: 10.1126/science.1114037. [DOI] [PubMed] [Google Scholar]
- 94.Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- 95.Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 96.Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, et al. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- 97.Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- 98.Piekarski G, Zippelius HM, Witting PA. Auswirkungen einer latenten Toxoplasma-Infektion auf das Lernvermogen von weissen Laboratoriumsratten and mausen. Z Parasitenkd. 1978;57:1–15. doi: 10.1007/BF00927625. [DOI] [PubMed] [Google Scholar]
- 99.Tohyama M, Takatsuji K. Atlas of neuroactive substances and their receptors in the rat. 1998. 337 Oxford University Press. Oxford.
- 100.Zástěra M, Pokorný J, Jíra J, Valkoun A. Amendment to standard laboratory methods for diagnosing of toxoplasmosis. Acta Hyg Epid Microb. 1987;3:3–14. [Google Scholar]
- 101.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 2001. 264 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of abbreviations.
(DOCX)